Back to Journals » Medical Devices: Evidence and Research » Volume 16
Safety and Performance of Hemostatic Powders
Authors Szymanski L, Gołaszewska K, Małkowska J, Kaczyńska J, Gołębiewska M, Gromadka B, Matak D
Received 9 February 2023
Accepted for publication 16 May 2023
Published 8 June 2023 Volume 2023:16 Pages 133—144
DOI https://doi.org/10.2147/MDER.S407838
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lukasz Szymanski,1,2 Kamila Gołaszewska,2 Justyna Małkowska,2 Judyta Kaczyńska,2 Małgorzata Gołębiewska,2 Bartosz Gromadka,2 Damian Matak2
1Department of Molecular Biology, Institute of Genetics and Animal Biotechnology, Polish Academy of Science, Magdalenka, 05-552, Poland; 2European Biomedical Institute, Jozefow, 05-410, Poland
Correspondence: Damian Matak, Email [email protected]
Background: Hemorrhage, a sudden and severe leakage of blood due to the disruption of blood vessels, is one of the most common causes of death from injuries worldwide. Severe bleeding accounts for more than 35% of pre-hospital deaths and about 40% of deaths recorded within 24 hours of injury. One of the methods for achieving homeostasis is the use of hemostatic powders. This study compares the basic safety and performance of the most popular hemostatic powders.
Methods: Basic safety of commercially available products were evaluated using MTT, MEM elution assay, and endotoxin testing. The in vitro performance was evaluated using water absorption capacity, water absorption rate, and adhesion strength assays.
Results: 4Seal, Starsil, and 4DryField extracts did not cause cytotoxicity in MTT and MEM elution assays. PerClot and SuperClot extracts demonstrated cytotoxic potential in MTT assay, while Arista extract was cytotoxic in both MEM elution and MTT assays. 4Seal has the lowest endotoxin contamination, followed by PerClot, 4DryField, SuperClot, Arista, and Starsil. 4Seal and Starsil showed significantly highest WAR among the tested samples, followed by 4DryField, Arista, PerClot, and SuperClot. Adhesion force is highest for 4Seal, followed by Starsil, PerClot, 4DryField Arista, and SuperClot.
Conclusion: 4Seal is the most versatile in terms of safety and functional properties compared to 4DryField, Arista, PerClot, Starsil, and SuperClot.
Keywords: hemostasis, hemostatic powder, bleeding, 4DryField, 4Seal, Arista, PerClot, Starsil, SuperClot
Introduction
Hemorrhage, which is a sudden and severe leakage of blood due to the disruption of blood vessels, is one of the most common causes of death from injuries in the world.1 It is estimated that, excluding armed conflicts, severe bleeding accounts for more than 35% of pre-hospital deaths and about 40% of deaths recorded within 24 hours of injury.2 However, death from bleeding often occurs much faster. For example, six deaths for every 10 bleeding deaths occur in the first three hours after the injury.3 The etymology distinguishes external hemorrhages caused by open wounds and internal hemorrhages that do not go beyond the body and are invisible to the eyes, which makes rescue procedures difficult.
However, any bleeding can be dangerous, especially if it occurs during surgery. Hemorrhagic shock is especially life-threatening, as it results from the loss of at least 25% of the blood volume and requires immediate assistance in the form of bleeding control.4 It has been shown that in patients who underwent reoperation for bleeding in the head and neck area, the risk of death within 30 days increased more than 5-fold.5 Therefore, maintaining hemostasis during surgical procedures is crucial in maintaining the vital functions of the patient and better visibility of the operated area of the body for the surgeon.6
Direct compression is the primary action to stop bleeding. If this method is not effective, a tourniquet is recommended. However, both methods are effective for hemorrhage in pressure areas such as the limbs.7 Research into hemostatic agents has developed since the beginning of the XXI century. One of the first hemostatic agents was chitosan and zeolite-based dressing that was used in the US Army.8 The dressing sealed the blood vessels and absorbed water, concentrating the blood coagulation factors. Hemostatic agents are most effective for non-compressible bleeding.9 The advantages of using hemostatic agents include ease and short time of application, low cost, negligible risk of immune responses and reduced need for transfusion of blood and blood products after surgery. What is more, this method is particularly helpful in cases where the bleeding area is extensive, and it is not possible to pinpoint the exact location of the bleeding.10 On the other hand, the biggest disadvantage of the use of hemostatic powders is that the performance of these materials relies on the natural homeostatic system of the body.11 The primary mechanism of action of polysaccharide-based hemostats, such as 4DryField, 4Seal, Arista, PerClot, SuperClot, and Starsil consists of absorbing water from the blood and concentrating the blood components at the injury site.
Over the years, many hemostatic powders have been developed and recognized as biocompatible. The main objective of biocompatibility testing is to minimize the potential risks associated with medical devices. However, it is important to note that biocompatibility outcomes for a product can fall anywhere within the permissible range as defined by relevant regulations. The results of biocompatibility testing for any given product are closely linked to the safety profile of the medical device in clinical settings. As a result, the higher the level of biocompatibility achieved, the lower the likelihood of adverse effects occurring.12
Therefore, the aim of this study is to evaluate and compare the cytotoxicity potential, endotoxin level and water absorption related performance of 4DryField, 4Seal, Arista, PerClot, SuperClot, and Starsil.12
Materials
Cell lines were purchased from ATCC, reagents for cell culture were purchased from Thermo Fisher Scientific, Poland, and all chemical compounds were purchased from Sigma, Poland, unless otherwise indicated. Detailed information about hemostatic powders are presented in Table S8 in Supplement Data and the morphology of the hemostatic powders is presented in Figure S4.
Methods
Statistical Analysis
All results are presented as the mean ± standard deviation (SD). Statistical evaluation was performed using the two-way ANOVA with Bonferroni’s multiple comparisons test for water absorption rate studies and one-way ANOVA with Bonferroni’s multiple comparisons test for other assays. GraphPad Prism software (version 9.3.1; GraphPad Software, Inc., La Jolla, CA, USA) was used for all evaluations. p < 0.05 was considered statistically significant. All tests were performed in triplicate unless otherwise stated. Additional statistical information is provided in Supplementary Materials.
Extraction
Before the cytotoxicity tests, hemostatic powders did not undergo any preparations. According to ISO 10993–12, extracts of the test items in the culture medium (MEM, 10% FBS, 1% P/S) were prepared by immersing the hemostatic powders in an extraction vehicle and extracted at 37°C for 24 ± 2 hours using an extraction ratio of 0.2 g/mL. The final volume of the extraction vehicle was adjusted to compensate for the material absorption.13 After extraction, the extracts of the test items were clear, with no visible particulates. Therefore, extracts were used immediately after preparation with no previous alternation.
Cytotoxicity – MTT Assay
The assay was performed according to the ISO 10993–5.14 Briefly, 100%, 50%, 33%, and 25% extracts of the hemostatic powders were added to quadruplicate monolayers of mouse fibroblast cells (L929) and incubated at 37°C, 95% humidity, 5 ± 0.1% CO2 for 24 ± 1 hours. Afterward, extracts were replaced with 50 µL of freshly prepared MTT solution and incubated at 37°C, 95% humidity and 5 ± 0.1% CO2 for 120 minutes. Then, 100 µL of isopropanol was pipetted to each well and incubated at 37°C, 95% humidity and 5 ± 0.1% CO2 for 10 minutes. Following the incubation, absorption at 570 nm (with a reference filter at 650 nm) was measured, and the percent viability was calculated from the blanks.
Cytotoxicity - MEM Elution Assay
The assay was performed according to the ISO 10993–5.14 After extraction, triplicate monolayers of L929 cells were dosed with 600 µL of extracts and incubated in the presence of 5 ± 0.1% CO2, 95% humidity for 24 ± 1 hours. The staining solution was prepared before use by mixing Trypan Blue solution with single strength MEM in a 1:1 ratio. Following the incubation, 100 μL of prepared staining solution was dispensed in each well. Afterward, cytotoxicity was assessed by microscopic observations according to Table 1 included in ISO 10993–5.14
Water Absorption Capacity
0.5 g of hemostatic powder was transferred in triplicate to previously weighted 50 mL falcon tubes. Subsequently, 40 mL of R.O. grade water was added to each tube. Afterward, all tubes were vortexed for 60seconds and then incubated at room temperature for 30 minutes. After incubation, samples were centrifuged (25°C, 3000g) for 10 minutes, and the supernatant fluid was decanted. Each tube was weighted, and water absorption capacity was calculated.15
Water Absorption Rate
The test system was set as indicated in Figure 1. First, a size-fitted filter was placed on the filter funnel. Afterward, the burette was filled with distilled water to reach level zero. Next, the burette was opened until the filter was thoroughly soaked with distilled water. Subsequently, the burette was adjusted to zero graduation. If necessary, distilled water was added to the burette.
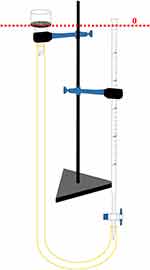 |
Figure 1 Test system to water absorption rate testing consisted of the burette, filter funnel, stand, clamps, and hose. |
Simultaneously, 0.1 g of sample powder was scattered evenly onto the filter, and the burette piston was opened. At the same time, the timer was started, and every 20seconds for a total of 60 seconds, the liquid level was recorded. Then, the water absorption speed was calculated. The test was performed in triplicate for every tested hemostatic powder.15
Adhesion/Cohesion Capacity
One gram of hemostatic powder and 20 mL of distilled water were mixed to obtain homogeneous solutions in a glass beaker. The samples were prepared in triplicate. Afterward, wells were filled entirely with gelled solutions and mounted in the test system, as shown in Figure 2. A metal probe was lowered to the middle of the well and immersed to be in line with the top of the gelled sample. The experimental speed was set to 15 mm/min in tension mode. Force (in N) at which the gel stopped adhering to the metal probe was recorded.15
Determination of Endotoxin Level
Extraction of hemostatic powders was conducted in water for injection at 37 ± 1°C for 72 ± 2 hours in regard to ISO 10993–12.13
Based on 85. Bacterial Endotoxin Test, US Pharmacopoeia, and Pierce Chromogenic Endotoxin Quant Kit was applied to measure endotoxin concentration.16 Tests were performed according to the manufacturer’s instructions. Briefly, a standard curve was prepared (R2=0.9811) and is presented in Figure 3. Also, the assay was internally validated by spiking the samples with 0.5 EU/mL of endotoxins to evaluate the consistency of the assay. All measurements were prepared in triplicate.
 |
Figure 3 Endotoxin standard curve (R2=0.9811). |
Results
MTT Cytotoxicity
4Seal Hemostatic powder, 4DryField Hemostatic powder, and Starsil Hemostatic powder cell culture medium extracts and their dilutions showed no cytotoxic potential to L-929 mouse fibroblasts using the quantitative MTT method in line with ISO 10993–5.
PerClot Hemostatic powder, Arista Hemostatic powder, and SuperClot Hemostatic powder cell culture medium extracts and their dilutions showed cytotoxic potential to L-929 mouse fibroblasts using the quantitative MTT method.
The cellular response observed from the positive and negative controls, systemic cell seeding errors, and absolute value of optical density confirmed the suitability of the test system. Results of cytotoxicity testing are presented in Figure 4. In addition, exact values for each extract are presented in Supplementary Data in Table S1.
 |
Figure 4 Cytotoxicity results. |
MEM Elution
4Seal Hemostatic powder, Starsil Hemostatic powder, PerClot Hemostatic powder, SuperClot Hemostatic powder, and 4DryField Hemostatic Powder cell culture medium extracts showed no cytotoxic potential to L-929 mouse fibroblasts using the MEM Elution method (grade 2 or lower), according to the ISO 10993–5 and USP <87>.
Arista Hemostatic powder cell culture medium extract showed cytotoxic potential to L-929 mouse fibroblasts using the MEM Elution method. Results of cytotoxicity testing are presented in Table 1 and Figure 5.
 |
Table 1 Results of Cytotoxic Potential Assessment |
 |
Figure 5 Cell culture images of cytotoxic potential assessment. |
Water Absorption Capacity (WAC)
4Seal and PerClot are characterized by the highest water absorption capacity of 22.456 mL and 19.827 mL per 0.5 g of powder, followed by Starsil (18.501) and 4DryField (18.008 mL). The lowest WAC was observed for Arista (8.705 mL) and SuperClot (6.463 mL). Results are present in Figure 6. Detailed measurements and statistics are presented in Table S2, Table S3 and Figure S1 in Supplement Data.
 |
Figure 6 Water absorption capacity (WAC) results per 0.5 g of product. p=0.1234; **p=0.0021; ****p<0.0001. Abbreviation: ns, non-significant. |
Water Absorption Rate (WAR)
4Seal and Starsil showed significantly highest WAR among the tested samples (1.97 mL in 20s, 2.4 mL in 40s, 2.57 in 60s and 1.93 mL in 20s, 2.33 mL in 40s, 2.43 mL in 60s, respectively). The water absorption rate of 4DryField is 0.97 mL in 20s, 1.1 mL in 40s, and 1.17 mL in 60s. Arista, PerClot and SuperClot are characterized by lowest WAR of 0.57 mL in 20s, 0.83 mL in 40s, 0.93 mL in 60s, 0.50 mL in 20s, 0.80 mL in 40s, 0.97 mL in 60s, and 0.37 mL in 20s, 0.50 mL in 40s, 0.57 mL in 60s respectively. Results are present in Figure 7. Detailed measurements and statistics are presented in Table S4, Table S5 and Figure S2 in Supplement Data.
 |
Figure 7 Results of water absorption rate (WAR) for 0.1 g of hemostatic powders. *p=0.0332; ***p=0.00002; ****p<0.0001. |
Adhesion Potential
Adhesion force was significantly highest for 4Seal (0.353 N), followed by Starsil (0.280 N). PerClot and 4DryField are characterized by an average adhesion force of 0.097 N and 0.093 N, respectively. Arista and SuperClot showed the lowest adhesion potential with an average force of 0.05 N. Results are presented in Figure 8. Detailed measurements and statistics are presented in Table S6, Table S7 and Figure S3 in Supplement Data.
 |
Figure 8 Results of adhesion assay of hemostatic powders.**p=0.0021; ****p<0.0001. |
Determination of Endotoxin Level
4Seal has lowest endotoxin contamination (0.684 EU/g) followed by PerClot (1.415 EU/g), 4DryField (2.070 EU/g), SuperClot (3.5 EU/g), Arista (4.917 EU/g), and Starsil (11/758 EU/g). Endotoxin testing results per mL and per 1 g finished device are presented in Table 2.
 |
Table 2 Endotoxins Concentration Results |
Discussion
In vitro tests performed in this study were chosen to evaluate the products in terms of basic safety and performance. Cytotoxicity testing is the primary method to evaluate medical devices in terms of cytotoxic potential according to ISO 10993–5 norm.14
Cytotoxicity testing allows for rapid biocompatibility assessment of the medical device. This study investigated tested products using both quantitative and qualitative tests–MTT and MEM elution assays. MTT assay is a quantitative assay with more strict cytotoxicity acceptance criteria than the MEM elution test, which is qualitative. For example, according to the ISO 10993–5, the device is considered non-cytotoxic if the viability of the cells after exposition to the full strength extract is more than 70%. In contrast, the devices pass the MEM elution assay if the cytotoxic score is ≤2, representing a viability of around 50%.17,18
4Seal, Starsil, and 4DryField extracts did not cause cytotoxicity in either test. PerClot and SuperClot extracts demonstrated cytotoxic potential in the MTT assay, while Arista extract was cytotoxic in both MEM elution and MTT assays. 4Seal and Starsil extracts being inert to cultured cells indicate that there should be no negative consequences of using these products in patients. 4DryField extract did not cause cytotoxicity as per ISO 10993–5 requirements however, the viability of the cells was significantly lower than in the case of 4Seal and Starsil extracts, suggesting that it may influence the tissue negatively in clinical use. The results of PerClot, SuperClot, and Arist suggest that the impact on the tissue might be even more severe. Cytotoxicity is a preliminary test using cultured cells, and the failure does not mean that the device is unsafe for clinical use since the results should be evaluated in the context of a full battery of biocompatibility tests.19 However, it is essential in the context of hemostatic powders as those products are commonly used on unprotected organs; therefore, the exposition of the cells building the organ is comparable to the one in the cytotoxicity test.
The presence of endotoxins in the medical device can lead to severe health complications such as impairment of amyloid-beta efflux, acute inflammation, and disturbed CSF distribution.20,21 Therefore, the level of endotoxic contamination was tested for each product. The general limit for medical devices is 20 EU/device.22 The result shows that 4Seal, PerClot, 4DryField, and SuperClot are well below the allowable limit for the maximal size of the product, which is 5 g. On the other hand, Arista meets the endotoxin-level requirements for 1 g and 3 g products, whereas Starsil only for the 1 g size of the device. Results also indicate that the 1 g PerClot and 4DryField as well as 1 g and 3 g 4Seal, may be used in procedures involving contact with cerebrospinal fluid, for which the limit is 2.15 EU/device.22
Tested products achieve homeostasis by absorbing water and concentrating coagulation factors. Therefore, the performance tests focused on water absorption capacity and rate as well as the adhesive force produced by the powder when mixed with water. The higher the water absorption rate and water absorption capacity, the quicker hemostasis is achieved, and more severe bleeding can be managed. Hemostatic powders work by absorbing water from the blood, causing an increase in the concentration of red blood cells, thrombocytes, leukocytes, and coagulation proteins, thus inducing instantly primary hemostasis and accelerating the blood clotting process.23 Thus, generally, higher water handling properties translate to superior clinical properties of the product. Moreover, the convenience of using the hemostatic powder is proportional to the adhesive force of the product. Therefore, hemostatic powders with higher adhesion force will adhere to the tissue more firmly, allowing for better control of the bleeding and visibility of the operation field. Interestingly, Li et al evaluated the water absorption properties of porous gelatin microsphere (PGE) hemostats and showed that during 3 hours of incubation, PGE is able to absorb water equal to 1500% of its original mass.24 Our results indicate that polysaccharide-based hemostats have, in general, better water-absorbing capabilities than PGE with 4Seal ability to absorb 4500% of its mass and SuperClot with 1300%.
Here, we evaluated hemostatic powders that primary mechanism of action consists of absorbing water from the blood and concentrating the blood components at the injury site, but other polysaccharide-based hemostatic materials are available. Chitosan, dextran, cellulose, hyaluronic acid, and alginate are used in the form of powders, films, sponges, wovens, and hydrogels as tissue adhesives, sealants, and hemostatic agents in medicine.25 Recently, hydrogels caught the attention and have been widely researched and developed to enable rapid control of hemorrhage and facilitate wound healing.26 Hydrogels hold many advantages over solid hemostats, such as injectability, the ability to adhere to irregular wounds, the possibility to act as delivery agents of therapeutic compounds or growth factors, and antibacterial properties.27,28 On the other hand, hydrogels have poor absorptive capabilities and may be easily eluted from the application site.29 Moreover, hemostats containing animal-derived components are susceptible to transferring allergic and viral contaminants, while those containing inorganic materials may cause a cytotoxic reaction and are usually non-biodegradable.30 Therefore, even though many innovative hemostatic agents are being developed, the commercial product must be cost-effective, easily portable, and effectively applied at the injury site under any conditions.31
Conclusion
Recently, as hemostatic powders gained popularity in the field of surgery, 16 key recommendations were proposed by an international, multidisciplinary expert group of surgeons regarding the use of hemostatic powders in surgical practice.32 One of the recommendations indicates the need for the wide dissemination of data about the performance of hemostatic powders. In that context, performance studies of commonly used hemostatic powders are of paramount importance. Furthermore, since the mechanism of action of studied medical devices is based on water absorption, the results presented in this study can be directly translated to clinical performance.
Results clearly show that 4Seal has superior functional properties, followed by Starsil and 4DryField hemostatic powders. On the other hand, PerClot, Arista, and SuperClot are characterized by the poorest water-handling properties among tested devices. Overall, in vitro studies indicate that 4Seal is the most versatile in terms of safety and functional properties among tested hemostatic powders.
Impact Statement
Hemorrhage is one of the most common causes of death from injuries worldwide. Severe bleeding accounts for more than 35% of pre-hospital deaths and about 40% of deaths recorded within 24 hours of injury.33 One of the most common methods for achieving homeostasis is the use of hemostatic powders. This study compares the basic safety and performance of the most popular hemostatic powders and can be used as a guideline for choosing the suitable powder for surgery.
List of Abbreviations
WAR, Water Absorption Rate; WAC, Water Absorption Capacity; MEM, Minimum Essential Media; P/S, penicillin/streptomycin; FBS, Fetal Bovine Serum; RO, Reverse Osmosis.
Data Sharing Statement
All of the raw data is presented in Supplementary Materials.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
European Biomedical Institute Sp. z o.o.
Disclosure
Dr Damian Matak and Dr Lukasz Szymanski have invented 4Seal Hemostatic powder. All tests were performed in ISO 17025 accredited and GLP certified laboratory to ensure data integrity. Dr Szymanski and Dr Matak have no financial interests in 4Seal Hemostatic powder. Other authors declare no conflicts of interest in this work.
References
1. Morrison CA. The prehospital treatment of the bleeding patient--dare to dream. J Surg Res. 2013;180(2):246–247. doi:10.1016/j.jss.2011.12.022
2. Chambers JA, Seastedt K, Krell R, et al. “Stop the bleed”: a U.S. military installation’s model for implementation of a rapid hemorrhage control program. Mil Med. 2019;184(3–4):67–71. doi:10.1093/milmed/usy185
3. Holcomb JB, Del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–136. doi:10.1001/2013.jamasurg.387
4. Mutschler M, Nienaber U, Brockamp T, et al. Renaissance of base deficit for the initial assessment of trauma patients: a base deficit-based classification for hypovolemic shock developed on data from 16,305 patients derived from the TraumaRegister DGU®. Crit Care. 2013;17(2):R42. doi:10.1186/cc12555
5. Haapio E, Kinnunen I, Airaksinen JKE, et al. Determinants of re-operation for bleeding in head and neck cancer surgery. J Laryngol Otol. 2018;132(4):336–340. doi:10.1017/S0022215118000294
6. Shah A, Palmer AJR, Klein AA. Strategies to minimize intraoperative blood loss during major surgery. BJS Br J Surg. 2020;107(2):e26–e38. doi:10.1002/bjs.11393
7. Liu L, Mei J, He J, et al. International expert consensus on the management of bleeding during VATS lung surgery. Ann Transl Med. 2019;7(23):712. doi:10.21037/atm.2019.11.142
8. Sung YK, Lee DR, Chung DJ. Advances in the development of hemostatic biomaterials for medical application. Biomater Res. 2021;25:37. doi:10.1186/s40824-021-00239-1
9. Kheirabadi B. Evaluation of topical hemostatic agents for combat wound treatment. US Army Med Dep J. 2011;2011:25–37.
10. Zhong Y, Hu H, Min N, et al. Application and outlook of topical hemostatic materials: a narrative review. Ann Transl Med. 2021;9(7):577. doi:10.21037/atm-20-7160
11. Nouri S, Sharif MR, Afzali H, et al. The advantages and disadvantages of methods used to control liver bleeding: a review. Trauma Mon. 2015;20(4):e28088. doi:10.5812/traumamon.28088
12. Szymanski L, Golaszewska K, Wiatrowska A, et al. ISO 10993 biological evaluation of novel hemostatic powder – 4SEAL®. Biomater Res. 2022;26(1):12. doi:10.1186/s40824-022-00258-6
13. Anonymous. ISO 10993-12:2021(En), biological evaluation of medical devices — part 12: sample preparation and reference materials. Available from: https://www.iso.org/obp/ui/#iso:std:iso:10993:-12:ed-5:v1:en.
14. Anonymous. ISO 10993-5:2009(En), biological evaluation of medical devices — part 5: tests for in vitro cytotoxicity. Available from: https://www.iso.org/obp/ui/#iso:std:iso:10993:-5:ed-3:v1:en.
15. Ji X, Xing C, Shi X. Modified starch material of biocompatible hemostasis; 2013.
16. Anonymous. Bacterial Endotoxins. USP. Available from: https://www.usp.org/harmonization-standards/pdg/general-methods/bacterial-endotoxins.
17. Räägel H, Turley A, Fish T, et al. Medical device industry approaches for addressing sources of failing cytotoxicity scores. Biomed Instrum Technol. 2021;55(2):69–84. doi:10.2345/0890-8205-55.2.69
18. Li W, Zhou J, Xu Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed Rep. 2015;3(5):617–620. doi:10.3892/br.2015.481
19. Liu X, Rodeheaver DP, White JC, et al. A comparison of in vitro cytotoxicity assays in medical device regulatory studies. Regul Toxicol Pharmacol RTP. 2018;97:24–32. doi:10.1016/j.yrtph.2018.06.003
20. Manouchehrian O, Ramos M, Bachiller S, et al. Acute systemic LPS-exposure impairs perivascular CSF distribution in mice. J Neuroinflammation. 2021;18(1):34. doi:10.1186/s12974-021-02082-6
21. Erickson MA, Hartvigson PE, Morofuji Y, et al. Lipopolysaccharide impairs amyloid beta efflux from brain: altered vascular sequestration, cerebrospinal fluid reabsorption, peripheral clearance and transporter function at the blood–brain barrier. J Neuroinflammation. 2012;9:150. doi:10.1186/1742-2094-9-150
22. Research C for DE and Guidance for Industry. Pyrogen and endotoxins testing: questions and answers. FDA; 2019. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-pyrogen-and-endotoxins-testing-questions-and-answers.
23. Bustamante-Balén M, Plumé G. Role of hemostatic powders in the endoscopic management of gastrointestinal bleeding. World J Gastrointest Pathophysiol. 2014;5(3):284–292. doi:10.4291/wjgp.v5.i3.284
24. Li L, Du Y, Yin Z, et al. Preparation and the hemostatic property study of porous gelatin microspheres both in vitro and in vivo. Colloids Surf B Biointerfaces. 2020;187:110641. doi:10.1016/j.colsurfb.2019.110641
25. Li D, Chen J, Wang X, et al. Recent advances on synthetic and polysaccharide adhesives for biological hemostatic applications. Front Bioeng Biotechnol. 2020;8:926. doi:10.3389/fbioe.2020.00926
26. Pourshahrestani S, Zeimaran E, Kadri NA, et al. Polymeric hydrogel systems as emerging biomaterial platforms to enable hemostasis and wound healing. Adv Healthc Mater. 2020;9(20):2000905. doi:10.1002/adhm.202000905
27. Wang T, Yi W, Zhang Y, et al. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloids Surf B Biointerfaces. 2023;222:113096. doi:10.1016/j.colsurfb.2022.113096
28. Chen Z, Yao J, Zhao J, et al. Injectable wound dressing based on carboxymethyl chitosan triple-network hydrogel for effective wound antibacterial and hemostasis. Int J Biol Macromol. 2023;225:1235–1245. doi:10.1016/j.ijbiomac.2022.11.184
29. Kibungu C, Kondiah PPD, Kumar P, et al. This review recent advances in chitosan and alginate‐based hydrogels for wound healing application. Front Mater. 2021;8. doi:10.3389/fmats.2021.681960
30. Yu P, Zhong W. Hemostatic materials in wound care. Burns Trauma. 2021;9:tkab019. doi:10.1093/burnst/tkab019
31. Santosh Shivaji B, Jianzhong S, Yifei S, et al. Polysaccharide-based hemostats: recent developments, challenges, and future perspectives. Cellulose. 2021;28(14):8899–8937. doi:10.1007/s10570-021-04132-x
32. Eden C, Buonomo OC, Busch J, et al. An international multidisciplinary peer-driven consensus on the optimal use of hemostatic powders in surgical practice. Updat Surg. 2021;73(4):1267–1273. doi:10.1007/s13304-021-01136-x
33. Donley ER, Munakomi S, Loyd JW. Hemorrhage Control. Treasure Island (FL): StatPearls StatPearls Publishing; 2023.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.