Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Safety and Efficacy of Transarterial Chemoembolization and Immune Checkpoint Inhibition with Camrelizumab for Treatment of Unresectable Hepatocellular Carcinoma
Authors Zhang JX, Chen P , Liu S, Zu QQ, Shi HB, Zhou CG
Received 16 January 2022
Accepted for publication 23 March 2022
Published 31 March 2022 Volume 2022:9 Pages 265—272
DOI https://doi.org/10.2147/JHC.S358658
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Imam Waked
Jin-Xing Zhang,1,* Pei Chen,2,* Sheng Liu,1 Qing-Quan Zu,1 Hai-Bin Shi,1 Chun-Gao Zhou1
1Department of Interventional Radiology, The First Affiliated Hospital with Nanjing Medical University, Nanjing, 210029, People’s Republic of China; 2Department of Basic Medicine, Jiangsu College of Nursing, Huai’an, 223005, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hai-Bin Shi; Chun-Gao Zhou, Department of Interventional Radiology, The First Affiliated Hospital with Nanjing Medical University, No. 300 Guangzhou Road, Nanjing, 210029, People’s Republic of China, Tel +86-25-68306316 ; +86-13770598792, Fax +86-25-83724440, Email [email protected]; [email protected]
Background: The clinical outcomes of hepatocellular carcinoma (HCC) patients who receive transarterial chemoembolization (TACE) and immunotherapy are not well characterized. The present study evaluates the safety and efficacy of TACE in combination with immune checkpoint inhibitor treatment for unresectable HCC.
Methods: A retrospective analysis of 34 HCC patients who received TACE and treatment with the immune checkpoint inhibitor (ICI), Camrelizumab, between July 2019 and May 2021, was performed. This included 21 patients who developed progressive disease and eight who remained stable after several sessions of TACE, along with five patients who were initially diagnosed with advanced HCC. Adverse events (AEs), objective response rate (ORR) according to modified response evaluation criteria in solid tumors, progression-free survival (PFS), and overall survival (OS) were evaluated.
Results: The median follow-up from ICI initiation was 10.6 months (range: 2.4– 25.0 months). Grade I/II and grade III/IV AEs from ICI treatment occurred in 20 (58.8%) and 2 patients (5.9%), respectively. Two to three months after ICI therapy, the ORR was 35.3% (12/34) and the median PFS and OS was 6.1 months (range: 1.1– 19.3 months) and 13.3 months (range: 2.4– 25.0 months), respectively.
Conclusion: TACE in combination with ICI could be a promising treatment approach for unresectable HCC patients.
Keywords: efficacy, hepatocellular carcinoma, immune checkpoint inhibition, safety, transarterial chemoembolization
Introduction
Transarterial chemoembolization (TACE) is a widely accepted treatment modality for patients with unresectable hepatocellular carcinoma (HCC).1,2 However, as the number of TACE sessions increases so does the number of patients with disease progression, especially for those with multiple nodules and larger tumors.3 Among patients with advanced HCC who receive TACE alone, long-term survival outcomes are unsatisfactory.4
Immunotherapy has provided a new therapeutic option for patients with unresectable HCC.5,6 A Phase 1/2 trial (CheckMate-040) found that HCC patients treated with nivolumab had an objective response rate (ORR) of 20% and progression-free survival (PFS) of 4.1 months.7 A Phase 3 trial showed that patients with advanced HCC who received pembrolizumab (KEYNOTE-240) had an ORR of 18.4% and a disease control rate of 62.2%.6 Preliminary results from a recent Phase 2 trial, which evaluated the antitumor activity and safety of the anti-PD-1 inhibitor, Camrelizumab, revealed manageable toxicity and improved survival for advanced HCC patients.8 The antitumor activity of immunotherapy is based on inhibiting the PD-1/PD-L1 axis and promoting tumor-specific T cell activation.8 It is shown that administration of immunotherapy after TACE may prolong and maximize the immune response to HCC by preventing T cell exhaustion.9,10 While several clinical trials using combination therapy with immune checkpoint inhibitors (ICIs) are currently underway, clinical outcomes have not yet been thoroughly evaluated. It is possible that ICIs enhance the anti-tumor response of TACE, which could provide hope for patients with aggressive intermediate or advanced stage HCC. The present study aimed to evaluate the safety and efficacy of use TACE in combination with ICI treatment for this patient population.
Methods
The procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital with Nanjing Medical University (Ethical review no. 2021-SR-013). Written informed consent was not required for this retrospective study. The data that support the findings of this study are available from the corresponding author (Chun-Gao Zhou) upon reasonable request.
Patients
Between July 2019 and May 2021, patients with unresectable HCC who received TACE in combination with ICI treatment (Camrelizumab for Injection, Jiangsu Hengrui, China) at our institution were retrospectively reviewed. HCC was pathologically or clinically diagnosed using the Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China.11 Patients were included in the study if they had (1) Barcelona Clinic Liver Cancer (BCLC) stage C, or BCLC stage B with progressive or stable disease after at least two consecutive TACE sessions, (2) Child–Pugh class ≤ B7, and (3) Eastern Cooperative Group (ECOG) performance status ≤ 1.
For unresectable HCC, therapeutic decisions were made in consultation with a multidisciplinary team of hepatobiliary surgeons, interventional radiologists, radiologists, oncologists, and radiation oncologists. TACE was routinely performed to manage intermediate and advanced stage HCC with preserved liver function. The recommendation of combination therapy was recommended for patients with refractory TACE or advanced stage HCC if immunotherapy induced an anti-tumor response and effectively synergized with TACE8–10 and if poor outcomes were achieved using TACE alone. Thirty-four patients were enrolled, including 21 with progressive disease and eight with stable disease after several sessions of TACE, along with five patients who had been initially diagnosed with advanced HCC resulting from vascular invasion or extrahepatic metastasis and were receiving immunotherapy.
Baseline clinical data, including age, gender, etiology, Child–Pugh class, α-Fetoprotein (AFP) level, Eastern Cooperative Oncology Group (ECOG) performance status score, number of tumors, maximum tumor diameter, bilobar involvement, type of vascular invasion,11 extrahepatic metastasis and Barcelona Clinic Liver Cancer (BCLC) stage, were collected.
TACE Therapy
TACE was performed via the right femoral artery under local anesthesia. A 5-F catheter was introduced, and angiography was conducted to assess the tumor number, size, localization, and tumor-feeding arteries. Doses of chemotherapeutic agents were infused through the hepatic artery based on body mass index (lobaplatin, 30–50 mg). Selective or super-selective embolization was performed with a microcatheter (2.7 F; Terumo Medical Corp., Tokyo, Japan; or 2.4 F; Merit Maestro, South Jordan, Utah, USA) using a conventional lipiodol-based technique. The emulsion, consisting of 10 mg epirubicin and 10 mL Lipiodol, was prepared just before injection by mixing from one syringe to another through a three-way stopcock. The standard emulsion dose is 5–20 mL and was adjusted based on tumor size and liver function. Embolization was then performed using gelatin sponge particles or polyvinyl alcohol particles to achieve tumor-feeding artery stasis. Post-TACE syndromes were recorded and liver function indexes were checked within 1 week after each TACE session.
The first immunotherapy was performed 3–5 days after the post-TACE syndrome had abated. Patients received ICI treatment with 3 mg/kg Camrelizumab intravenously every 3 or 4 weeks. Treatment was continued until disease progression based on the Modified Response Evaluation Criteria for Solid Tumors (mRECIST), unacceptable adverse events (AEs), patient refusal, or clinician decision.
Follow Up and Definition
Regular follow-up occurred until the patient died or the study ended. Blood tests, including complete blood count, liver function, renal function, thyroid function, and myocardial enzyme were performed every 3–4 weeks in order to monitor AEs from ICI treatment. Tumor markers were assessed and imaging examinations were conducted every 1–2 months to evaluate treatment response. When residual viable tumors were identified or new lesions had developed, treatment was recommended after multidisciplinary consensus by the clinical team based on the patient’s tumor status and general condition.
Primary outcomes were AEs from Camrelizumab therapy using the Common Terminology Criteria for Adverse Events, version 5.12 Secondary outcomes included the objective response rate (ORR), progression-free survival (PFS), and overall survival (OS). Treatment response was evaluated according to the mRECIST, which included complete response, partial response, stable disease, and progression disease. The ORR was defined as the proportion of patients who achieved a complete or partial response 2–3 months after initiation of Camrelizumab treatment.13 The disease control rate was defined as the proportion of patients who achieved a complete or partial response and had stable disease 2–3 months after initiation of Camrelizumab treatment.13 PFS was defined as the period between TACE prior to initiation of Camrelizumab treatment and disease progression or death. The OS time was defined as the period between TACE prior to initiation of Camrelizumab treatment and death or August 31, 2021.
Statistical Analysis
Continuous data are presented as the mean and standard deviation if normally distributed and the median (range) if the distribution was skewed for continuous variables. Categorical data are presented as frequencies and percents. OS was estimated using the Kaplan–Meier method. Statistical analysis was performed using SPSS (Version 25.0, Chicago, IL) and GraphPad Software (Prism 8.0.1, San Diego, California).
Results
Patient Characteristics
Thirty-four patients were enrolled in this study. The median age was 56 years, and 88.2% (30/34) of the patients were male. All patients had hepatitis B infection. Twenty-one (61.8%) and 13 (38.2%) of patients had an ECOG performance status of 0 and 1, respectively. Twenty-eight (82.4%) and 6 (17.6%) patients were Child-Pugh class A and class B, respectively. Sixteen (47.1%) and 18 (52.9%) of patients had BCLC stage B and stage C, respectively. Patient characteristics are summarized in Table 1.
 |
Table 1 Patients Characteristics (n = 34) |
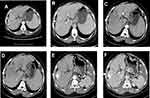
Each patient received ≥3 Camrelizumab injection cycles. The median number of Camrelizumab injections was 5.9 (range, 3–24). During immunotherapy, 83 TACE procedures were conducted at an average of 2.4 sessions per patient (range, 1–8 sessions) (Figure 1). An additional super-selective embolization trans-internal iliac artery with PVA particle (350–560 μm) was performed for one patient with right iliac bone metastasis.
Adverse Events
No major complications from TACE occurred in this study. During a median follow-up of 10.6 months (range, 2.4–25.0) from the time of initial immunotherapy, 20 patients (58.8%) experienced grade I/II AEs, and 2 patients (5.9%) experienced grade III/IV AEs (Table 2). Grade I/II AEs included reactive cutaneous capillary endothelial proliferation (RCCEP, n = 13), fatigue (n = 3), transaminitis (n = 2), and hypothyroidism (n = 2). Grade III/IV AEs included RCCEP (n = 1) in the nasal mucosa and severe pneumonitis (n = 1). The patient with RCCEP in the nasal mucosa developed epistaxis after the third cycle of immunotherapy. Nasal endoscopy showed mucosa erosion and hemorrhage. His symptoms were controlled by endoscopic electrocoagulation. The patient with pneumonitis was treated with endotracheal intubation and hormone therapy in the intensive care unit for 4 days and recovered completely. Thereafter, Camrelizumab treatment was discontinued for this patient. There were no deaths associated with this treatment.
 |
Table 2 Camrelizumab-Related Adverse Events |
Treatment Response and Overall Survival
A partial response to treatment was achieved in 12 patients (35.3%), stable disease was achieved in 13 patients (38.2%), and disease progression occurred in 9 patients (26.5%) (Figure 2). The ORR was 35.3% (12/34). Tumor response based on mRECIST is shown in Table 3. The treatment response based on intrahepatic lesions outside of the embolized area and extrahepatic lesions is shown in Supplementary Table 1, and the tumor response based on RECIST v1.1 is shown in Supplementary Table 2.
 |
Table 3 Response to Combined TACE and Camrelizumab Therapy |
During follow-up, 31 of the 34 patients (91.2%) discontinued Camrelizumab treatment because of disease progression (n = 22), patient refusal (n = 8) or an inability to tolerate the adverse event (n = 1). Of the 22 patients with disease progression, 17 received subsequent treatment with sorafenib (n = 7), lenvatinib (n = 5), apatinib (n = 3), atezolizumab plus bevacizumab (n = 1), or radiotherapy (n = 1). After the last follow-up visit, 20 of the 34 patients (58.8%) died. The median PFS was 6.1 months (range: 1.1–19.3 months; Figure 3) and the median OS was 13.3 months (range: 2.4–25.0 months; Figure 4).
 |
Figure 3 Kaplan-Meier curve for progression free survival (PFS) time in all patients (median PFS, 6.1 months). |
 |
Figure 4 Kaplan-Meier curve for overall survival (OS) time in all patients (median OS, 13.3 months). |
Discussion
This study evaluated the safety and efficacy of TACE in combination with ICI treatment among patients with unresectable HCC. The findings indicated that immunotherapy following TACE achieved effective tumor control (ORR of 35.5%), improved survival, and was associated with manageable ICI-related AEs. The ORR of this combination treatment modality led to better outcomes than treatment with anti-PD-1 inhibitors alone; the ORRs for nivolumab (CheckMate-040) and pembrolizumab (KEYNOTE-240) were 15–20% and 18.4%, respectively.6,7
Intra and extra-hepatic metastases and vascular invasion occurred among almost all patients following several TACE sessions. Hepatic artery embolization alone was shown to reduce the antitumor immune response by enhancing PD-L1 expression.14 In the tumor microenvironment, PD-1/PD-L1 overexpression is an important mechanism for tumor immune escape and progression.15 Blocking the interaction between PD-1 and its ligands is considered a promising treatment strategy for HCC.8 The concept that TACE combined with anti-PD-1/anti-PD-L1 inhibitor treatment could improve disease outcomes has prompted the launch of several clinical studies to evaluate this modality.
The use of anti-PD-1 inhibitors has resulted in a significant breakthrough in systemic treatment for advanced HCC. While ICIs have a manageable safety profile and durable response, however, they have a low ORR of 10–20% when used alone.6,8 A recent phase 2 trial showed that advanced HCC patients who received Camrelizumab had a median OS of 13.8 months,8 which was comparable to the OS observed in the current study. It is likely that differences in the patient population and the time at which these studies were initiated, as well the relatively short follow-up time in the current study might have weakened the survival benefit from combination therapy. To enhance the therapeutic efficacy of combination treatment, several strategies have been explored. Early-phase trials show that ICI treatment in combination with anti-angiogenic agents results in an ORR of 24.0–33.2% for unresectable HCC.5,16 In addition, Xu et al reported that the combination of Camrelizumab and Apatinib achieved an ORR of 34.3% in advanced HCC patients.17 TACE in combination with Camrelizumab resulted in an ORR of 35.3% in the current study. This is likely because TACE induced tumor-specific T cells by activating neo-tumor-associated antigens,9,18 while targeting PD-1 pathways reversed T cell exhaustion and restored antitumor immune responses.10,19 Compared with other combination modalities that include PD-1 inhibitors where ORRs were 24.0–33.2% and disease control rates were 72.3%–73.0%,5,17 TACE and Camrelizumab had a comparable or improved impact on tumor control. Given the characteristics of the study population, this combination modality maintained its high anti-tumor activity and could be used as a second-line treatment option for advanced HCC. These results indicate that there is a synergistic anti-tumor immunomodulatory effect between TACE and Camrelizumab that could be most effective for these patients.
Camrelizumab had a safety profile and its predominant AE was the occurrence of RCCEP on the skin, along with a few instances in the oral and nasal mucosa and palpebral conjunctiva. Other Camrelizumab-related AEs included asthenia, transaminitis, hypothyroidism, and pneumonitis. In the current study, the incidence of AEs was 64.7%, comparable to that found in other studies of HCC.8 Most AEs were gradeI/II and were relieved or eliminated after comprehensive treatment. Two patients experienced grade III/IV AEs, and received immediate treatment. The safety profile of Camrelizumab was similar to that reported in a previous study.8 Importantly, TACE did not appear to worsen ICI-related AEs in this study.
This study has some limitations. First, it was a retrospective study with a small sample size. However, the findings still confirmed that combination treatment for unresectable/progressive disease had high efficacy. Second, the heterogeneity of the baseline patient characteristics and TACE procedure may influence interpretation of the tumor response and survival outcomes.
In summary, TACE in combination with Camrelizumab treatment had an acceptable safety profile and encouraging efficacy against unresectable HCC. These findings require confirmation using additional prospective studies.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author (Chun-Gao Zhou) upon reasonable request.
Ethics Statement
This retrospective study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional ethics committee of the First Affiliated Hospital with Nanjing Medical University (Ethical review no. 2021-SR-013). Considering that patient medical data was analysed retrospectively, all informed consents were waived by the ethics committee. Of note, no patients-identifiable information was utilised.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
Jin-Xing Zhang and Pei Chen are co-first authors for this study. Hai-Bin Shi and Chun-Gao Zhou are co-correspondence authors for this study. Jin-Xing Zhang, Pei Chen, Sheng Liu, Qing-Quan Zu, Hai-Bin Shi, and Chun-Gao Zhou have no conflicts of interest or financial ties to disclose in this work.
References
1. Kudo M, Han KH, Ye SL, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9:245–260. doi:10.1159/000507370
2. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention, and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi:10.1038/s41575-019-0186-y
3. Peck-Radosavljevic M, Kudo M, Raoul J-L, et al. Outcomes of patients (pts) with hepatocellular carcinoma (HCC) treated with transarterial chemoembolization (TACE): global OPTIMIS final analysis. J Clin Oncol. 2018;36:4018. doi:10.1200/JCO.2018.36.15_suppl.4018
4. Kan X, Liang B, Zhou G, et al. Transarterial chemoembolization combined with apatinib for advanced hepatocellular carcinoma: a propensity score matching analysis. Front Oncol. 2020;10:970. doi:10.3389/fonc.2020.00970
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med. 2020;382:1894–1905. doi:10.1056/NEJMoa1915745
6. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, Phase III trial. J Clin Oncol. 2020;38:193–202. doi:10.1200/JCO.19.01307
7. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi:10.1016/S0140-6736(17)31046-2
8. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–580. doi:10.1016/S1470-2045(20)30011-5
9. Chew V, Lee YH, Pan L, et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut. 2019;68:335–346. doi:10.1136/gutjnl-2017-315485
10. Tampaki M, Ionas HE, Hadziyannis E, Deutsch M, Malagari K, Koskinas J. Association of TIM-3 with BCLC stage, serum PD-L1 detection, and response to transarterial chemoembolization in patients with hepatocellular carcinoma. Cancers. 2020;12:212. doi:10.3390/cancers12010212
11. Department of Medical Administration, National Health and Health Commission of the People’s Republic of China. [Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2019. Edition)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28. 112–128. Chinese. doi:10.3760/cma.j.issn.1007-3418.2020.02.004
12. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5; 2017.
13. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72:288–306. doi:10.1016/j.jhep.2019.09.026
14. Takaki H, Hirata Y, Ueshima E, et al. Hepatic artery embolization enhances expression of programmed cell death 1 ligand 1 in an orthotopic rat hepatocellular carcinoma model: in vivo and in vitro experimentation. J Vasc Interv Radiol. 2020;31:1475–1482.e2. doi:10.1016/j.jvir.2020.03.023
15. Kole C, Charalampakis N, Tsakatikas S, et al. Immunotherapy for hepatocellular carcinoma: a 2021 update. Cancers. 2020;12:2859. doi:10.3390/cancers12102859
16. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22:977–990. doi:10.1016/S1470-2045(21)00252-7
17. Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, Phase II Trial. Clin Cancer Res. 2021;27:1003–1011. doi:10.1158/1078-0432.CCR-20-2571
18. Flecken T, Schmidt N, Hild S, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415–1426. doi:10.1002/hep.26731
19. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi:10.1084/jem.20100643
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.