Back to Journals » Drug Design, Development and Therapy » Volume 8
Safety and efficacy of S-1 chemotherapy in recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-based chemotherapy: multi-institutional retrospective analysis
Authors Peng P, Cheng H, Ou X, Zeng L, Wu X, Liu Y, Lin Z, Tang Y, Wang S, Zhang H , Chen Z
Received 12 May 2014
Accepted for publication 31 May 2014
Published 14 August 2014 Volume 2014:8 Pages 1083—1087
DOI https://doi.org/10.2147/DDDT.S67592
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Pei-Jian Peng,1,* Hua Cheng,2,* Xue-Qing Ou,3 Lin-Juan Zeng,1 Xuan Wu,4 Yu-Meng Liu,5 Zhong Lin,1 Yan-Na Tang,1 Si-Yang Wang,3 Hong-Yu Zhang,1 Zhi-Bin Chen3
1Department of Medical Oncology, 2Department of Surgical Oncology, 3Department of Radiation Oncology, The Fifth Affiliated Hospital of Sun-Yat-Sen University, Zhu Hai, Guangdong Province, People's Republic of China; 4Department of Medical Oncology, Cancer Center, Sun-Yat-Sen University, Guangzhou, People's Republic of China; 5Department of Oncology, the People's Hospital of Zhongshan City, Zhongshan, Guangdong Province, People's Republic of China
*These authors contributed equally to this work
Purpose: This retrospective study evaluates the efficacy and safety of S-1 chemotherapy for recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-based chemotherapy.
Patients and methods: Thirty-nine patients with recurrent and metastatic nasopharyngeal carcinoma who failed previous platinum-based chemotherapy received oral S-1 chemotherapy (twice daily from day 1 to 14) every 3 weeks. The dose of S-1 was determined according to the body surface area (BSA): 40 mg twice a day for BSA <1.25 m2; 50 mg twice a day for 1.25 m2 ≤BSA<1.5 m2; and 60 mg twice a day for BSA ≥1.5 m2.
Results: Treatment was well tolerated. Most adverse events were mild. Grade 3 hematological toxicity occurred in 7.7%. There was one complete response (2.6%) and 12 partial responses (30.7%), giving an overall response rate of 33.3% (95% CI [confidence interval], 21.7–50.8). Median time-to-progression was 5.6 months, and median survival was 13.9 months. One- and 2-year survival rates were 60% and 26%, respectively.
Conclusion: S-1 monotherapy is considered a safe and effective treatment option for recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-based chemotherapy.
Keywords: S-1, nasopharyngeal carcinoma, chemotherapy, platinum
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant tumor in South China.1,2 NPC is highly responsive to chemotherapy and prolongation of survival can often be achieved after chemotherapy for recurrent and metastatic disease.3–5 Patients with recurrent and/or metastatic NPC are generally not candidates for further multimodality curative treatment. Palliative chemotherapy has been performed mostly with platinum-based regimens as first-line chemotherapy.3,6 However, for those with disease progression after prior platinum-based chemotherapy, there is no standard second-line chemotherapy. Moreover, in patients who received multiple lines of chemotherapy, toxicity became a major issue. So, the identification of active regimen with a favorable toxicity profile is necessary and important.
S-1 is an oral anticancer agent containing a combination of two modulators and tegafur, which is a pro-drug of 5-fluorouracil (5-FU). One of the modulators is 5-chloro-2,4-dihydroxypyridine, which causes prolonged retention of 5-FU in the blood. Furthermore, it enhances the pharmacological actions of 5-FU by competitively inhibiting its degradation. The other modulator is potassium oxonate, which is localized in the mucosa of the gastrointestinal tract after oral administration and alleviates gastrointestinal toxicities induced by 5-FU.7,8 S-1 has shown promising results for the treatment of a variety of solid cancers, including head and neck cancer.8,9 However, the clinical role of S-1 in patients with NPC is still uncertain. To address this issue, we conducted the following multi-institutional retrospective analysis to evaluate the efficacy and safety of S-1 chemotherapy for recurrent and metastatic NPC patients after failure of platinum-based chemotherapy.
Patients and methods
Patients
Eligibility criteria included histologically confirmed recurrent and/or metastatic NPC, ECOG (Eastern Cooperative Oncology Group) performance status of 0–2, and at least one measurable disease as assessed by response evaluation criteria in solid tumor (RECIST). Inclusion also required disease progression after one or more palliative chemotherapies of platinum-regimen. Prior chemotherapy with capecitabine was not permitted. However, prior chemotherapy with 5-FU was allowed. S-1 chemotherapy was administered at a planned standard dose and schedule (40 mg twice a day for BSA <1.25 m2; 50 mg twice a day for 1.25 m2 ≤ BSA <1.5 m2; and 60 mg twice a day for BSA ≥1.5 m2, S-1 was given for 14 consecutive days, followed by a 7-day rest period). Patients had to have adequate bone marrow (hemoglobin level ≥9 g/dL, white blood cell count ≥3000/mm3, neutrophil count ≥1500/mm3, and platelet count ≥100,000/mm3), hepatic function (total bilirubin level ≤1.5 mg/dL and aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels ≤2.5 times the upper limit of normal), and renal function (serum creatinine level ≤1.5 mg/dL). Minimum patient age was 18 years, and minimum life expectancy was 12 weeks. Written informed consent was obtained from all patients. The Institutional Review Board of each participating center approved the study.
Evaluation of treatment and statistical analysis
Tumor response was assessed according to the RECIST. Evaluation of response was performed every two cycles during the chemotherapy and then every 3 months after the completion of the chemotherapy. Adverse events (AEs) were evaluated before each treatment cycle according to National Cancer Institute Common Toxicity Criteria (version 3.0). Treatment was continued until disease progression, presence of unacceptable toxicity, or patient refusal.
All patients were included in efficacy and safety analysis. The following endpoints were used in the evaluation of efficacy: response rate, progression-free survival (PFS) and overall survival (OS). PFS and OS probabilities were calculated by the Kaplan–Meier method. Time-to-progression (TTP) was calculated from the date of first dose of S-1 to the date of documented progression or most recent follow-up in patients without disease progression. Survival time was calculated from the date of first dose of S-1 to the date of death or most recent follow-up for surviving patients. The statistical data were obtained using an SPSS (SPSS Inc., Chicago, IL, USA) software package, version 11.5.
Results
Patient characteristics
Between March 2010 to October 2013, 39 patients received S-1 monotherapy for recurrent and metastatic NPC after failure of platinum-based chemotherapy at the Fifth Affiliated Hospital of Sun-Yat-Sen University, Cancer Center of Sun-Yat-Sen University and the People’s Hospital of Zhongshan City. The characteristics of the patients are summarized in Table 1. Thirty-five patients were previously treated with radiotherapy or chemoradiotherapy. The prescribed radiotherapy doses were 70 Gy to the planning target volume (PTV) including the primary gross tumor volume, 60–62 Gy to the PTV enclosing clinical target volume 1 (ie, high-risk regions), 54 Gy to the PTV enclosing clinical volume 2 (ie, low-risk regions and neck nodal regions), and 60–64 Gy to the nodal primary gross tumor in 33 fractions. Treatment was delivered once daily with five fractions per week.
  | Table 1 Patients’ characteristics |
Treatment efficacy
A total of 162 cycles of S-1 were administered to the 39 patients, with the median number of cycles administered per patient of four (range, 1–10 cycles). Ten patients (25.7%) discontinued S-1 due to disease progression. In the remaining 29 patients (74.3%), treatment was discontinued in the absence of disease progression due to patient decision, either because of lack of further response or economic issue. One patient (2.6%) had complete response (CR), twelve patients (30.7%) had partial response (PR), 16 patients (41.0%) had stable disease (SD), and ten patients (25.7%) had progressive disease (PD). The overall response rate (CR + PR) was 33.3% (95% confidence interval [CI], 21.7–50.8), and the disease control rate (CR + PR + SD) was 74.3%. With a median follow-up period of 12 months (range 3–42 months), the median TTP was 5.6 months (95% CI, 3.6–7.5 months) (Figure 1), median OS for all patients was 13.9 months (95% CI, 9.8–18.2 months) (Figure 2), with 1-year survival rate of 60% and 2-year survival rate of 26%.
  | Figure 1 Kaplan–Meier curve of progression-free survival. |
  | Figure 2 Kaplan–Meier curve of overall survival. |
Toxicity of treatment
Most AEs were mild. Table 2 summarizes the toxicity of S-1 treatment. Hematological toxicity was mild, with only three patients developing Grade 3 toxicity (7.7%), including Grade 3 neutropenia in two patients and Grade 3 anemia in one patient. The most common non-hematological toxicity was nausea (30.7%), vomiting (20.5%), diarrhea (10.2%), fatigue (10.3%), and stomatitis (15.4%). No Grade 3/4 non-hematological toxicity was observed. Hand–foot syndrome was observed in two patients (5.1%), with grade 1 toxicity. Hepatic, renal and other toxicities were mild. None of the patients developed any Grade 4 toxicity, and there were no treatment-related deaths. The dosage was adjusted between 80 and 120 mg/day in eleven patients (28.2%), depending on diarrhea, stomatitis, or bone marrow function.
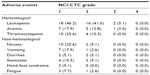  | Table 2 Treatment-related adverse events (n=39) |
Discussion
Chemotherapy fills an important role in recurrent and/or metastatic NPC treatment.3,10 Platinum-based combination regime (typically cisplatin + 5-FU) is the most commonly used chemotherapy regimen.3,10,11 Despite the well-documented sensitivity of NPC to a variety of chemotherapeutic agents, there is no consensus on second-line chemotherapy treatment for patients who failed platinum-based regimen chemotherapy. For this reason, other options with improved efficacy and safety profiles are thus highly desirable.6,12,13
5-FU, which has been recognized as one of the most promising anticancer agents, is used for the treatment of various cancers including NPC. A variety of methods of administration have been studied. Continuous intravenous infusion results in high serum concentrations of 5-FU, thus increasing its efficacy.14,15 However, this method is restraining for the patient and can result in a high rate of complications, especially those that are catheter-related. The past 5–10 years have seen an increasing trend for the substitution of conventional 5-FU with oral prodrugs of 5-FU, including capecitabine and S-1.7,16,17
S-1, as a single agent, is active for the treatment of gastric, colorectal, head and neck, breast, and pancreatic cancers.8,9,18,19 The current study is the first report evaluating the safety and efficacy of S-1 chemotherapy in recurrent and metastatic NPC patients after failure of platinum-based chemotherapy. In this study, this treatment showed a response rate of 33.3%, with the median TTP and OS of 5.6 and 13.9 months, and 1- and 2-year survival rates were 60% and 26%, respectively. Chua et al20 reported a Phase II study in which 49 Chinese patients with platinum-refractory advanced NPC received capecitabine (at a dose of 1,000–1,250 mg/m2 twice daily for 14 days) in a 3-week cycle. The overall response rate was 37%, with a median TTP of 5 months and median OS of 14 months. Treatment-related AEs were reported to be generally mild except for the hand–foot syndrome, which occurred in 86% of patients.20 Recent studies also showed other drugs (paclitaxel, docetaxel, gemcitabine, and irinotecan) as monotherapy in patients treated with cisplatin previously, had response rates within the range of 14%–48% and median PFS between 3.9 and 14 months.20–23 In contrast to these clinical trials and other combination chemotherapy regimens,24,25 S-1 monotherapy seems to be a promising regimen as second-line chemotherapy in platinum-pretreated refractory NPC. Moreover, it is encouraging to note that in our study, one patient with multiple lung metastases, who was treated with S-1 and achieved PR, survived more than 42 months and is still alive. However, from our current study results, whether S-1 is indeed comparable or superior to other regimens as a second-line treatment in terms of efficacy has not yet been fully demonstrated and requires further prospective randomized study.
In our experience, S-1 was well tolerated by patients. None of the patients was prematurely withdrawn from the study due to treatment-related adverse effects and there were no treatment-related deaths. Compared with capecitabine or capecitabine-based regimens, S-1 monotherapy showed lower hand–foot syndrome toxicity, which offered an advantage in NPC patients after previous radiotherapy and chemotherapy. Myelosuppression is the only common adverse effect observed in the current study, with an incidence similar to those reported in large series of gastric, colorectal, and head and neck cancer patients. Two cases of grade 3 leukopenia were seen and were reversed with granulocyte colony-stimulating factor (G-CSF) treatment. All patients with leukopenia or other adverse reactions recovered with G-CSF treatment, dose reduction, or postponement of S-1 administration, without hospitalization. These results suggest that the AEs caused by S-1 were temporary and easily reversible. S-1 can be used relatively safely for patients who have received prior treatments. Because of relatively mild side-effects, most of the patients could be treated in the outpatient settings with comparatively long-term drug administration. Thus, the patients could undergo anticancer therapy while maintaining their quality of life.
Although S-1 is given as a single agent in this study, combining the drug with other chemotherapeutic agents may further improve the overall response rate in NPC and is worth investigating in future trials. Combining S-1 and taxane or S-1 and gemcitabine is particularly interesting because of the encouraging effect in our current clinical experience.
In conclusion, S-1 is an effective salvage regimen for platinum-pretreated refractory recurrent and metastatic NPC. S-1 seems to be not only active but also favorable in keeping the quality of life high. S-1 as a single agent or in combination with other chemotherapeutic agents or treatment modalities should be further studied in NPC.
Disclosure
The authors declare no conflicts of interest in this work.
References
Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12(6):421–429. | |
Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765–1777. | |
Ma BB, Chan AT. Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma. Cancer. 2005;103(1):22–31. | |
Fandi A, Bachouchi M, Azli N, et al. Long-term disease-free survivors in metastatic undifferentiated carcinoma of the nasopharyngeal type. J Clin Oncol. 2000;18(6):1324–1330. | |
Zhang L, Chen QY, Liu H, et al. Emerging treatment options for nasopharyngeal carcinoma. Drug Des Devel Ther. 2013;7:37–52. | |
Suarez C, Rodrigo JP, Rinaldo A, et al. Current treatment options for recurrent nasopharyngeal cancer. Eur Arch Otorhinolaryngol. 2010;267(12):1811–1824. | |
Shirasaka T, Shimamoto Y, Oshima H, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7(5):548–557. | |
Saif MW, Syrigos KN, Katirtzoglou NA. S-1: a promising new oral fluoropyrimidine derivative. Expert Opin Investig Drugs. 2009;18(3):335–348. | |
Tsukuda M, Kida A, Fujii M, et al; Chemotherapy Study Group of Head and Neck Cancer. Randomized scheduling feasibility study of S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br J Cancer. 2005;93(8):884–889. | |
Ma BB, Hui EP, Chan AT. Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: current and future directions. Cancer Sci. 2008;99(7):1311–1318. | |
Au E, Ang PT. A Phase II trial of 5-fluorouracil and cisplatinum in recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol. 1994;5(1):87–89. | |
Lee AW, Ng WT, Chan YH, et al. The battle against nasopharyngeal cancer. Radiother Oncol. 2012;104(3):272–278. | |
Lee AW, Sze WM, Au JS, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61(4):1107–1116. | |
Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988;6(10):1653–1664. | |
Iyer L, Ratain MJ. 5-fluorouracil pharmacokinetics: causes for variability and strategies for modulation in cancer chemotherapy. Cancer Invest. 1999;17(7):494–506. | |
Cunningham D, Coleman R. New options for outpatient chemotherapy – the role of oral fluoropyrimidines. Cancer Treat Rev. 2001;27(4):211–220. | |
Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generate 5-fluorouracil selectively in tumors by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34(8):1274–1281. | |
Schoffski P. The modulated oral fluoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs. 2004;15(2):85–106. | |
Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a Phase III trial. Lancet Oncol. 2008;9(3):215–221. | |
Chua D, Wei WI, Sham JS, et al. Capecitabine monotherapy for recurrent and metastatic nasopharyngeal cancer. Jpn J Clin Oncol. 2008;38(4):244–249. | |
Ngeow J, Lim WT, Leong SS, et al. Docetaxel is effective in heavily pretreated patients with disseminated nasopharyngeal carcinoma. Ann Oncol. 2011;22(3):718–722. | |
Zhang L, Zhang Y, Huang PY, et al. Phase II clinical study of gemcitabine in the treatment of patients with advanced nasopharyngeal carcinoma after the failure of platinum-based chemotherapy. Cancer Chemother Pharmacol. 2008;61(1):33–38. | |
Poon D, Chowbay B, Cheung YB, et al. Phase II study of irinotecan (CPT-11) as salvage therapy for advanced nasopharyngeal carcinoma. Cancer. 2005;103(3):576–581. | |
Wang CC, Chang JY, Liu TW, et al. Phase II study of gemcitabine plus vinorelbine in the treatment of cisplatin-resistant nasopharyngeal carcinoma. Head Neck. 2006;28(1):74–80. | |
Pei-Jian Peng, Xue-Qing Ou, Zhi-Bin Chen, et al. Multicenter phase II study of capecitabine combined with nedaplatin for recurrent and metastatic nasopharyngeal carcinoma patients after failure of cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2013;72(2):323–328. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
