Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Safety and Efficacy of Drug-Eluting Beads Trans-Arterial Chemoembolization for Hepatocellular Carcinoma in Taiwan (SERENADE-T)
Authors Liu YS, Chang PY, Liang PC, Ou MC, Hwang JI, Chen CH
Received 13 May 2022
Accepted for publication 9 August 2022
Published 15 August 2022 Volume 2022:9 Pages 811—821
DOI https://doi.org/10.2147/JHC.S374555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Manal Hassan
Yi-Sheng Liu,1 Pi-Yi Chang,2 Po-Chin Liang,3 Ming-Ching Ou,4 Jen-I Hwang,5,6 Chien-Hung Chen7,8
1Department of Medical Imaging, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan; 2Department of Radiology, Taichung Veterans General Hospital, Taichung City, Taiwan; 3Department of Radiology, National Taiwan University Hospital, Taipei City, Taiwan; 4Department of Diagnostic Radiology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan; 5Department of Medical Imaging, Tungs’ Taichung MetroHarbor Hospital, Taichung City, Taiwan; 6Department of Radiology, School of Medicine, National Defense Medical Center, Taipei City, Taiwan; 7Department of Internal Medicine, National Taiwan University Hospital Yunlin Branch, Douliu City, Yunlin County, Taiwan; 8Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei City, Taiwan
Correspondence: Chien-Hung Chen, Department of Internal Medicine, National Taiwan University Hospital Yunlin Branch, No. 579, Sec. 2, Yunlin Road, Douliu City, Yunlin County, 640, Taiwan, Tel +886-2-23123456 ext. 65923, Fax +886-2-23819723, Email [email protected]
Purpose: The aim of this retrospective study was to evaluate the safety and efficacy of patients with hepatocellular carcinoma treated with drug-eluting bead with doxorubicin transarterial chemoembolization (DEBDOX-TACE) in Taiwan.
Patients and Methods: We retrospectively investigated 630 hepatocellular carcinoma patients who underwent DEBDOX-TACE in multiple institutions from 2011 to 2016 in Taiwan. Tumor response was assessed per modified response evaluation criteria in solid tumors, overall survival, and safety.
Results: This study included 630 patients who underwent DEBDOX-TACE, participants’ mean age was 66 years, 68.1% males and 15.6% females. The mean doxorubicin dose administered via DEBDOX-TACE was 56 mg. Complete and partial response rates were 14.6% and 49.2%, respectively, with a disease control rate of 84.6%. The median overall survival was 29.2 months. The most common post-embolization symptom was abdominal pain (22.4%). No hepatic encephalopathy and no procedure-related death were found.
Conclusion: Real-world data from Taiwan demonstrated that DEBDOX-TACE for hepatocellular carcinoma can achieve high tumor response rate with low adverse events.
Keywords: chemoembolization, DEBDOX, doxorubicin, liver cancer
Introduction
Hepatocellular carcinoma (HCC) is one of the major cancers. Worldwide, it ranks the 5th most common cancer and the 3rd most common cause of cancer-related deaths.1 Taiwan has reported increasing incidence of HCC where it may have been due to increased awareness of reporting and better screening service.2,3 Liver cirrhosis is a major risk factor for HCC. The major etiologies are hepatitis B virus (HBV) or hepatitis C virus (HCV) infections. Fatty liver disease (alcoholic or non-alcoholic), obesity, diabetes, smoking, aflatoxin, family history and genetic factors are also listed as possible risk factors.2,4
Transarterial chemoembolization (TACE), disrupting the blood supply to the tumor thus inducing tumor ischemia, is the treatment of choice for patients with intermediate-stage Barcelona Clinic Liver Cancer (BCLC) stage B HCC.5 These patients are asymptomatic and have unresectable multinodular tumors with adequate preservation of liver function. Conventional TACE (cTACE) is performed by injecting a chemotherapeutic drug mixed with an embolic agent into the tumor feeding artery. The feeding artery is then embolized to obtain the synergistic effect of drug cytotoxic activity and ischemia.6,7 The outcome of cTACE was variable. The most probable reasons are that cTACE does not have a well-standardized procedure or protocol.6,8
Drug-eluting bead (DC Bead, Boston Scientific, Massachusetts, USA) with doxorubicin (DEBDOX) represents the second generation of TACE where the liquid embolic agent is substituted with size calibrated hydrogel microsphere beads ranging from 70 to 700 µm that are biocompatible, hydrophilic, non-resorbable, and capable of loading chemotherapeutic agent, most commonly with doxorubicin in Taiwan.9–11 The landmark PRECISION V, multicenter randomized controlled trial (RCT) demonstrated that tumor response for DEBDOX-TACE significantly outperformed cTACE in the Eastern Cooperative Oncology Group (ECOG) 1, Child-Pugh B, bilobar, and recurrent disease subgroups. DEBDOX-TACE demonstrated a better safety profile with a significant decrease in serious liver-related adverse events and systemic side effects versus cTACE.12 This study paved the way for subsequent research with DEBDOX-TACE, where meta-analyses of these studies established the benefits of DEBDOX-TACE in improving survival, improved tumor response, while reducing adverse events.13–16 We published the multidisciplinary Taiwan consensus recommendations for the use of DEBDOX-TACE in HCC Treatment in 20189 to better guide the clinical application of this procedure. However, real-world large patient sample and long-term follow-up data remained lacking for HCC patients treated with DEBDOX-TACE.
The purpose of this retrospective study was to evaluate the safety and efficacy of DEBDOX-TACE treatment in HCC patients in Taiwan.
Materials and Methods
Patient Selection
The study was designed as a retrospective, multicenter analysis of patient data from prospectively maintained databases. Patients were treated with DEBDOX-TACE in three Taiwan institutions, namely, National Taiwan University Hospital, National Cheng Kung University Hospital, and Taichung Veterans General Hospital from 2011 to 2016 with at least 12 months of follow-up. Institutional review board approval was acquired before initiation of the study in each institution (Research Ethics Committee of the National Taiwan University Hospital, No: 201708028RINC; Institutional Review Board of National Cheng-Kung University Hospital, No: B-ER-106-166; Institutional Review Board of Taichung Veterans General Hospital, No: CE17306A). Informed patient consent was waived due to the retrospective nature of the study and all the data were existing medical records. All patient records were stored in the de-linked format. Passwords were required to open the databases. The waive was approved by the Research Ethics Committee or Institutional Review Board for each institution. All pre-treatment assessments including medical history, physical examination, laboratory evaluation, and imaging were completed for each patient.
The inclusion criteria were as follows: HCC diagnosed by pathology by non-invasive criteria according to American Association for the Study of Liver Diseases guidelines; and HCC patient at least 18 years old treated with at least one DEBDOX-TACE. The exclusion criteria were as follows: concurrent with any other cancer except non-melanomatous skin cancer; tumor invasion of the main portal vein, hepatic vein, or biliary duct, extrahepatic arterial supply to tumor; patient diagnosed with atypical HCC (eg infiltrative); concurrent antineoplastic treatment (i.e radiotherapy, radiofrequency ablation, sequential intra-arterial chemotherapy, sorafenib, etc.). Patients had received at least 2 consecutive sessions of TACE (at least 2 TACE at same admission, irrespective of cTACE or DEBDOX-TACE). There were lack of data before or after DEBDOX-TACE due to incomplete medical records. To reduce confounding factors, this study focused only on DC Bead. The study was registered at clinicaltrials.gov (ID code NCT03283956). The conduct of the study was in accordance with the World Medical Association Declaration of Helsinki.
DEBDOX-TACE Treatment Protocol
Treatment was based on the liver multidisciplinary board consensus and deemed to have unresectable disease by experienced hepatobiliary surgeons, subsequently performed by experienced interventional radiologists at the respective institutions. All patients received a standardized review of medical history, physical examination, laboratory and imaging examinations (either contrast-enhanced computer tomography (CE-CT) or contrast enhanced magnetic resonance imaging (CE-MRI)) within 6 weeks pre-procedure. All patients should have received a standardized DEBDOX-TACE treatment. Detailed DEBDOX-TACE technique has been previously described by Chang et al.9 Segmental or lobar DEBDOX-TACE was performed according to a local procedure with DC Bead of appropriate size, loaded with 50 to 75 mg of doxorubicin per 2 mL DC Bead. The total dose of doxorubicin administered depends on tumor size and tumor number by the same embolization endpoint. Additional embolic agent (ex: bland microsphere, Lipiodol, gelfoam pledgets) would be used to achieve the embolization endpoint.
Data Collection, Follow-Up and Response Evaluation
Patient characteristics, medical history, baseline physical examination, laboratory and imaging examinations (either CE-CT or CE-MRI) were collected up to 12 months after DEBDOX-TACE. The tumor response was assessed at the first imaging follow-up after DEBDOX-TACE, usually 1–3 months after DEBDOX-TACE.
Response evaluation was assessed with the modified Response Evaluation Criteria in Solid Tumors (mRECIST). Complete response (CR) was defined as the disappearance of any intratumoral arterial enhancement in all target tumors. Partial response (PR) was defined as at least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target tumors, taking as reference the baseline sum of the diameters of target tumors. Stable disease (SD) was defined as any case that did not meet the definition of either PR or progressive disease (PD). PD was defined as an increase of at least 20% in the sum of the diameters of viable (enhancing) target tumors, taking as reference the smallest sum of the diameters of viable (enhancing) target tumors recorded since treatment baseline.17,18 Disease control rate was the proportion of patients achieving CR and PR, and SD. Images were evaluated by an experienced IR at the respective institutions.
Safety Evaluation
Adverse Events (AEs) of DEBDOX-TACE were evaluated based on the incidence of Grade 3 and Grade 4 toxicities up to 8 weeks following the procedure, according to the National Cancer Institute’s Common Terminology Criteria for adverse events (CTCAE) Version 4.0319.
Statistical Analysis
Descriptive statistics were computed for categorical variables and means ± standard deviations or medians with interquartile ranges for continuous variables. For survival analysis, Kaplan-Meier was used. Tumor response and adverse events were tabulated in tables. All effects were considered significant at p < 0.05. Statistical analysis was performed using SAS Software (Version 9.4, SAS Institute, North Carolina, USA).
Results
Patient Population
A total of 630 adult patients who received DEBDOX-TACE were included in the final analysis. The mean age was 65.7 ± 11.7 (range 54 to 77.4) years, among which were 429 (68.1%) males and 267 (15.6%) females. The numbers of BCLC stage 0, A, B, C, and D patients were 83 (13.2%), 452 (71.8%), 92 (14.6%), and 3 (0.48%), respectively. A total of 122 patients (19.4%) had single lesion, 67 patients (10.7%) had 2 lesions, 58 patients (9.24%) had 3 lesions, and 381 patients (60.7%) had at least 4 lesions. The largest tumor size was 4.62 ± 3.57 cm. Among them, 106 patients were non-cirrhotic. Child-Pugh A including non-cirrhosis, and Child-Pugh B were numbered at 532 (84.4%), and 98 (15.6%) patients, respectively, with no patient with Child-Pugh C status. Other disease status, laboratory parameters, and patient characteristics are listed in Table 1.
 |
Table 1 Patients Characteristics |
DEBDOX-TACE Treatment
The mean doxorubicin dose delivered via DEBDOX-TACE was 56.1 ± 27.6 mg. A total of 588 patients (93.3%) had segmental embolization whilst 42 patients (6.67%) had lobar embolization. DC Bead sizes, 75–150 µm, 100–300 µm, 300–500 µm, 500–700 µm, were used in 10 (1.7%), 256 (43.03%), 322 (54.1%), 7 (1.2%) patients, respectively (shown in Table 2).
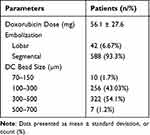 |
Table 2 DEBDOX-TACE Treatment Characteristics |
Treatment Responses and Overall Survival
Complete response was achieved in 92 (14.6%) patients, 310 (49.2%) attained PR, and 131 (20.8%) had SD. Disease control rate was 84.6%. The overall survival rate post DEBDOX-TACE was 82.5% at 1 year, 57.1% at 2 years, 42.4% at 3 years, 34.3% at 4 years, and 26.5% at 5 years. The smaller tumor size and few tumor numbers had higher complete response rate (Table 3). For those patients (N = 385) with tumor burden beyond up-to-7, complete response was achieved in 59 (15.3%) patients, 79 (35.8%) attained PR, and 196 (50.9%) had SD. The objective tumor response was lower for tumor burden beyond up-to-7, but not reaching statistically significant difference. Regarding the bead size, there were only 10 patients received 70–150 µm size bead and only 7 patients received 500–700 µm size bead. Thus, we compared the tumor response only between 100 and 300 µm vs 300–500 µm beads. There was no statistical difference in the tumor response between these two groups. The median overall survival was 29.2 months (Figure 1).
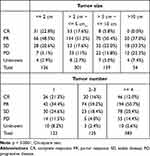 |
Table 3 Tumor Responses After DEBDOX-TACE Treatment According to Tumor Size and Tumor Number |
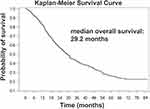 |
Figure 1 Kaplan–Meier Survival curve of patients received DEBDOX-TACE treatment. |
Multivariate Analysis for Tumor Response and Overall Survival
To investigate the parameters used to predict tumor response and overall survival, multivariate logistic regression (for tumor response) and Cox regression (for overall survival) were performed. As shown in Table 4, male, tumor size, Barcelona Clinic Liver Cancer (BCLC) stage, serum albumin level, aspartate aminotransferase (AST), and alpha-fetoprotein (AFP) were predictors for tumor response. Age, hemoglobin, white blood cell count, BCLC Stage, AST, tumor number, and AFP level were predictors for overall survival.
 |
Table 4 Multivariate Analysis for Parameters to Predict Tumor Response or Overall Survival |
Safety Evaluation
The most common post-embolization syndrome was abdominal pain (22.4%). Bacteremia (0.3%) and liver abscess (0.8%) were rare. No hepatic encephalopathy was noted. No procedure-related mortality was found (Table 5). Approximately 40% to 50% patients had elevated aminotransferase of any grade. However, grade 3 and above adverse events for alanine aminotransferase (ALT) and AST only, respectively, charted at 2.86% and 6.19%. Grade 3 and higher bilirubin elevation took place only in 1.3% of the studied subjects (Table 5). Patients of albumin-bilirubin (ABLI) grade 3 had a higher probability of developing fever, loss of appetite, ascites, bacteremia, hepatic encephalopathy, and grade 3 INR prolongation after DEBDOX-TACE. Among the study subjects, Child-Pugh scores increased in 16% and ALBI grade increased in 22.0% of the patients after DEBDOX-TACE. Only 4 subjects had significant decreased liver function (Child-Pugh scores increase >2 points) after DEBDOX-TACE (Table 6).
 |
Table 5 Adverse Events Up to 8 Weeks Post DEBDOX-TACE Treatment |
 |
Table 6 Changes of Child-Pugh Score and ALBI Grade Post DEBDOX-TACE |
Discussion
Development of DEBDOX-TACE was used to advance the efficacy and safety of conventional drug chemoembolization, commonly associated with highly variable technique and clinical outcomes.6,8,20 The technique of DEBDOX-TACE using DC Bead allows higher and more sustained concentration of drugs to target tumor and minimizes systemic exposure to chemotherapy agents, which increases efficacy while reducing the potential for AEs.21 Over two decades of continuous effort in exploring DEBDOX-TACE efficacy and safety in treating HCC patients has now culminated in a bevy of promising state-of-the-art clinical data, in tandem with the ongoing spirited debate of the procedure against other available treatment modalities.
This study found that DEBDOX-TACE can achieve a commendable objective response rate (ORR) of 63.8%, of which there were 14.6% CR and 49.2% PR, comparable with previous studies reporting ORR of DEBDOX-TACE ranging from 52% to 94.4%.12,22 ORR achieved in this study was higher than that of Precision V’s (63.8% vs 52%).12 However, in a recent study by Kang et al, where among 36 patients who received DEBDOX-TACE, ORR was 94.4%, with 69.4% CR and 25% PR. The higher ORR reported by Kang et al was probably attributed to the procedure of DEBDOX-TACE being done using smaller sized (70–150 µm) DC Bead M1 (Boston Scientific, Massachusetts, USA).22 Our study demonstrated that the smaller tumor size and few tumor numbers had higher CR rate. No CR was found for tumor larger than 10 cm. High tumor burden, such as up-to-7 criteria,23,24 is presumed to have poor response to TACE. However, the CR rate to DEBDOX-TACE in our patients beyond up-to-7 was similar to that in patients within up-to-7. Hung et al recently proposed 7–11 criteria, not up-to-7 might be better to define high tumor burden.25 These data could provide reference for future patient selection for DEBDOX-TACE.
This study demonstrated a median overall survival of 29.2 months, substantially higher than reported by some studies.26,27 Kloeckner et al in their drug-eluting chemoembolization arm reported a median survival of 12.3 months.26 Whilst median survival of 15 months was reported in the drug-eluting chemoembolization arm by Gomes et al, with higher doxorubicin dose than this study.27 Contrarily, higher survival than in our study was previously reported by Burrel et al with median survival of 40.2 months and 31.9 months respectively for BCLC A and B patients28. This was again demonstrated recently by Aliberti et al, where despite the fact that there were 45.8% patients with BCLC C stage, median survival was 42 months.29 Notably, these two papers had highly selected patients (ie 95% Child-Pugh A),28 and the use of smaller sized drug-eluting microspheres beads (70–150 µm).29
While there have been multiple systematic reviews and meta-analyses published to date in an effort to consolidate the available literature surrounding the superiority of DEBDOX-TACE, it is still controversial.13 A meta-analysis of 4 randomized control trials and 8 observational studies, which included 1449 patients, alluded to the non-superiority of DEBDOX-TACE in terms of tumor response and survival.30 Conversely, a recent meta-analysis by Yang et al included 17 retrospective studies, 3 prospective studies, and 8 randomized controlled trials suggested that DEBDOX-TACE is superior to both transarterial radioembolization and cTACE in terms of overall survival.13 The median overall survival was 19.4 months after cTACE, and the overall survival rate post cTACE was 70.3% at 1 year, 51.8% at 2 years, 40.4% at 3 years, and 32.4% at 5 years.31 The median survival of patients with HCC after TACE treatment might be lower in Asia patients, 6.3–11.5 months.32 Our patients had slightly longer median overall survival and one-year survival rate compared with the historical data after cTACE. However, we did not collect cTACE patients in the present study. Thus, we could not conclude that DEBDOX-TACE had the survival superiority.
Given the heterogeneity of publications, caution needs to be exercised when conducting cross-trial comparisons and subsequently drawing definitive conclusions. However, taken all these data together, there is evidence suggesting that DEBDOX-TACE, is effective in patients with advanced stage disease with improved tumor response, disease control, and survival benefit, to which our results from real-life setting were in concordance with those findings. These results infer that proper patient selection such as those with Child–Pugh B and ECOG 1 disease,7,12 and usage of smaller sized drug eluting microsphere beads may achieve better treatment outcomes with DEBDOX-TACE.22,33,34 The ALBI grade has been claimed to be better for evaluating the underlying liver function than Child-Pugh score because it was a reproducible and objective measure.35 High baseline ALBI grade was associated with poorer prognosis in patients with HCC undergoing TACE therapy.36 Our multi-variate analysis did not find ALBI grade was an independent predictor for tumor response or overall survival. This might be partially explained by the low percentage (2%) of ALBI grade 3 in our patient cohort.
There was approximately one fourth of patients with post-embolization syndrome reported in this study. Relevant symptoms occurrence was comparable to the Precision V DC Bead arm: abdominal pain (22.4% vs 21.5%), nausea (11.6% vs 16.1%), and vomiting (8.3% vs 10.5%).37 This result could be attributed to the fact that the chemistry of the DC Bead matrix, as demonstrated by Hagan et al21 where it has slower drug release in vitro and a lower plasma Cmax in animal pharmacokinetic study, suggested gradual loco-regional exposure to chemotherapeutic agents. However, it has not established whether this difference is substantial to translate into clinically meaningful differences in lowering post-embolization syndrome and AEs.38 Furthermore, post-embolization syndrome may represent a reaction to treatment instead of a reflection of true AEs.34 Hepatobiliary injury is a well-identified complication associated with DEBDOX-TACE.22,39 The overall incidence of hepatobiliary serious adverse events was as low as 10.3%. No hepatic encephalopathy and no procedure-related death were found in this study. These findings were comparable to literatures where DEBDOX-TACE was found to be associated with a significantly lower post-embolization syndrome and AEs.22,40,41 As current standard of care progresses towards super-selective, application of smaller drug-eluting microsphere beads, maximizing chemotherapeutic delivery to tumor via DEBDOX-TACE while sparing normal liver parenchyma, coupled with improvement in angiographic technology (eg C-arm mounted cone-beam CT), could potentially enhance local tumor control and further reduce the severity of post-embolization syndrome and AEs.42
This study had several limitations. The first is selection bias in this retrospective study design. Specifically, the lack of a comparative arm with other embolization modalities, to which no meaningful comparator inference can be made. Finally, while the sample size in this study was of reasonable number, larger data sample will enable better powering of the study to make conclusions about the outcomes.
Conclusion
In conclusion, we found that DEBDOX-TACE achieved high treatment response and few adverse events. Further well-designed randomized control studies are warranted to validate our observations. Overall, these findings further augment the burgeoning real-world clinical evidence for the case of DEBDOX-TACE in patients with HCC.
Acknowledgments
The authors thank Lee Hong, Low, RPh, for providing medical writing support, in accordance with Good Publication Practice (GPP3) guidelines.
Disclosure
This work was supported by an unrestricted investigator-initiated study grant by BTG International Asia Ltd, a Boston Scientific Company. The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953.
2. Kuo CN, Liao YM, Kuo LN, Tsai HJ, Chang WC, Yen Y. Cancers in Taiwan: practical insight from epidemiology, treatments, biomarkers, and cost. J Formos Med Assoc. 2020;119:1731–1741.
3. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370.
4. Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. 2018;27:205–212.
5. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
6. Nouri YM, Kim JH, Yoon HK, Ko HK, Shin JH, Gwon DI. Update on Transarterial Chemoembolization with Drug-Eluting Microspheres for Hepatocellular Carcinoma. Korean J Radiol. 2019;20:34–49.
7. Vogl TJ, Gruber-Rouh T. HCC: transarterial Therapies-What the Interventional Radiologist Can Offer. Dig Dis Sci. 2019;64:959–967.
8. Craig P, Young S, Golzarian J. Current Trends in the Treatment of Hepatocellular Carcinoma with Transarterial Embolization: variability in Technical Aspects. Cardiovasc Intervent Radiol. 2019;42:1322–1328.
9. Chang PY, Huang CC, Hung CH, et al. Multidisciplinary Taiwan Consensus Recommendations for the Use of DEBDOX-TACE in Hepatocellular Carcinoma Treatment. Liver Cancer. 2018;7:312–322.
10. Ou MC, Liu YS, Chuang MT, Lin CY, Huang LT, Lin CY. Time-to-progression following conventional compared with drug-eluting-bead transcatheter arterial chemoembolisation in patients with large hepatocellular carcinoma. Clin Radiol. 2019;74:295–300.
11. Liu YS, Lin CY, Chuang MT, et al. Five-year outcome of conventional and drug-eluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. BMC Gastroenterol. 2018;18:124.
12. Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52.
13. Yang B, Liang J, Qu Z, Yang F, Liao Z, Gou H. Transarterial strategies for the treatment of unresectable hepatocellular carcinoma: a systematic review. PLoS One. 2020;15:e0227475.
14. Chen P, Yuan P, Chen B, Sun J, Shen H, Qian Y. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41:75–85.
15. Xie ZB, Wang XB, Peng YC, et al. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res. 2015;45:190–200.
16. Han T, Yang X, Zhang Y, et al. The clinical safety and efficacy of conventional transcatheter arterial chemoembolization and drug-eluting beads-transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a meta-analysis. Biosci Trends. 2019;13:374–381.
17. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72:288–306.
18. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
19. U.S. Department of Health and Human Services NIH NCI. Common terminology criteria for adverse events: CTCAE v4.03.
20. Young S, Craig P, Golzarian J. Current trends in the treatment of hepatocellular carcinoma with transarterial embolization: a cross-sectional survey of techniques. Eur Radiol. 2019;29:3287–3295.
21. Hagan A, Phillips GJ, Macfarlane WM, Lloyd AW, Czuczman P, Lewis AL. Preparation and characterisation of vandetanib-eluting radiopaque beads for locoregional treatment of hepatic malignancies. Eur J Pharm Sci. 2017;101:22–30.
22. Kang YJ, Lee BC, Kim JK, et al. Conventional Versus Small Doxorubicin-eluting Bead Transcatheter Arterial Chemoembolization for Treating Barcelona Clinic Liver Cancer Stage 0/A Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2020;43:55–64.
23. Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–359.
24. Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: proposal of Modified Bolondi’s Subclassification (Kinki Criteria). Dig Dis. 2015;33:751–758.
25. Hung YW, Lee IC, Chi CT, et al. Redefining Tumor Burden in Patients with Intermediate-Stage Hepatocellular Carcinoma: the Seven-Eleven Criteria. Liver Cancer. 2021;10:629–640.
26. Kloeckner R, Weinmann A, Prinz F, et al. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer. 2015;15:465.
27. Gomes AS, Monteleone PA, Sayre JW, et al. Comparison of Triple-Drug Transcatheter Arterial Chemoembolization (TACE) With Single-Drug TACE Using Doxorubicin-Eluting Beads: long-Term Survival in 313 Patients. AJR Am J Roentgenol. 2017;209:722–732.
28. Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–1335.
29. Aliberti C, Carandina R, Lonardi S, et al. Transarterial Chemoembolization with Small Drug-Eluting Beads in Patients with Hepatocellular Carcinoma: experience from a Cohort of 421 Patients at an Italian Center. J Vasc Interv Radiol. 2017;28:1495–1502.
30. Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: current state of the art. World J Gastroenterol. 2018;24:161–169.
31. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64:106–116.
32. Torimura T, Iwamoto H. Treatment and the prognosis of hepatocellular carcinoma in Asia. Liver Int. 2021;1:548.
33. Malagari K, Moschouris H, Kiakidis T, et al. Five-Years Outcome Analysis of 142 Consecutive Hepatocellular Carcinoma Patients Treated with Doxorubicin Eluting Microspheres 30-60 mum: results from a Single-Centre Prospective Phase II Trial. Cardiovasc Intervent Radiol. 2019;42:1551–1562.
34. Urbano J, Echevarria-Uraga JJ, Ciampi-Dopazo JJ, et al. Multicentre prospective study of drug-eluting bead chemoembolisation safety using tightly calibrated small microspheres in non-resectable hepatocellular carcinoma. Eur J Radiol. 2020;126:108966.
35. Demirtas CO, D’Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021;3:100347.
36. Mishra G, Majeed A, Dev A, et al. Clinical utility of albumin bilirubin grade as a prognostic marker in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: a systematic review and meta-analysis. J Gastrointest Cancer. 2022.
37. Vogl TJ, Lammer J, Lencioni R, et al. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197:W562–70.
38. Hagan A, Caine M, Press C, et al. Predicting pharmacokinetic behaviour of drug release from drug-eluting embolization beads using in vitro elution methods. Eur J Pharm Sci. 2019;136:104943.
39. Monier A, Guiu B, Duran R, et al. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: comparison between drug-eluting beads and lipiodol emulsion. Eur Radiol. 2017;27:1431–1439.
40. Zhang ZS, Li HZ, Ma C, Xiao YD. Conventional versus drug-eluting beads chemoembolization for infiltrative hepatocellular carcinoma: a comparison of efficacy and safety. BMC Cancer. 2019;19:1162.
41. Karalli A, Teiler J, Haji M, et al. Comparison of lipiodol infusion and drug-eluting beads transarterial chemoembolization of hepatocellular carcinoma in a real-life setting. Scand J Gastroenterol. 2019;54:905–912.
42. Khalaf MH, Sundaram V, AbdelRazek Mohammed MA, et al. A Predictive Model for Postembolization Syndrome after Transarterial Hepatic Chemoembolization of Hepatocellular Carcinoma. Radiology. 2019;290:254–261.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
