Back to Journals » Cancer Management and Research » Volume 11
Safety and efficacy of combination therapy with apatinib and doxorubicin in metastatic soft tissue sarcomas: an observational study from multiple institutions
Authors Tian Z , Wang X, Liu Z, Wang J, Yao W, Zhao Y, Gao S, Zhang P, Ge H
Received 1 March 2019
Accepted for publication 13 May 2019
Published 6 June 2019 Volume 2019:11 Pages 5293—5300
DOI https://doi.org/10.2147/CMAR.S207150
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Zhichao Tian,1 Xin Wang,1 Zhiyong Liu,1 Jiaqiang Wang,1 Weitao Yao,1 Yao Zhao,2 Songtao Gao,3 Peng Zhang,1 Hong Ge4
1Department of Orthopedics, the Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou 450008, Henan Province, People’s Republic of China; 2Department of Orthopedics, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, Henan Province, People’s Republic of China; 3Department of Orthopedics, the Affiliated People’s Hospital of Zhengzhou University, Zhengzhou 450003, Henan Province, People’s Republic of China; 4Department of Radiation Oncology, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, Henan Province, 450008, People’s Republic of China
Purpose: Apatinib has shown effectiveness in treating sarcoma. This study aimed to assess the safety and efficacy of apatinib and doxorubicin combination therapy in metastatic soft tissue sarcomas (STS) and to compare the therapeutic effects of two treatments (apatinib after doxorubicin vs apatinib plus doxorubicin) on STS.
Patients and methods: A total of 76 patients with metastatic STS who received apatinib and doxorubicin between May 2016 and June 2017 were retrospectively reviewed. Patients were divided into either the apatinib after doxorubicin group (in which apatinib was used after six cycles of doxorubicin chemotherapy) or the apatinib plus doxorubicin group (in which apatinib was used in combination with doxorubicin chemotherapy).
Results: There were 55 patients in the apatinib after doxorubicin group and 21 patients in the apatinib plus doxorubicin group. There were significant differences between the apatinib plus doxorubicin group and the apatinib after doxorubicin group in the objective response rate (57.14% vs 25.45%, respectively, p=0.016) and average change from baseline in the target lesion size (−41.71±43.75% vs −1.89±51.61%, respectively, p=0.03). There were no significant differences in disease control rate (85.71% vs 63.64%, p=0.093) and median progression-free survival (8.8 months vs 10.3 months, p=1). Grade 3–4 adverse events were more common with apatinib plus doxorubicin than with apatinib after doxorubicin, and these included leukopenia (5.45% vs 38.1%, respectively, p=0.001), anemia (7.27% vs 28.57%, respectively, p=0.023), oral mucositis (3.64% vs 19.05%, respectively, p=0.046), transaminase increases (0% vs 14.29%, respectively, p=0.011).
Conclusion: Our results do not support the use of apatinib plus doxorubicin for metastatic STS unless the specific objective is tumor shrinkage.
Keywords: chemotherapy, apatinib, tyrosine-kinase inhibitor, sarcoma, adverse events
Introduction
Soft tissue sarcoma (STS) is a rare mesenchymal malignancy with more than 50 subtypes.1 The incidence of STS is very low, with just over 10,000 new cases per year in the United States,2 and only 20,000–30,000 new cases annually in People’s Republic of China.3 The most common subtypes of STS include undifferentiated pleomorphic sarcoma (UPS), gastrointestinal stromal tumor, synovial sarcoma, liposarcoma, and leiomyosarcoma. The most common primary sites are the extremities (43%), trunk (10%), viscera (19%), retroperitoneum (15%), or head and neck (9%).3,4 Surgery and adjuvant radiotherapy were the main treatments for non-metastatic STS.4,5 Retroperitoneal and gastrointestinal sarcomas most often metastasize to the liver.6 Extremities and head and neck sarcomas most commonly metastasize to the lungs.7 Most of these metastases are multiple and cannot be completely resected, and chemotherapy is therefore preferred for the treatment of metastatic STS.4,7,8 The most commonly used chemotherapy drug is doxorubicin, with response rates of approximately 20%.4,7,9,10 Second-line chemotherapy drugs include ifosfamide, gemcitabine, and docetaxel, with response rates of approximately 18%.4,9,11 The median overall survival (OS) of patients with metastatic STS is approximately 12 months.7,12
For decades, patients with metastatic STS have had no more effective treatment than chemotherapy, until the emergence of molecular targeted drugs has made a breakthrough in the treatment of such patients. One of the molecular targeted drugs is Pazopanib. As a broad-spectrum vascular endothelial growth factor receptor tyrosine kinase inhibitor (VEGFR TKI),13 pazopanib was approved by the Food and Drug Administration (FDA) in 2012 to treat metastatic STS. Since then, a growing number of reports have shown that other broad-spectrum TKIs, similar to pazopanib, are effective in treating STS. These include regorafenib, sorafenib, sunitinib, anlotinib, imatinib, and apatinib.14–20 Apatinib (known as AiTanTM in People’s Republic of China and Rivoceranib worldwide) is a broad-spectrum VEGFR TKI that was approved in People’s Republic of China in 2014 for the treatment of advanced or metastatic gastric cancer. It has been reported to be effective in the treatment of osteosarcoma and soft tissue sarcoma.14,21,22 Patients in People’s Republic of China with metastatic STS have been prescribed apatinib off-label for more than 3 years, and we began using apatinib in such patients in May 2016. Most of these patients began using apatinib treatment after doxorubicin chemotherapy failure, and some chose to take apatinib orally in parallel with doxorubicin chemotherapy. We retrospectively analyzed the clinical data of these patients, and evaluated the efficacy and safety of apatinib in combination with doxorubicin in patients with STS.
Material and methods
Patients
This is a multicenter retrospective study that was performed at three hospitals: The Affiliated Cancer Hospital of Zhengzhou University, The Affiliated People’s Hospital of Zhengzhou University, and The First Affiliated Hospital of Zhengzhou University. Enrollment began in May 2016 and finished in June 2017. Inclusion criteria were as follows: 1) patients aged between 16 and 65 years, 2) histologically confirmed STS, 3) presence of multiple metastases, 4) absence of treatment with other targeted drugs, 5) acceptable hepatic, hematologic, and renal function, 6) Eastern Cooperative Oncology Group performance status (ECOG) score of 0 or 1,23 and 7) measurable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST).
This study was approved by the Institutional Review Board of the Ethics Committee for Clinical Investigation of The Affiliated Cancer Hospital of Zhengzhou University. All patients or children’s legal parent had signed informed consent for data collection and research purposes. The study protocol followed all appropriate guidelines according to the Declaration of Helsinki.
Treatment
According to the treatment sequence of doxorubicin and apatinib, patients were divided into the apatinib after doxorubicin group (in which apatinib was used after six cycles of doxorubicin chemotherapy) and the apatinib plus doxorubicin group (in which apatinib was used in parallel with doxorubicin chemotherapy).
In the apatinib after doxorubicin group, patients were administered 37.5 mg/m2 doxorubicin per day via intravenous bolus on days 1 and 2. Treatment was repeated every 3 weeks until disease progression or the development of unacceptable adverse events (AEs) occurred, up to a maximum of six cycles. Patients then received 500 mg oral apatinib (Jiangsu Hengrui Medicine, Lianyungang, People’s Republic of China) once daily. The dose of apatinib administered was reduced to 250 mg per day for patients who could not tolerate AEs. Apatinib was continued until disease progression or unacceptable toxicity.
In the apatinib plus doxorubicin group, patients were administered 37.5 mg/m2 doxorubicin per day via intravenous bolus on days 1 and 2. Cycles were scheduled every 21 days until disease progression or the development of unacceptable AEs, up to a maximum of six cycles. Patients in parallel received 500 mg apatinib (oral dose) once daily continuously, starting on day 1. The dose of apatinib administered was reduced to 250 mg per day for patients who could not tolerate AEs. Apatinib was continued until disease progression or unacceptable toxicity.
Patients were assessed for toxicity, according to the National Cancer Institute (NCI)’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. If severe toxicity occurred, apatinib and doxorubicin treatment was delayed until patient recovery, for a maximum of two weeks.
Evaluation
Tumor response was assessed every 1 or 2 months via magnetic resonance imagingor computed tomography, and was categorized as complete response (CR), partial response, stable disease (SD), and progressive disease (PD) according to RECIST criteria. Differences in the objective response rate (ORR), disease control rate (DCR), and median progression-free survival (m-PFS) between the two groups (apatinib after doxorubicin vs apatinib plus doxorubicin) were also assessed.
PFS was defined as the time from the initiation of apatinib to the occurrence of PD or death, whichever occurred first. AEs were classified and graded based on the NCI-CTCAE version 4.0.
Statistical analysis
All statistical analyses were performed using SPSS 21.0 software for Windows (IBM, Armonk, NY, USA). PFS was estimated using the Kaplan–Meier method, with 95% CI. Group-wise comparison was conducted using Fisher’s exact test and Wilcoxon rank sum test with continuity correction. Quantitative variables are presented as medians (range) or number of patients (percentage). All statistical analyses were two-sided, and p<0.05 was considered statistically significant. The database was locked for statistical analysis in January 2019, and this is a descriptive analysis.
Results
Patients’ characteristics
A total of 82 patients with metastatic STS underwent doxorubicin and apatinib treatment from May 2016 to June 2017. Six patients were lost to follow-up and 76 patients completed the study. The median follow-up period was 25 months (range, 19–32 months) for the apatinib after doxorubicin group versus 22 months (range, 19–30 months) for the apatinib plus doxorubicin group.
The main characteristics of the patients are presented in Table 1. There were 55 patients in the apatinib after doxorubicin group, and 21 in the apatinib plus doxorubicin group. The average age of the patients was 40.25±14.44 years (apatinib after doxorubicin) and 41.43±13.34 years (apatinib plus doxorubicin). Most patients showed good performance status (ECOG 0 or 1) and had undergone surgery. The most common metastatic location was the lungs, and the distribution of histological subtypes was as follows: leiomyosarcoma, fourteen patients; UPS, twelve; synovial sarcoma, ten; angiosarcoma, eight; rhabdomyosarcoma, eight; malignant peripheral nerve sheath tumor (MPNST), five; liposarcoma, five; fibrosarcoma, four; clear cell sarcoma, three; epithelioid sarcoma, three; alveolar soft part sarcoma, two; malignant granular cell tumor, one; and epithelioid hemangioendothelioma, one. Comparison of various patient characteristics revealed that there were no statistically significant differences between the two groups (Table 1).
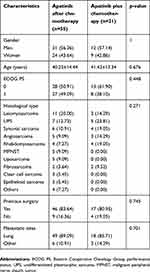 | Table 1 Patient demographics and clinical characteristics |
Efficacy of therapy
None of the 55 patients in the apatinib after doxorubicin group achieved CR, although 14 patients showed PR and 21 patients showed SD (Tables 2 and 3). The ORR was 25.45% and the DCR was 63.64%. M-PFS was 10.3 months (95% CI, 3.8–13.5 months). In the apatinib plus doxorubicin group, ORR was 57.14%, DCR was 85.71%, and m-PFS was 8.8 months (95% CI, 6.5–12.0 months; Table 3). There were significant differences between the apatinib plus doxorubicin group and the apatinib after doxorubicin group with regard to ORR (57.14% vs 25.45%, respectively, p=0.016), and average change from baseline in target lesion size (−41.71±43.75% vs −1.89±51.61%, p=0.03; Figure 1). There was no significant difference in DCR (63.64% vs 85.71%; p=0.093) and m-PFS (10.3 months vs 8.8 months, p=1; Table 3).
 | Table 2 Responses of various histological subtypes to treatment |
 | Table 3 Clinical efficacy |
Toxicity and safety
The occurrence of AEs differed between treatment groups. Patients receiving apatinib plus doxorubicin were more likely to require doxorubicin dose reduction than those receiving apatinib after doxorubicin (8 of 21 patients vs 13 of 55 patients, respectively). There was no significant difference in toxicity-related apatinib dose reduction between the groups (5 of 21 patients in the apatinib plus doxorubicin group vs 12 of 55 patients in the apatinib after doxorubicin group).
AEs were significantly more prevalent in patients treated with apatinib plus doxorubicin than in those treated with apatinib after doxorubicin (Table 4). Most patients experienced grade-1 or 2 AEs, a few patients experienced grade-3 or 4 AEs, and no drug-related deaths occurred. Grade-3/4 AEs were significantly more common in the apatinib plus doxorubicin group than in the apatinib after doxorubicin group, and these included leukopenia (5.45% of vs 38.1%, respectively, p=0.001), anemia (7.27% vs 28.57%, respectively, p=0.023), oral mucositis (3.64% vs 19.05%, respectively, p=0.046), and transaminase increases (0% vs 14.29%, respectively, p=0.011; Table 4).
 | Table 4 Adverse events |
Discussion
In this observational study, 55 patients received apatinib after six cycles of doxorubicin chemotherapy (apatinib after doxorubicin group), and 21 patients received oral apatinib in parallel with doxorubicin chemotherapy (apatinib plus doxorubicin group). Compared with those receiving apatinib after doxorubicin, patients treated with apatinib plus doxorubicin showed significantly increased ORR and AEs (Tables 3 and 4), but no significant prolongation of PFS (Figure 2). Both of these treatments achieved better results than treatment with doxorubicin chemotherapy alone.10,11,24 The results of this study also indicate that different histological types of STS respond differently to apatinib and chemotherapy (Figure 1).
 | Figure 2 Kaplan–Meier estimates of progression-free survival for both treatment groups. |
Doxorubicin monotherapy is the first-line chemotherapy regimen recommended by the National Comprehensive Cancer Network,4 and treatment of metastatic STS with this drug achieved an ORR of 14–20% and a m-PFS of 4.6–6 months.11,24 In the past decades, no regimen superior to doxorubicin monotherapy has been developed.10,11 With the emergence of small molecule-targeted drugs,25 pazopanib became the first broad-spectrum TKI approved by FDA for the treatment of metastatic STS, and achieved an ORR of 6–17% and a m-PFS of 4.6–6 months.26–28 As a broad-spectrum TKI similar to pazopanib, apatinib has been used off-label for the treatment of STS in People’s Republic of China since its launch. At present, many reports have confirmed the efficacy of apatinib in the treatment of metastatic STS.21,22,29 And others have shown the synergistic effects of TKIs combined with chemotherapy.19,30 In order to determine whether apatinib shows synergistic effects with chemotherapy, we combined apatinib with doxorubicin in the treatment of metastatic STS. Most of the patients in this study received other treatments after PD, consequently we could not calculate the exact OS of the cases in this study. ORR, DCR, and PFS were instead selected for statistical analysis.
Our study is the first to combine apatinib with doxorubicin. The results of this study showed that compared with apatinib after doxorubicin, the ORR of apatinib plus doxorubicin treatment was significantly higher (Table 3), and the diameter of the target lesion was significantly lower (Figure 1). This suggests that apatinib can synergistically interact with doxorubicin. However, the combination of the two drugs did not result in significant prolongation of PFS, which was even lower than that of the treatment with apatinib after doxorubicin (Figure 2). We considered that this may be related to drug resistance to apatinib. The reported m-PFS of STS treated with apatinib is approximately 4 months.21,22 Patients in apatinib plus doxorubicin group were administered apatinib at the start of the treatment, which led to the early onset of drug resistance. In the apatinib after doxorubicin group, apatinib was used late and drug resistance therefore appeared late, resulting in a longer m-PFS. As a broad spectrum TKI similar to apatinib, sorafenib showed the same effect. Compared with the efficacy of treating STS with ifosfamide alone (ORR, 11%; m-PFS, 6.9 months) reported by Noujaim et al,31 Garcia et al reported that sorafenib plus ifosfamide showed increased ORRs (17%) and decreased m-PFS (4.8 months).19 This also occurred when combining two chemotherapy drugs in the treatment of STS, as Judson et al showed that compared to treatment with doxorubicin alone, treatment with doxorubicin plus ifosfamide showed a significant increase in ORR (26% in the doxorubicin and ifosfamide group vs 14% in the doxorubicin group). However, OS was not significantly prolonged (median OS, 14.3 months in the doxorubicin and ifosfamide group vs 12.8 months in the doxorubicin group).24 Based on the above, we can conclude that the in parallel use TKI and chemotherapy drugs will not necessarily increase PFS and OS.
The results of this study showed that treatment with apatinib plus doxorubicin increased AEs compared with apatinib after doxorubicin treatment (Table 4). In these AEs, there were statistically significant differences associated with leukopenia, anemia, oral mucositis, and transaminase increases. Increased AEs resulted in more patients requiring reduced doxorubicin doses in the apatinib plus doxorubicin group, which also increased risks for these patients, especially those who are frail or older than 60 years of age. The treatment of those AEs increased costs obviously. Therefore, in patients with metastatic STS, treatment with apatinib after doxorubicin resulted in fewer complications and was significantly superior to the apatinib plus doxorubicin regimen.
However, apatinib plus doxorubicin presents certain advantages. Currently, it has been reported that preoperative chemotherapy can reduce the difficulty of surgery and prolong OS after tumor volume reduction.32 Therefore, apatinib plus preoperative chemotherapy may be considered in the selected non-metastatic STS, as the probability and degree of tumor shrinkage are greater. For patients with metastatic STS, the primary objective of treatment is to maximize survival and improve quality of life. Apatinib plus doxorubicin did not extend survival (and even shortened it) and resulted in a significant increase in AEs. Therefore, apatinib after doxorubicin is preferable for use in patients with metastatic STS.
The main limitation of this study is that it was a retrospective study without a control group, and the sample size was small, thus decreasing the level of evidence. In addition, because of the rarity of some types of sarcoma, we had insufficient numbers to permit subset analyses, which may have reduced the statistical power.
Conclusion
Compared with apatinib after doxorubicin, patients treated with apatinib plus doxorubicin showed significantly increased ORR and AEs, but no significant prolongation of PFS. Apatinib plus doxorubicin as preoperative treatment may benefit patients with resectable high-grade STS if the specific objective is tumor shrinkage.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer. 2014;120(12):1763–1774. doi:10.1002/cncr.28657
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
3. Sarcoma Committee of Chinese Anti-Cancer Association, Chinese Society of Clinical Oncology.Chinese expert consensus on diagnosis and treatment of soft tissue sarcomas (Version 2015). Zhonghua Zhong Liu Za Zhi. 2016;38(4):310–320. doi:10.3760/cma.j.issn.0253-3766.2016.04.013
4. von Mehren M, Randall RL, Benjamin RS
5. Blay JY. Getting up-to-date in the management of soft tissue sarcoma. Future Oncol. 2018;14(10s):3–13. doi:10.2217/fon-2018-0074
6. Trans-Atlantic Retroperitoneal Sarcoma Working Group (TARPSWG). management of metastatic retroperitoneal sarcoma: a consensus approach from the Trans-Atlantic Retroperitoneal Sarcoma Working Group (TARPSWG). Ann Oncol. 2018;29(4):857. doi:10.1093/annonc/mdx807
7. Morgan SS, Cranmer LD. Systematic therapy for unresectable or metastatic soft-tissue sarcomas: past, present, and future. Curr Oncol Rep. 2011;13(4):331–349. doi:10.1007/s11912-011-0182-z
8. Zer A, Prince RM, Amir E, Abdul Razak AR. Multi-agent chemotherapy in advanced soft tissue sarcoma (STS) - A systematic review and meta-analysis. Cancer Treat Rev. 2018;63:71–78. doi:10.1016/j.ctrv.2017.12.003
9. Villalobos VM, Byfield SD, Ghate SR, Adejoro O. A retrospective cohort study of treatment patterns among patients with metastatic soft tissue sarcoma in the US. Clin Sarcoma Res. 2017;7:18. doi:10.1186/s13569-017-0084-4
10. Gronchi AFS, Quagliuolo V, Broto JM, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18(6):812–822. doi:10.1016/S1470-2045(17)30334-0
11. Beatrice Seddon SJS, Whelan J, Leahy M, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18(20):1397–1410. doi:10.1016/S1470-2045(17)30622-8
12. Lindner LH, Litiere S, Sleijfer S, et al. Prognostic factors for soft tissue sarcoma patients with lung metastases only who are receiving first-line chemotherapy: an exploratory, retrospective analysis of the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Int J Cancer. 2018;142(12):2610–2620. doi:10.1002/ijc.31286
13. Nakano K, Takahashi S. Current molecular targeted therapies for bone and soft tissue sarcomas. Int J Mol Sci. 2018;19(3):739. doi:10.3390/ijms19030739
14. Xie L, Xu J, Sun X, et al. Apatinib for advanced osteosarcoma after failure of standard multimodal therapy: an open label Phase II clinical trial. Oncologist. 2018. doi:10.1634/theoncologist.2018-0542
15. Munhoz RDA SP, Gounder MM, Dickson MA. A phase Ib/II study of gemcitabine and docetaxel in combination with pazopanib for the neoadjuvant treatment of soft tissue sarcomas. Oncologist. 2015;20(11):1245–1246. doi:10.1634/theoncologist.2015-0245
16. OB T M, Italiano A, Penel N. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17(12):1732–1742. doi:10.1016/S1470-2045(16)30507-1
17. Schuetze SBV, Choy E, Baker LH. Phase 2 study of dasatinib in patients with alveolar soft part sarcoma, chondrosarcoma, chordoma, epithelioid sarcoma, or solitary fibrous tumor. Cancer. 2017;123(1):90–97. doi:10.1002/cncr.30379
18. Yihebali Chi ZF, Hong X, Yao Y, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft tissue sarcoma. Clin Cancer Res. 2018;24(21):5233–5238. doi:10.1158/1078-0432.CCR-17-3766
19. Garcia Del Muro X, Maurel J, Martinez TJ, et al. Phase II trial of ifosfamide in combination with the VEGFR inhibitor sorafenib in advanced soft tissue sarcoma: a Spanish group for research on sarcomas (GEIS) study. Invest New Drugs. 2018;36(3):468–475. doi:10.1007/s10637-018-0583-z
20. Liu W, Jiang Q, Zhou Y. Advances of systemic treatment for adult soft-tissue sarcoma. Chin Clin Oncol. 2018;7(4):42. doi:10.21037/cco
21. Xie L, Guo W, Wang Y, Yan T, Ji T, Xu J. Apatinib for advanced sarcoma: results from multiple institutions’ off-label use in China. BMC Cancer. 2018;18(1):396. doi:10.1186/s12885-018-4242-8
22. Baorang Zhu JL, Xie Q, Diao L, Gai L, Yang W. Efficacy and safety of apatinib monotherapy in advanced bone and soft tissue sarcoma: an observational study. Cancer Biol Ther. 2018;19(3):198–204. doi:10.1080/15384047.2017.1416275
23. Park CM, Koh Y, Jeon K, et al. Impact of Eastern Cooperative Oncology Group Performance Status on hospital mortality in critically ill patients. J Crit Care. 2014;29(3):409–413. doi:10.1016/j.jcrc.2014.01.016
24. Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15(4):415–423. doi:10.1016/S1470-2045(14)70063-4
25. Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488–497. doi:10.1016/S0140-6736(16)30587-6
26. Kawai A, Araki N, Hiraga H, et al. A randomized, double-blind, placebo-controlled, Phase III study of pazopanib in patients with soft tissue sarcoma: results from the Japanese subgroup. Jpn J Clin Oncol. 2016;46(3):248–253. doi:10.1093/jjco/hyv184
27. van der Graaf WTA, Blay J-Y, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886. doi:10.1016/S0140-6736(12)60651-5
28. Yoo KH, Kim HS, Lee SJ, et al. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: a retrospective case series. BMC Cancer. 2015;15:154. doi:10.1186/s12885-015-1584-3
29. Feng LZL, Zhao J, Zhao G, et al. Efficacy and safety of Apatinib in stage IV sarcomas: experience of a major sarcoma center in China. Oncotarget. 2017;8(38):64471–64480. doi:10.18632/oncotarget.16293
30. Martin-Liberal J, Lopez-Pousa A, Broto JM, et al. Phase I trial of sorafenib in combination with ifosfamide in patients with advanced sarcoma: a Spanish group for research on sarcomas (GEIS) study. Invest New Drugs. 2014;32(2):287–294. doi:10.1007/s10637-013-9989-9
31. Noujaim J, Constantinidou A, Messiou C, et al. Successful ifosfamide rechallenge in soft-tissue sarcoma. Am J Clin Oncol. 2015;1. doi:10.1097/COC.0000000000000243
32. Gronchi A, Jones RL. The value of neoadjuvant chemotherapy in localized high-risk soft-tissue sarcoma of the extremities and trunk. JAMA Oncol. 2018;4(9):1167–1168. doi:10.1001/jamaoncol.2018.1392
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

