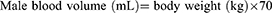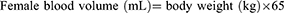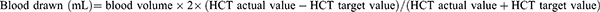Back to Journals » Journal of Pain Research » Volume 16
Safe and Effective Blood Preservation Through Acute Normovolemic Hemodilution and Low-Dose Tranexamic Acid in Open Partial Hepatectomy
Authors Yang J, Zhang J, Luo J, Ouyang J, Qu Q, Wang Q, Si Y
Received 20 June 2023
Accepted for publication 21 October 2023
Published 15 November 2023 Volume 2023:16 Pages 3905—3916
DOI https://doi.org/10.2147/JPR.S426872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Jian Yang,1,* Jing Zhang,1,* Jiayan Luo,2 Jie Ouyang,1 Qicai Qu,1 Qitao Wang,1 Yongyu Si1
1Department of Anesthesiology, Second Affiliated Hospital of Kunming Medical University, Kunming, 650101, People’s Republic of China; 2Department of Anesthesiology, People’s Hospital of Yanting, Sichuan, 621600, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yongyu Si, Department of Anesthesiology, Second Affiliated Hospital of Kunming Medical University, No. 374 of Dianmian Road, Wuhua District, Kunming, 650101, People’s Republic of China, Tel +86 10 65333681, Fax +86 10 65334416, Email [email protected]
Objective: In this study, we evaluated the efficacy of tranexamic acid (TXA) and acute normovolemic hemodilution (ANH) with 6% hydroxyethyl starch (130/0.4) in minimizing blood loss during open partial liver resection. Coagulation function was assessed using thromboelastography (TEG) and hemostasis tests, while renal function changes were tracked through serum creatinine values post-surgery.
Methods: Thirty patients undergoing open partial liver resection were allocated to two groups: Group T received TXA + ANH, and Group A received ANH alone. Blood was drawn from the radial artery under general anesthesia. Both groups received peripheral vein injections of 6% hydroxyethyl starch 130/0.4. Group T additionally received intravenous TXA. Primary outcomes included blood loss and allogeneic blood transfusions. TEG assessed coagulation status and renal function was monitored.
Results: Group T demonstrated superior outcomes compared to Group A. Group T had significantly lower intraoperative blood loss (700 mL vs 1200 mL) and a lower bleeding rate per kilogram of body weight (13.3 mL/kg vs 20.4 mL/kg). Coagulation parameters favored Group T, with higher TEG maximum amplitude (55.91 mm vs 45.88 mm) and lower activated partial thromboplastin time (38.04 seconds vs 41.49 seconds). Neither group experienced acute renal injury or kidney function deficiency during hospitalization.
Conclusion: TXA and ANH in a small dose during liver resection stabilize clotting, reduce blood loss by 6% compared to hydroxyethyl starch 130/0.4, and do not affect renal function.
Keywords: acute normovolemic hemodilution, coagulation function, liver resection, thromboelastography, tranexamic acid
Introduction
The liver is a highly vascularized organ with delicate tissue. Its basic anatomical units, hepatic lobules, are centered around the central vein. Operating on the liver presents challenges due to its role in synthesizing most coagulation factors, making it tricky to manage effectively with a single approach. Managing blood loss and the need for transfusions has always been a concern in liver surgery. Simultaneously, using allogeneic blood transfusions during the perioperative period can lead to adverse patient reactions, including transfusion reactions, the potential transmission of viruses, immune suppression, and transfusion-related lung injuries.
Studies have demonstrated that perioperative allogeneic blood transfusions are associated with poorer patient outcomes, especially in those undergoing tumor resection procedures.1,2 To address these issues, clinicians are focusing on increasing hemoglobin levels in patients, carefully determining when blood transfusions are necessary, and minimizing blood loss during surgery. This involves implementing strategies like maintaining low central venous pressure, using blood clotting techniques, employing various surgical tools, adjusting anesthesia methods, and administering specific medications to reduce blood loss during liver surgeries.3 Acute normovolemic hemodilution is a blood conservation technique used prior to surgery after anesthesia. It involves replacing the extracted whole blood with an equal volume of artificial colloidal solution, all without increasing the return of blood to the liver. As a result, there is no increase in the pressure within the right atrium and inferior vena cava, leading to a reduction in blood stagnation within the liver. This, in turn, helps to minimize blood loss during partial liver resection. Additionally, following hepatectomy, transfusing whole blood with the same proportion of coagulation factors may yield some improvement in coagulation. Research studies have verified that acute normovolemic hemodilution can effectively decrease blood loss during surgery and reduce the need for blood transfusions both during and after the procedure. Moreover, postoperative complications and the duration of hospital stays do not exhibit significant changes as a result of this approach.4–6 When acute normovolemic hemodilution is combined with maintaining a low central venous pressure during hepatectomy, it can further reduce blood loss and the requirement for allogeneic transfusions. However, it does not have a substantial impact on coagulation function.7 It is important to note that changes in coagulation function can occur during surgery due to intraoperative blood loss and fluid resuscitation. Research has indicated that acute normovolemic hemodilution may prolong the values of PT, APTT, and TT while reducing plasma fibrinogen concentrations.8 Tranexamic acid is a commonly used antifibrinolytic medication in various medical fields, including trauma, orthopedic surgery, and obstetric surgery.9 Studies have demonstrated its effectiveness in reducing blood loss and the need for blood transfusions in hepatectomy procedures.10,11 The primary objective of this study was to investigate whether combining tranexamic acid with acute isovolemic hemodilution could result in greater blood loss reduction compared to acute normovolemic hemodilution. Additionally, we aimed to closely monitor coagulation function to establish a theoretical foundation for implementing blood-saving strategies during hepatectomy. It is worth noting that both acute normovolemic hemodilution and tranexamic acid are associated with potential kidney-related side effects.12,13 We also tracked the occurrence of acute kidney injury and renal insufficiency from 48 hours post-surgery until the discharge of patient.
Materials and Methods
Patients
Thirty patients who were scheduled to have a liver resection using laparotomy (not hemi-hepatectomy) and acute normovolemic hemodilution (ANH), with a scope of liver resection equal to or greater than three segments or a diameter for any single lesion equal to or greater than 5 cm, were included in our study. Patients or their representatives signed the consent form after being informed of the study. The study was approved by the hospital’s ethics committee.
Inclusion Criteria
① Patients who were willing to undergo the liver resection with ANH, and who signed the consent form and the ANH consent form themselves or had them signed by their family members.
② Patients who fall within the age range of 18 and 75 years, have an ASA (American Society of Anesthesiologists) grading of I or II, and weigh more than or equal to 50 kg.
③ Patients with a Child-Pugh grade of A or B, ≥ 3 liver segments removed via laparotomy, or any single lesions with diameters ≥ 5 cm.
④ Hematocrit (HCT, also known as packed cell volume [PCV]) ≥ 33%, platelet count ≥ 100×109/L, and hemoglobin ≥ 110 g/L prior to operation.
⑤ Patients without serious liver or renal failure, apparent defective coagulation functioning, coagulation-related diseases, or a history of anticoagulant usage, and without heart- or lung-related ailments.
Exclusion Criteria
①Patients with cirrhosis and portal hypertension.
② Patients who may have an inferior vena cava tumor thrombus or a portal vein tumor thrombus.
③ Patients who experienced surgically-induced vascular invasion or unintentional vascular damage.
Methods
ANH
Based on the results of preoperative exams and tests (T0) of the 30 participants, they were randomly divided into two groups—Group A and Group T. Under general anesthesia, blood was collected from the radial artery of each group, and a sodium chloride solution with 6% hydroxyethyl starch 130/0.4 was administered equally to each group. Subsequently, Group T received 1% TXA intravenously dripped for 30 minutes at a loading dose of 10 mg/kg, then 1% TXA was continuously pumped at a rate of 1 mg/(kg h) until the skin suture was completed, while Group A received normal saline administered at the same rate after blood was drawn. The blood volume of the patients was calculated based on their body weights:
Either the hemoglobin level was 100 g/L or the HCT target value was 30%. The maximum blood drawn was ≤ 30% of the blood volume.
The ACD (A: citric acid, C: trisodium citrate, and D: dextrose) blood storage bag was positioned on a swinging balancer during blood collection. The blood was drawn, labelled with the patient’s name, gender, age, blood type, hospital case number, and precise collection time, and kept refrigerated at 4 °C. When connecting and replacing blood storage bags, certain hygienic procedures were followed. During surgery, a 1:1 ratio of crystalloid to colloid was infused. The same chief surgeon carried out each operation. With intermittent closure of the first hepatic portal (the Pringle maneuver), the liver was severed using the forceps clamp technique. Peripheral venous blood was collected and monitored for hemoglobin and platelet count using blood cell analysis before blood was drawn (T1), after blood was drawn (T2), after liver parenchyma dissection and hemostasis but before autotransfusion (T3), after autotransfusion but before skin suture (T4), after skin suture (T5), within 24 hours after surgery (T6), and within 48 hours after surgery (T7). Peripheral venous blood was collected to assess thromboelastography (TEG) after liver parenchyma dissection and hemostasis but before autotransfusion (T3). We noted the duration of the procedure, the amount of blood lost during the procedure, the amount of blood transfused, and the amount of urine produced.
Intraoperative Transfusion Strategies
① Red blood cell transfusion: Red blood cell transfusions were done following liver resection hemostasis or when a patient’s hemoglobin level was below 70 g/L. Allogeneic blood was then transfused after the autologous blood.
② Fresh frozen plasma: Prothrombin time (PT) or activated partial thromboplastin time (APTT) took 1.5 times longer than expected, or there was substantial intraoperative hemorrhage.
③Cryoprecipitation: Plasma fibrinogen levels below 1.5 g/dL indicated a lack of functional fibrinogen, as determined by TEG monitoring.
④ The body temperature was maintained between 35.5 °C and 37 °C and the nasopharyngeal temperature was regularly monitored.
⑤ The calcium ion level was maintained at Ca2+ ≥ 0.9 mmol/L.
⑥ CVP (central venous pressure) was maintained at a level of < 5 mmHg throughout the dissection of the liver parenchyma.
⑦MAP (mean arterial pressure) was maintained at ≥ 65 mmHg.
Observation Results
Primary Outcomes
Blood loss and transfusion
① Number of transfusion cases and allogeneic blood transfusion volume during operation; intraoperative blood losses.
Secondary Outcomes
① Coagulation function: TEG and hemostasis assessments are conducted to assess the performance of coagulation functions. ② Kidney function: Renal insufficiency was evaluated through serum creatinine levels within the first 48 hours following surgery and monitored for any signs of occurrence between the postoperative period and discharge.
TEG
A TEG device known as kaolin thromboelastography, using the TEG® 5000 system from Haemoscope (Haemonetics), operates with a setup where a pin is suspended from a torsion wire and immersed in a cup containing whole blood. This cup is placed within a heating block and continuously oscillates at an angle of 4°45’ every 5 seconds. Any alterations in the viscoelastic strength of the clot directly affect the torsion wire, which is then detected by an electromechanical transducer. This system offers real-time analysis of the viscoelastic characteristics of clot formation and dissolution within whole blood. Clinically, four parameters are typically employed:
R, referring to the reaction time or clotting time, represents the moment of initial significant clot formation.
Angle signifies the kinetics of clot development, indicating the speed at which thrombin generation, fibrin deposition, and cross-linking occur.
MA, denoting Maximum Amplitude, represents the peak strength of the clot.
Ly30, or percent Lysis 30 minutes after MA, quantifies the degree of clot dissolution 30 minutes following its maximum strength.
Statistical Analysis
The statistical software SPSS 26.0 was used to process and analyze all the data. Normally distributed measurement data are reported as mean ± standard deviation and subjected to statistical analysis using independent samples t-test and paired t-test. Non-normally distributed measurement data were subjected to a nonparametric rank sum test and expressed as the median (the minimum value to the maximum value). Two-factor repeated measures analysis of variance was used to examine blood cell analysis and creatinine value analysis at each time point. Gender and transfusion rate were analyzed using the Fisher’s exact test or the chi-squared test. A statistically significant difference was observed by a p-value of less than 0.05 (p < 0.05).
Results
Demographic Data, Tests, and Examinations Before the Surgery (T0)
Groups T and A did not differ statistically from one another in terms of demographic information, hemoglobin, platelet count, hemostasis tests, and TEG (Table 1).
 |
Table 1 Demographic Data and Preoperative Tests, Examinations |
Comparison of Intraoperative Condition
The median of total blood loss in Group T was 700 mL, which was lower than the 1200 mL in Group A (U = 61.500, P = 0.033) as shown by the results of the Mann–Whitney U-test; the median blood loss in Group T was 13.3 mL/kg, which was lower than the 20.4 mL/kg in Group A (U = 54.500, P = 0.015). In terms of the volume of blood drawn, the duration of the procedure, the volume of intraoperative blood transfusions, and the volume of intraoperative urine, there was no statistically significant difference between Groups T and A (Table 2).
 |
Table 2 Volume of Blood Drawn, Blood Loss, Operation Duration, Urine Volume, Allogeneic Blood Transfusion |
TEG at the Completion of Liver Resection
At the completion of liver resection (T3, after the severing of liver parenchyma), the maximum amplitude (MA) value of TEG in Group T was 55.91 ± 7.97 mm, which was higher than the 45.88 ± 19.79 mm in Group A, with a difference of 10.023 (95% confidence interval [CI]: −1.531–21.576). The results of independent samples t-test showed t′ = 1.820, P = 0.043 with a statistically significant difference, and MA in Group A was lower than the normal lower limit (50 mm). There was no statistically significant difference with respect to the other TEG data (Table 3).
 |
Table 3 TEG at the Time of Hepatic Parenchyma Was Severed and Hemostasis Was Completed |
Comparison of Postoperative Coagulation Function
There was no statistically significant difference in preoperative coagulation function between Groups T and A (P > 0.05). Both groups had statistically significant differences (P < 0.05) in coagulation function, significantly prolonged PT, APTT, INR (international normalized ratio), decreased Fib (fibrillation), increased D-dimer, and decreased FDPs (fibrin degradation products) when their postoperative data were compared to those before surgery, as shown by the results of the intra-group paired t-test. By the end of the procedure (T5), the APTT in Group T was 38.04 ± 3.59 s (seconds), which was 3.450 s (95% CI: −6.785 - −0.115) shorter than the 41.49 ± 5.18 s in Group A, were within normal threshold. The results of the independent sample t-test revealed a statistically significant difference of −2.119, P = 0.043. Based on the data displayed in Table 4, there was no statistically significant difference in the other coagulation function characteristics between Groups T and A (P > 0.05).
 |
Table 4 Coagulation Function at the End of Surgery |
Evaluation of Blood Cell Count Results
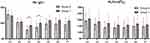
The following comparison was made between the Hb (hemoglobin) readings of the two groups: The Hb values in Groups T and A were at their lowest points at the conclusion of the liver dissection (T3), began to rise at T4, and exhibited no statistically significant difference at T5, T6, or T7 (P > 0.05). T3 signaled the start of Group T’s Hb value exceeding that of Group A. At T3, the Hb value for Group T was 93.2 ±14.4 g/L, which was significantly higher than the Hb value for Group A, which was 78.8 ±13.8 g/L (T = 2.816, P = 0.009). At T4, the Hb value for Group T was 100.2 ±15.4 g/L, which was significantly higher than the Hb value for Group A, which was 87.2 ±14.1 g/L (T = 2.411, P = 0.023). Comparison of PLT (platelet count) values between Groups T and A: There was no statistically significant difference in the PLT value between the two groups at T1, T2, T3, T4, T5, and T7 (P > 0.05), and all PLT values at T1, T2, T3, T4, T5, and T6 were within the normal range (125–350 x 109/L) (Figure 1).
Variation in Kidney Function Between Groups T and a
The serum creatinine readings in Group T after surgery, within 24 hours following surgery, and within 48 hours following surgery, were all within a normal range, and were greater than those in Group A. There was no statistically significant difference in the blood creatinine value before and after surgery at each time point based on intra-group comparisons within Groups T and A. The Cr (creatinine) values in Group A showed a downward trend, while the Cr values in Group T showed an upward trend. During postoperative hospitalization, neither group experienced renal function impairment (Table 5).
 |
Table 5 Comparison of Serum Creatinine Cr (umol/L) |
Discussion
Open partial hepatectomy remains a commonly employed surgical procedure in clinical practice, particularly in cases where laparoscopic surgery is challenging due to limited visibility, restricted space, or unique lesion locations, such as those in the caudate lobe or large tumors, including but not limited to hepatocellular carcinoma, cholangiocarcinoma, hepatic hemangioma, and intrahepatic bile duct calculus.
The liver, given its vital role in clotting factor synthesis and its proximity to major blood vessels like the inferior vena cava and abdominal aorta, poses a risk of significant blood loss and necessitates potential allogeneic transfusions during liver resection. To mitigate these risks, various blood-saving techniques such as maintaining low central venous pressure, blood flow obstruction methods, ANH, and TXA have gained widespread acceptance in partial hepatectomy procedures.
In our research, we employed a low CVP approach (CVP < 5 mm Hg, equivalent to 1.33 cm H2O) prior to liver parenchyma dissection. Previous studies have already demonstrated the efficacy of maintaining low CVP in reducing intraoperative blood loss.14,15 ANH, either alone or in conjunction with a low CVP strategy, significantly decreased the need for allogeneic blood transfusions during hepatectomy.16,17
We specifically found that when TXA was combined with ANH, the median blood loss was 700 mL (13.3 mL/kg), a substantial reduction compared to the 1200 mL (20.4 mL/kg) observed in the ANH-only group, with statistical significance (P < 0.05). While two out of the 15 patients in the TXA and ANH group required blood transfusions and plasma infusions during the operation, this figure was lower than the 4 patients in the ANH-only group, although this difference did not reach statistical significance (P > 0.05), likely due to the small sample size. These findings underscore the effectiveness of combining TXA and ANH as strategies to minimize blood loss in open partial hepatectomy, resulting in fewer patients requiring allogeneic blood transfusions. Receiving allogeneic transfusions during perioperative hepatectomy is associated with an unfavorable prognosis.18 To mitigate the need for such transfusions during partial hepatectomy, the primary focus is on minimizing blood loss. Surgeons and anesthesiologists are actively exploring strategies to reduce surgical blood loss.
One viable approach is ANH, a blood preservation technique commonly used in cardiac surgery with cardiopulmonary bypass (CPB). ANH entails the extraction of a fraction of the patient’s blood immediately following the induction of anesthesia, followed by its substitution with either an artificial colloid solution or a volume of crystalloid solution equivalent to three times the amount of blood withdrawn. The stored blood is then reintroduced to the patient when a specific hematocrit (Hct) level is reached or when clinically necessary. It is crucial to achieve an appropriate level of blood dilution to maximize the benefits of ANH while avoiding circulation overload, which can lead to excessive surgical blood loss due to coagulation factor dilution.
In our study, we aimed for a target hemoglobin level of 100g/L (Hct 33%). Using an equivalent amount of colloid to supplement the circulating blood volume helped prevent high central venous pressure during hepatectomy. A study by Jones et al19 compared various diluents for ANH (Ringer’s solution, 5% albumin, succinyl gelatin, and hydroxyethyl starch) with a target hemoglobin of 90g/L. The results indicated that hydroxyethyl starch and succinyl gelatin caused increased APTT, decreased MA value, and decreased α angle of TEG.
Notably, blood dilution with 6% hydroxyethyl starch (130/0.4) can negatively impact clotting function, possibly due to its effect on platelet activity via von Willebrand factor and FVIII factor.20 Studies on cardiopulmonary bypass heart surgery have shown reduced platelet aggregation function in ANH-collected blood samples, although thrombin production remains unaffected.21 While ANH-collected blood stored at room temperature for up to 8 hours has a hemostatic effect,22 preserving it at 4 °C helps maintain platelet activity.23
In our study, we used a 4 °C refrigerator to maximize blood clotting function preservation. Transfusing fresh whole blood can partially enhance coagulation function. Clinical trials in hepatectomy patients have demonstrated that ANH can reduce the need for red blood cell and plasma transfusions post-surgery.5 The blood collected in our study was transfused back into the patient after liver resection and hemostasis, resulting in increased hemoglobin and platelet values. There were no significant differences in preoperative hemoglobin levels or blood collections between the two groups.
Following hepatic parenchymal separation and hemostasis, the hemoglobin level in the group that received TXA in combination with ANH was significantly higher at 93.2±14.4 g/L compared to the ANH-only group at 78.8±13.8 g/L (P < 0.05). After blood transfusion, the Hb value in the TXA combined with ANH group further increased to 100.2±15.4 g/L, significantly surpassing the ANH group with 87.2±14.1 g/L (P < 0.05). For patients undergoing ANH, the addition of TXA proves to be an effective blood preservation intervention, resulting in higher hemoglobin levels during the perioperative period. Tranexamic acid, a synthetic derivative of lysine, functions as an antifibrinolytic medication. It is widely employed to both prevent and treat significant blood loss in various medical contexts such as cardiac surgery, orthopedic surgery, and trauma cases. The mechanism of action of tranexamic acid involves its strong affinity for the lysine binding site of plasminogen, thereby obstructing the interaction between fibrin and plasminase. Although plasminase can continue to be produced, it becomes incapable of binding to fibrin or fibrin monomers due to the presence of tranexamic acid. Consequently, tranexamic acid effectively prevents plasminase from breaking down the formed fibrin, ultimately achieving hemostasis.
The hemostatic effect of tranexamic acid varies depending on the dosage. Low doses of tranexamic acid inhibit the activation of plasminogen, while higher doses directly inhibit plasminogen activity, leading to a more pronounced hemostatic effect. Findings from prior research has demonstrated that tranexamic acid can significantly reduce blood loss during liver resection.10,24 In a systematic review, it was found that tranexamic acid partially inhibits fibrinolysis at concentrations ranging from 5 to 10mg/L, and it effectively inhibits fibrinolysis at concentrations ranging from 10 to 15mg/L.25
In our study, we administered tranexamic acid intravenously in group T at a loading dose of 10 mg/kg immediately following AHN. Subsequently, tranexamic acid was continuously administered at a rate of 1 mg/kg/h until the procedure concluded. This dosing regimen aligns with the recommended prophylactic administration of tranexamic acid. Comparing the coagulation function between the two groups, we observed differences. According to the TEG results obtained after liver resection, the MA value of TEG in Group T was notably higher compared to Group A (55.91 ± 7.97mm in Group T vs 45.88 ± 19.79 mm in Group A, with a significance of P < 0.05). This discrepancy may be attributed to the use of TXA in Group T, which reduces fibrinolysis within formed blood clots.
Additionally, when measuring whole blood with TEG, Group T experienced reduced blood loss, with fewer losses of clotting factors and higher blood cell and hemoglobin levels, resulting in stronger clot intensity. Towards the end of the operation, both groups exhibited similar trends in their hemostasis tests. In comparison to pre-operation values, both groups displayed simultaneous elongation of R value, a decrease in α angle, a reduction in MA value, and an increase in Ly30. D-dimer showed a slight increase, FDPs increased slightly, and fibrinogen slightly decreased. These changes remained within the range of normal values without clinical significance.
Notably, APTT in Group T was 38.04 ± 3.59 s, which was shorter than the 41.49 ± 5.18 s in Group A (t = −2.119, P < 0.05). It is possible that neither ANH nor the blood loss from hepatectomy triggered hyperfibrinolysis, and the dose of TXA administered may not have been sufficient to inhibit fibrinolysis significantly.
However, our study had limitations. Thrombin antithrombin complex (TAT) and plasmin-α2-antiplasmin complex (PAP) were not determined, and sensitive and specific markers such as thrombomodulin and tissue-type plasminogen activator-inhibitor-1 complex (tPAI. C) were not used to assess the clotting status. Additionally, the concentration and activity of clotting factors were not measured. Monitoring TXA concentration in blood in conjunction with clinical conditions may be necessary for future research. In our research, we utilized a 130/0.4 hydroxyethyl starch solution as the diluent for ANH, and it is worth noting that this solution has the potential to impede platelet function. Findings from prior studies have demonstrated that tranexamic acid can enhance platelet function in patients, regardless of whether they are undergoing antiplatelet therapy.26,27 Additionally, tranexamic acid not only has the capacity to inhibit fibrinolysis in individuals with chronic renal failure but also to ameliorate platelet function in such patients.28
It is important to mention that the MA value of 80% is indicative of platelet function, whereas approximately 20% of MA pertains to fibrin function.29,30 In cases where there is no clear evidence of substantial hyperfibrinolysis and fibrinolysis inhibition, it is plausible to hypothesize that tranexamic acid might enhance platelet function, thereby contributing to the stability of already formed blood clots. Nonetheless, further investigations are necessary to validate this hypothesis. Existing research suggests that low CVP, ANH, and TXA are all risk factors that may compromise kidney function.31–33 In order to better protect the patients, we set the maximum limit for high CVP at 5 mmHg, a reduced loading dose of TXA of 10 mg/kg, a lower maintenance dose of TXA of 1 mg/kg per hour, a higher target hemoglobin value of ANH at 100 g/L, and a MAP of greater than 65 mmHg. According to an animal study, the myocardium, brain, and spinal cord are able to maintain oxygen supply during ANH as the heart index rises and peripheral vascular resistance falls, but renal oxygen supply starts to decline at an HCT of 30% and is only 25% of the baseline value at an HCT of 10%.32,34 Patients who were administered high-dose TXA [at a loading dose of 100 mg/kg and a maintenance dose of 10 mg/kg per hour] and had normal kidney function did not have substantial renal impairment.35 This aligns with our results indicating that individuals in both cohorts exhibited no indications of clinical or laboratory-related acute or chronic kidney dysfunction within 48 hours following the surgical procedure and throughout their stay in the postoperative care unit.
Conclusion
In summary, the combined utilization of low-dose TXA and ANH appears to offer potential benefits for patients undergoing open hepatectomy surgery. These potential advantages encompass diminished blood loss, stabilized clotting and coagulation functions, and the absence of adverse effects on renal function. To validate and further substantiate these findings, additional randomized double-blind studies are warranted to ensure the robustness and precision of these results.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Approval and Consent to Participate
I confirm that I have read the Editorial Policy pages. This study was conducted with approval from the Ethics Committee of Second Affiliated Hospital of Kunming Medical University. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study. Jian Yang and Jing Zhang were Co-first authors.
Funding
This study was funded by Hospital Science and Technology Foundation (NO. 2021yk005). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Disclosure
The authors declare that they have no competing interests.
References
1. Saxena A, Valle S, Liauw W, et al. Allogenic blood transfusion is an independent predictor of poorer peri-operative outcomes and reduced long-term survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a review of 936 cases. J Gastrointest Surg. 2017;21(8):1318–1327. doi:10.1007/s11605-017-3444-8
2. Wada H, Eguchi H, Nagano H, et al. Perioperative allogenic blood transfusion is a poor prognostic factor after hepatocellular carcinoma surgery: a multi-center analysis. Surg Today. 2018;48(1):73–79. doi:10.1007/s00595-017-1553-3
3. Moggia E, Rouse B, Simillis C, et al. Methods to decrease blood loss during liver resection: a network meta-analysis. Cochrane Database Syst Rev. 2016;10(10):CD010683. doi:10.1002/14651858.CD010683.pub3
4. Zhou X, Zhang C, Wang Y, et al. Preoperative acute normovolemic hemodilution for minimizing allogeneic blood transfusion: a meta-analysis. Anesth Analg. 2015;121(6):1443–1455. doi:10.1213/ANE.0000000000001010
5. Jarnagin WR, Gonen M, Maithel SK, et al. A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Ann Surg. 2008;248(3):360–369. doi:10.1097/SLA.0b013e318184db08
6. Matot I, Scheinin O, Jurim O, et al. Effectiveness of Acute Normovolemic Hemodilution to Minimize Allogeneic Blood Transfusion in Major Liver Resections. Anesthesiology. 2002;97(4):794–800. doi:10.1097/00000542-200210000-00008
7. Guo J-R, Shen H-C, Liu Y, et al. Effect of acute normovolemic hemodilution combined with controlled low central venous pressure on blood coagulation function and blood loss in patients undergoing resection of liver cancer operation. Hepatogastroenterology. 2015;62(140):992–996.
8. de Souza MAB, Klamt JG, Garcia LV. Effects of acute normovolemic hemodilution on blood coagulation: comparison between tests of an in vivo and an in vitro model. Rev Bras Anestesiol. 2010;60(4):363–375. doi:10.1016/S0034-7094(10)70045-3
9. Wong J, George RB, Hanley CM, et al. Tranexamic acid: current use in obstetrics, major orthopedic, and trauma surgery. Can J Anaesth. 2021;68(6):894–917. doi:10.1007/s12630-021-01967-7
10. Wu CC, Ho WM, Cheng SB, et al. Perioperative parenteral tranexamic acid in liver tumor resection: a prospective randomized trial toward a “blood transfusion”-free hepatectomy. Ann Surg. 2006;243(2):173–180. doi:10.1097/01.sla.0000197561.70972.73
11. Jaffer AA, Karanicolas PJ, Davis LE, et al. The impact of tranexamic acid on administration of red blood cell transfusions for resection of colorectal liver metastases. HPB. 2020;23(2):245–252. doi:10.1016/j.hpb.2020.06.004
12. Konrad FM, Mik EG, Bodmer SIA, et al. Acute normovolemic hemodilution in the pig is associated with renal tissue edema, impaired renal microvascular oxygenation, and functional loss. Anesthesiology. 2013;119(2):256–269. doi:10.1097/ALN.0b013e31829bd9bc
13. Ko DH, Kim TH, Kim JW, et al. Tranexamic acid-induced acute renal cortical necrosis in post-endoscopic papillectomy bleeding. Clin Endosc. 2017;50(6):609–613. doi:10.5946/ce.2017.021
14. Liu TS, Shen QH, Zhou XY, et al. Application of controlled low central venous pressure during hepatectomy: a systematic review and meta-analysis. J Clin Anesth. 2021;75:110467. doi:10.1016/j.jclinane.2021.110467
15. Hughes MJ, Ventham NT, Harrison EM, et al. Central venous pressure and liver resection: a systematic review and meta-analysis. HPB. 2015;17(10):863–871. doi:10.1111/hpb.12462
16. Ye H, Wu H, Li B, et al. Application of cardiovascular interventions to decrease blood loss during hepatectomy: a systematic review and meta-analysis. BMC Anesthesiol. 2023;23(1):89. doi:10.1186/s12871-023-02042-y
17. Gal R, Cundrle I, Seidlova J, et al. Anaesthesia management of major hepatic resections without application of allogeneic blood. Bratisl Lek Listy. 2003;104(7–8):243–246.
18. Martin AN, Kerwin MJ, Turrentine FE, et al. Blood transfusion is an independent predictor of morbidity and mortality after hepatectomy. J Surg Res. 2016;206(1):106–112. doi:10.1016/j.jss.2016.07.013
19. Jones SB, Whitten CW, Despotis GJ, et al. The influence of crystalloid and colloid replacement solutions in acute normovolemic hemodilution: a preliminary survey of hemostatic markers. Anesth Analg. 2003;96(2):363–368, table of contents. doi:10.1097/00000539-200302000-00012
20. Stump DC, Strauss RG, Henriksen RA, et al. Effects of hydroxyethyl starch on blood coagulation, particularly factor VIII. Transfusion. 1985;25(4):349–354. doi:10.1046/j.1537-2995.1985.25485273815.x
21. Scott KJ, Shteamer JW, Szlam F, et al. Platelet function, but not thrombin generation, is impaired in acute normovolemic hemodilution (ANH) blood. J Clin Anesth. 2019;58:39–43. doi:10.1016/j.jclinane.2019.04.032
22. Kinoshita H, Saito J, Nakai K, et al. Clotting functional stability of withdrawing blood in storage for acute normovolemic hemodilution: a pilot study. J Anesth. 2021;35(1):35–42. doi:10.1007/s00540-020-02856-x
23. Kusudo E, Murata Y, Matsumoto T, et al. Platelet function of whole blood after short-term cold storage: a prospective in vitro observational study. Transfusion. 2023;63(2):384–392. doi:10.1111/trf.17216
24. Karanicolas PJ, Lin Y, Tarshis J, et al. Major liver resection, systemic fibrinolytic activity, and the impact of tranexamic acid. HPB. 2016;18(12):991–999. doi:10.1016/j.hpb.2016.09.005
25. Picetti R, Shakur-Still H, Medcalf RL, et al. What concentration of tranexamic acid is needed to inhibit fibrinolysis? A systematic review of pharmacodynamics studies. Blood Coagul Fibrinolysis. 2019;30(1):1–10. doi:10.1097/mbc.0000000000000789
26. Weber CF, Görlinger K, Byhahn C, et al. Tranexamic acid partially improves platelet function in patients treated with dual antiplatelet therapy. Eur J Anaesthesiol. 2011;28(1):57–62. doi:10.1097/EJA.0b013e32834050ab
27. Van Aelbrouck C, Jorquera-Vasquez S, Beukinga I, et al. Tranexamic acid decreases the magnitude of platelet dysfunction in aspirin-free patients undergoing cardiac surgery with cardiopulmonary bypass: a pilot study. Blood Coagul Fibrinolysis. 2016;27(8):855–861. doi:10.1097/mbc.0000000000000485
28. Mezzano D, Panes O, Muñoz B, et al. Tranexamic acid inhibits fibrinolysis, shortens the bleeding time and improves platelet function in patients with chronic renal failure. Thromb Haemost. 1999;82(4):1250–1254. doi:10.1055/s-0037-1614370
29. Hartmann J, Hermelin D, Levy JH. Viscoelastic testing: an illustrated review of technology and clinical applications. Res Pract Thromb Haemost. 2023;7(1):100031. doi:10.1016/j.rpth.2022.100031
30. Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014;89(2):228–232. doi:10.1002/ajh.23599
31. Tk-w M, Chow KM, Kwan BC-H, et al. Manifestation of tranexamic acid toxicity in chronic kidney disease and kidney transplant patients: a report of four cases and review of literature. Nephrology. 2017;22(4):316–321. doi:10.1111/nep.12762
32. Crystal GJ. Regional tolerance to acute normovolemic hemodilution: evidence that the kidney may be at greatest risk. J Cardiothorac Vasc Anesth. 2015;29(2):320–327. doi:10.1053/j.jvca.2014.06.014
33. Correa-Gallego C, Berman A, Denis SC, et al. Renal function after low central venous pressure-assisted liver resection: assessment of 2116 cases. HPB. 2015;17(3):258–264. doi:10.1111/hpb.12347
34. Crystal GJ, Czinn EA, Salem MR. The mechanism of increased blood flow in the brain and spinal cord during hemodilution. Anesth Analg. 2014;118(3):637–643. doi:10.1213/ane.0000000000000078
35. Xie J, Lenke LG, Li T, et al. Preliminary investigation of high-dose tranexamic acid for controlling intraoperative blood loss in patients undergoing spine correction surgery. Spine J. 2015;15(4):647–654. doi:10.1016/j.spinee.2014.11.023
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.