Back to Journals » Drug Design, Development and Therapy » Volume 15
Ruxolitinib for Treatment of Steroid-Refractory Graft-versus-Host Disease: Real-World Data from Chinese Patients
Authors Wei C , Zhang X, Liang D, Yang J, Du J, Yue C, Deng L
Received 17 September 2021
Accepted for publication 8 November 2021
Published 30 November 2021 Volume 2021:15 Pages 4875—4883
DOI https://doi.org/10.2147/DDDT.S338752
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Cong Wei,1,* Xiaoting Zhang,1,* Dan Liang,1 Jilong Yang,1 Jingwen Du,1 Chunyan Yue,1 Lan Deng1,2
1Department of Hematology, Zhujiang Hospital, Southern Medical University, Guangzhou, 510280, People’s Republic of China; 2Department of Hematology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200011, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lan Deng
Department of Hematology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200011, People’s Republic of China
Tel +86 13724862939
Email [email protected]
Chunyan Yue
Department of Hematology, Zhujiang Hospital, Southern Medical University, Guangzhou, 510280, People’s Republic of China
Tel +86 15521117300
Email [email protected]
Background: Graft-versus-host disease (GVHD) is a main complication following allogeneic hematopoietic stem cell transplantation and is a leading cause of non-relapse-related death. Unsatisfactory response to standard first-line therapy with glucocorticoids is a predictor of a poor prognosis in patients with GVHD. Ruxolitinib is a selective Janus kinases 1/2 inhibitor which has been shown to control acute (a) and chronic (c) GVHD while maintaining graft-versus-tumor effects.
Objective: This study aims to evaluate the efficacy and safety of ruxolitinib in the treatment of steroid-refractory GVHD (SR-GVHD) in a population of Chinese patients.
Methods: We report the results of 55 patients, including 23 patients with aGVHD and 32 patients with cGVHD, who were treated with ruxolitinib as salvage therapy between August, 2017 and December, 2020.
Results: In patients with aGVHD, the overall response rate (ORR) was 86.9%, and the 1-year overall survival (OS) was 82.6% (95% CI, 67.1– 98.1%). The 1-year OS was significantly improved in responders than in non-responders (90.0% vs 33.3%, P=0.004). In patients with cGVHD, the ORR was 78.1%, and the 1-year OS was 81.3% (95% CI, 67.8– 94.8%). There was no significant difference in the 1-year OS between responders and non-responders (84.0% vs 71.4%, P=0.327). Cytopenia, cytomegalovirus-reactivation and infections were common adverse events, particularly in patients with aGVHD.
Conclusion: Our real-world data from Chinese patients further confirm that ruxolitinib is a safe and effective treatment for SR-GVHD.
Keywords: ruxolitinib, allogeneic hematopoietic stem cell transplantation, graft-versus-host disease, steroid-refractory
Introduction
Graft-versus-host disease (GVHD) is a major cause of morbidity and mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT).1,2 It is also a substantial barrier to the expansion of allo-HSCT in the field of hematological diseases. Systemic glucocorticoid therapy is widely accepted as first-line treatment. However, half of the patients are resistant to glucocorticoids, which is associated with poor prognosis. Currently, no standard second-line treatment for the treatment of steroid-refractory GVHD (SR-GVHD) is available. Based on clinical experience, treatment options include cyclosporine A, sirolimus, tacrolimus, mycophenolate mofetil, methotrexate, Anti-T lymphocyte globulin, extracorporeal photopheresis, among others.3,4 However, no evidence from large prospective trials exists to support the clinical use of these regimens. In addition, due to the widespread use of glucocorticoids and immunosuppressants, there has been an increase in infection complications and a decrease in the effect on graft-versus-tumor (GVT). Therefore, it is imperative to explore new effective treatment with less side effects.
Ruxolitinib is a selective Janus kinases (JAK) 1/2 inhibitor. Preclinical and clinical studies have confirmed the role of the JAK-STAT signaling pathway in the pathogenesis of GVHD.5–7 Mechanistically, ruxolitinib blocks the JAK1/2 signaling and leads to a series of effects, including inhibited antigen-presenting cell function, down-regulated expression of major histocompatibility complex class II in dendritic cells, reduced proliferation of effector T cells, suppressed production of proinflammatory cytokine and promoted production of tolerogenic Treg cells. Preclinical studies have shown that ruxolitinib improved survival and reduced histopathological GVHD grading in murine models. More importantly, ruxolitinib treatment was associated with the retention of the GVT effect.8,9
To date, several retrospective clinical studies reported promising results using ruxolitinib in patients with SR-GVHD.10–15 What’s more, based on findings of a open-label Phase 2 trial (REACH1), ruxolitinib has been FDA-approved for SR-aGVHD.16,17 REACH2 trial compared the efficacy and safety of ruxolitinib with best available therapy (BAT) in patients with SR-aGVHD.18 The trial results suggested that ruxolitinib was significantly more effective than BAT, suggesting that ruxolitinib is a promising agent in the treatment of SR-aGVHD. Additional studies are important and necessary to determine the efficacy and safety of ruxolitinib in the treatment of SR-GVHD. To date, there are few studies on Chinese patients. Therefore, we conducted a retrospective real-world study to analyze our experience with ruxolitinib for treating SR-GVHD in a population of Chinese patients.
Methods
Patients
This was a single-center, retrospective clinical study. Patients diagnosed with SR-GVHD who received ruxolitinib as salvage treatment at Zhujiang Hospital of Southern Medical University, Guangzhou, China between August, 2017 and December, 2020 were eligible for this study. The study was conducted in accordance with the Declaration of Helsinki. Approval for this study was granted by the Ethics Board of Zhujiang Hospital of Southern Medical University (2021-KY-068-01). Informed consent was obtained from all patients or guardians.
Inclusion Criteria
Patients with SR-GVHD after allo-HSCT were considered for inclusion in this study. aGvHD was graded according to the Glucksberg grading standards and the grade of cGVHD was based on the National Institutes of Health (NIH) consensus criteria.19,20 Steroid-refractory of aGVHD was defined as the lack of response of any involved organ to prednisone (≥2 mg/kg/day) for at least 7 days, or an increase of at least one grade within 3 days, or an incomplete response after more than 28 days of immunosuppressive treatment, including steroids. In patients with cGvHD, steroid resistance was defined as progression while on prednisone (≥1 mg/kg/day) for 2 weeks, or stable disease while on prednisone (≥0.5 mg/kg/day) for 1–2 months.
Treatment with Ruxolitinib and Evaluation Criteria
All patients received oral ruxolitinib 5–10 mg twice a day as a salvage therapy. Based on the physician’s experience and the assessment of the patient’s status, additional immunosuppressants may have been administered. Organ scoring and GVHD grading were performed as baseline levels prior to ruxolitinib administration. Patients were scored for their best response at any time after starting ruxolitinib. In aGVHD, complete response (CR) was defined as the absence of both symptoms and signs of GVHD, partial response (PR) as a decrease in the organ score of at least 1 grade at one or more sites without progression in any other organs, and no response (NR) as no change in the GVHD grade and/or progressive worsening of GVHD. Patients were considered as responding to ruxolitinib if they exhibited either PR or CR. In cGVHD, the therapeutic effect was evaluated using the NIH cGVHD response evaluation standards.21 Overall survival (OS) was defined as the cumulative probability of survival at the time of last follow-up, regardless of disease status.
Adverse Events
The levels of Cytomegalovirus (CMV) DNA and Epstein-Barr virus (EBV) DNA were monitored before and after ruxolitinib treatment. Other treatment-related adverse events were identified by review of follow-up records. Cytopenia was graded based on the Common Terminology Criteria for Adverse Events version 5.0.
Statistical Analysis
Descriptive statistics were used for patient characteristics. Mann-Whitney test was used for nonparametric data univariate analysis. OS was estimated and displayed using the Kaplan-Meier method and evaluated the significance of differences with the Log-rank test. The level of statistical significance was defined as P<0.05. Statistical analyses were performed using SPSS Statistics 25 and GraphPad Prism 7.
Results
Patients
A total of 55 patients received ruxolitinib therapy were enrolled in this study. Among patients with aGVHD (n=23), 60.9% were grade III or grade IV. Similarly, among patients with cGVHD (n=32), 62.5% had a severe case. The median number of previous second-line treatment agents for aGVHD and cGVHD was two (range 1–4) and two (range 1–6), respectively (Supplemental Table 1). The median time from GVHD diagnosis to ruxolitinib treatment was 5 days (range 1–79) and 17 days (range 7–1239), in aGVHD and cGVHD, respectively. Patient characteristics are summarized in Table 1.
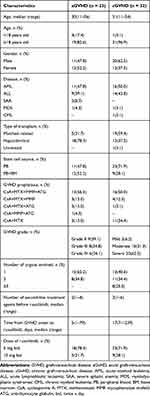 |
Table 1 Patient Characteristics |
Response to Ruxolitinib and Survival
Table 2 shows the effectiveness of ruxolitinib treatment in patients with aGVHD. The median duration from ruxolitinib to initial response was 5 days (range 2–18). The overall response rate (ORR) was 86.9% in patients with aGVHD, including 13 CR and 7 PR. The ORRs for skin, gastrointestinal (GI) tract, and liver aGVHD were 88.2%, 92.3%, and 66.7%, respectively (Figure 1A). All patients with grade II responded to ruxolitinib. The ORRs for grade III and grade IV were 87.5% and 66.7%, respectively. Over the course of treatment, 12 patients reduced steroids use and 11 patients discontinued steroids.
 |
Table 2 Clinical Response to Ruxolitinib |
 |
Figure 1 Organ-specific response of patients with SR-GVHD to ruxolitinib treatment: (A) site of aGVHD; (B) site of cGVHD. |
The effectiveness data of ruxolitinib for the treatment of cGVHD are summarized in Table 2. The median duration from ruxolitinib to initial response was 10 days (range 2–59). In cGVHD, the ORR was 78.1%, including 8 CR and 17 PR. All patients with skin, GI tract, joints and fascia involvement showed significant responses. The ORRs for mouth, eyes, and liver cGVHD were 90.9%, 80.0%, and 80.0%, respectively, whereas, the ORR for lung cGVHD was only 44.4% (Figure 1B). The ORR for both mild and moderate cGVHD was 100%, whereas for severe cGVHD was 65.0%. A total of 12 patients reduced steroids use and 19 patients discontinued steroids. However, one patient increased the dosage; thus was a case of pulmonary cGVHD that continued to progress rapidly after treatment with ruxolitinib.
Univariate analysis of response in all 55 patients suggested that patients who received both peripheral blood (PB) and bone marrow (BM) hematopoietic stem cells had a better response than those who received PB only (p=0.044; Figure 2A). Moreover, patients who initially presented with less severe of GVHD had a significantly better response than those who presented with severe GVHD, (p=0.012; Figure 2B). The ORRs for the 5 mg and the 10 mg bid group were 85.4% and 71.4%, respectively, with no statistically significant differences observed (p=0.247). Likewise, there were no significant differences between the response and the non-response group when stratified by age, gender, donor type, number of involved organs.
 |
Figure 2 Clinical response rate of ruxolitinib in SR-GVHD according to: (A) stem cell source; (B) GVHD grade. |
The median follow-up after ruxolitinib in patients with aGVHD and cGVHD was 433 days (range 25–1466) and 495 days (range 65–1364), respectively. 39 patients were still alive at the time of the last follow-up (August 31, 2021). For the aGVHD group, the 1-year and 2-year OS was 82.6% (95% CI, 67.1–98.1%) and 75.1% (95% CI, 55.3–94.9%), respectively. The 1-year OS was 90.0% (95% CI, 76.9–100%) and 33.3% (95% CI, 0–86.6%), for the response and the non-response group, respectively (P=0.004; Figure 3A). For the cGVHD group, the 1-year and 2-year OS was 81.3% (95% CI, 67.8–94.8%) and 72.8% (95% CI, 56.3–89.3%), respectively. The 1-year OS was 84.0% (95% CI, 69.7–98.3%) and 71.4% (95% CI, 37.9–100%) for the response and the non-response group, respectively (P=0.327; Figure 3B).
Adverse Events, Relapse and Mortality
The reactivation rate of CMV and EBV in aGVHD was 52.2% and 8.7%, respectively. In cGVHD, the reactivation rate of CMV and EBV was 10.0% and 6.7%, respectively. In addition, infection complications including bacterial, fungal, viral and other pathogenic infections such as tuberculosis, were also monitored during ruxolitinib treatment. The incidence of infection in patients with aGVHD and cGVHD was 82.6% and 68.8%, respectively (Supplemental Table 2). Cytopenia was observed in all patients with aGVHD and in 62.5% patients with cGVHD. Additional details are shown in Table 3.
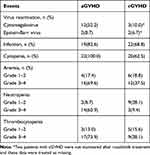 |
Table 3 Adverse Events |
Nine patients had a relapse of primary disease, of which five had aGVHD and four had cGVHD. The median duration from initial response to relapse in patients with aGVHD and cGVHD was 196 days (range 156–555) and 554 days (range 343–861), respectively. In addition, five patients with aGVHD developed cGVHD after discontinuation of ruxolitinib; two of them, who presented, with severe cGVHD resumed ruxolitinib. In total, 16 patients died: seven patients with aGVHD and nine patients with cGVHD died. The causes of death included severe pulmonary infection, relapse of primary disease, GVHD progression, cerebral hemorrhage and heart failure (Table 4).
 |
Table 4 Relapse and Mortality |
Discussion
Patients with SR-GVHD usually have poor outcomes. Effective therapies for these patients are limited, and novel therapeutic approaches are urgently needed. Ruxolitinib, a selective protein kinase inhibitor that targets JAK1/2, was shown to improve aGVHD in murine models, and, when used for the first time in patients with SR-GVHD, led to remission.5,9 Since then, an increasing number of trials to analyze the mechanism and confirm its effectiveness in the treatment of SR-GVHD have been conducted. In this retrospective study, we analyzed the efficacy and safety of ruxolitinib in the treatment of SR-GVHD in our center.
The main objective of our study was to confirm the efficacy of ruxolitinib in the treatment of SR-GVHD. Our results indicate that, despite more than half of the patients having grade III–IV aGVHD or severe cGVHD and receiving multiple immunosuppressive therapies, ruxolitinib still leads to a promising response. In patients with aGVHD, the ORR was 86.9% and the 1-year and 2-year OS were 82.6% and 75.1%. Moreover, the 1-year OS was significantly better in responders than in non-responders in the aGVHD group (P=0.004). Similarly, in patients with cGVHD, the ORR was 78.1% and the 1-year and 2-year OS were 81.3% and 72.8%. However, there was no significant difference in the 1-year OS between responders and non-responders in cGVHD group. Our results are comparable to those reported by other retrospective clinical studies. Zeiser et al reported a similar ORR of 81.5% and 85.4% for patients with aGVHD and cGVHD, respectively.10 Gomez et al reported an ORR of 69.5% and 57.1% for patients with aGVHD and cGVHD, respectively.15 Moreover, Zhao et al, who reported outcomes of 34 multidrug-resistant GVHD (MDR-GVHD) patients who received ruxolitinib as salvage therapy, including an ORR of 60.0% and 89.5% for patients with aGVHD and cGVHD, respectively.22 Although the definitions of MDR-GVHD and SR-GVHD are slightly different, both refer to the use of ruxolitinib in cases where multiple second-line treatments have failed. Additional studies have reported ORRs in the range of 45–100% and 43.4–100% for aGVHD and cGVHD, respectively.11–14,22–28 Altogether, the currently available studies suggest that ruxolitinib improves response in patients with SR-GVHD, resulting in improved OS.
It is worth noting that in the REACH2 trial, Zeiser et al reported an ORR at day 28 that was significantly higher in the ruxolitinib group than in the BAT group (62% vs 39%, P<0.001).18 In this trial, the median failure free survival (FFS) was significantly longer in the ruxolitinib group than in the BAT group (4.86 months vs 1.02 months, P<0.0001). Moreover, results from the REACH3 trial indicated that the ORR of cGVHD at 24 weeks was significantly higher in the ruxolitinib group than in the BAT group (49.7% vs 25.6%; P<0.0001), whereas the median FFS was significantly longer (unreached vs 5.7 months; P < 0.0001).29 Altogether, these two prospective trials suggest efficacy and safety of ruxolitinib in acute and chronic SR-GVHD, respectively, although additional randomized controlled trials are needed to further confirm these findings.
It is worth mentioning that all patients included in this study had received multiple second-line therapies, including immunosuppressants and mesenchymal stem cells, before receiving ruxolitinib. Some immunosuppressants were also used in combination with ruxolitinib, which was gradually reduced to discontinuation. Through comparison, we found that the ORR observed in our study was similar to that other retrospective studies, but improved in comparison to those from the REACH2/REACH3 trials. Therefore, the combination of immunosuppressive therapy can result in partial remission of GVHD, affecting the efficacy evaluation of ruxolitinib. However, with ruxolitinib, patients can achieve remission while reducing the withdrawal of prednisone and immunosuppressants, thus overall reducing the incidence of adverse events such as infection, which is also an encouraging result.
The efficacy of ruxolitinib appears to be organ specific. Among the patients with aGVHD, 65.2% had involvement of a single organ, mostly in the skin and GI tract, and achieved ORR of 88.2% and 92.3%, respectively. However, the ORR of liver aGVHD was 66.7%; one patient developed new liver involvement during treatment and did not respond to ruxolitinib. In agreement with this finding, Jagasia et al and Zhao et al reported ORRs for patients with liver aGVHD of 26.7% and 53.3%, respectively.17,22 The REACH2 trial showed similar results, with no significant remission on day 28, despite low liver and GI tract involvement scores.18 Ruxolitinib treatments appears less effective in liver aGVHD. In the cGVHD group, more than half of patients had severe cGVHD or multiple organ involvement. The ORR of lung cGVHD was 44.4%, while all the other involved organs showed good response. Only one patient with an initial lung score of 2 achieved CR; the pulmonary symptoms completely disappeared after treatment with ruxolitinib with a long-time survival of more than 3 years. Modi et al and Zeiser et al reported an ORR of 10% and 8.6% in patients with lung involvement.14 In the context of higher ORRs of other affected organs, the efficacy of ruxolitinib in lung involvement is far from optimal; whether it is related to the pharmacokinetics of ruxolitinib or the pathophysiology of liver and lung GVHD requires further investigation.
Univariate analysis indicated that response to ruxolitinib treatment was associated with the stem cell source and GVHD grade. Patients who received both PB and BM appear to have a better response than those who received PB only. In addition, our study found that patients who initially presented with less severe of GVHD had a significantly better response that those who presented with severe GVHD. To the best of our knowledge, these results have not yet been confirmed by other reports, and indeed large-sample prospective studies are needed to validate the findings.
Notably, there no significant differences in the ORR between the 5 mg and the 10 mg bid group were observed. Isberner et al suggested that ruxolitinib exposure is increased in GVHD patients in comparison to myelofibrosis patients due to a significant reduction in ruxolitinib clearance.30 In agreement with our results, no significant differences in the mean ruxolitinib concentrations in GVHD patients after dose reduction were observed. So far, no guidelines exist for the optimal dose of ruxolitinib for clinical use. Most clinical studies have used 10 mg bid, and reported ORRs from 73.2% to 81%. These results are comparable to those of the present study. Therefore, we had hypothesized that patients receiving the 5 mg bid would have had the same benefit as those receiving the 10 mg bid, hence the lower dose would be more economical. However, it is important to note, that further prospective studies are needed to clarify the optimal dose.
The association between ruxolitinib treatment and viremia has been explored in prior studies. According to our study, the reactivation rate of CMV was 52.2% in aGVHD but only 10.0% in cGVHD. Zeiser et al and Jagasia et al reported lower reactivation rates in patients with aGVHD than our study did (33.3% in the Zeiser et al and 19.7% in the Jagasia et al study).10,17 Additionally, Gomez et al has reported a CMV reactivation rate of 52.2% and 26.0% in patients with aGVHD and cGVHD, respectively.15 Although in our study the CMV reactivation rate was high in patients with aGVHD, no severe CMV virus disease and no CMV infection-related deaths occurred. Moreover, previous studies have suggested that most CMV infections occur within 3 months post-transplantation, and it is important to note that immunosuppressants as standard prophylaxis for GVHD also increase the risk of viral infection.31 Therefore, it is difficult to attribute CMV reactivation to administration of ruxolitinib. Cytopenia is the most common toxic effect of ruxolitinib.4,18,26 We observed a 100% incidence of hematological toxicities in the aGVHD group, with a high incidence of grade 3–4 anemia, neutropenia and thrombocytopenia. These findings may be linked to use of ruxolitinib in some patients without complete hematopoietic system recovery following transplantation. Symptoms in these patients improved after symptomatic supportive therapy such as blood transfusion, with no patient discontinuing ruxolitinib due to cytopenia. Therefore, in order to prevent and treat potentially serious complications, CMV-DNA and blood routine tests should be routinely monitored during ruxolitinib therapy, particularly in aGVHD patients.
In our study, the relapse rates of primary disease were 21.7% and 12.5% for aGVHD and cGVHD, respectively. Relapse was the leading cause of death in patients with aGVHD. Other studies have reported similar relapse rates.23,25,32 Choi et al and Carniti et al demonstrated that ruxolitinib treatment reduced GVHD and preserved the beneficial of GVT effect in murine models.8,9 More prospective clinical trials are needed to determine whether ruxolitinib reduces the risk of relapse in patients compared with other immunosuppressants. In cGVHD, 15.6% of patients died from infection; in our study the rate of infection complications was high in both aGVHD and cGVHD groups. Therefore, infection is very common during the treatment of SR-GVHD, and prevention and aggressive treatments should be carefully considered.
Our study also has some limitations. First, because of the retrospective nature of the study, selection bias and recall bias were difficult to avoid. This could have impacted monitoring and recording of adverse events. Second, our study was a single center study and the sample size was relatively small and had a relatively short follow-up. Third, we cannot rule out the effect of combination therapies.
Conclusion
Ruxolitinib treatment could be a promising option for the treatment of SR-GVHD. Based on our results, ruxolitinib treatment in patients with SR-GVHD resulted in a high ORR and increased OS. The efficacy of ruxolitinib at a dose of 5 mg bid seem to be not inferior to that at 10 mg bid, a finding worth of further exploration. Patients who received both PB and BM and initially presented with less severe GVHD had a better response. Regarding safety, although our study reported a high incidence of cytopenia and infection, all adverse events were within expectations, and highlight the importance of blood routine monitoring and infection prophylaxis. High-quality and large-sample prospective clinical trials are needed to confirm the efficacy, safety and optimal dose of ruxolitinib treatment in SR-GVHD.
Acknowledgments
Clinical data collection was supported by the department of Hematology of Zhujiang Hospital. All the authors contributed to the development of the manuscript draft, including approval of the final draft for submission. Finally, the authors appreciate the unknown referee’s valuable and profound comments.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Penack O, Marchetti M, Ruutu T, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7(2):e157–e167. doi:10.1016/s2352-3026(19)30256-x
2. Mohty M, Holler E, Jagasia M, et al. Refractory acute graft-versus-host disease: a new working definition beyond corticosteroid refractoriness. Blood. 2020;136(17):1903–1906. doi:10.1182/blood.2020007336
3. Wolff D, Schleuning M, von Harsdorf S, et al. Consensus conference on clinical practice in chronic GVHD: second-line treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17(1):1–17. doi:10.1016/j.bbmt.2010.05.011
4. Malard F, Huang XJ, Sim JPY. Treatment and unmet needs in steroid-refractory acute graft-versus-host disease. Leukemia. 2020;34(5):1229–1240. doi:10.1038/s41375-020-0804-2
5. Spoerl S, Mathew NR, Bscheider M, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123(24):3832–3842. doi:10.1182/blood-2013-12-543736
6. Betts BC, Bastian D, Iamsawat S, et al. Targeting JAK2 reduces GVHD and xenograft rejection through regulation of T cell differentiation. Proc Natl Acad Sci U S A. 2018;115(7):1582–1587. doi:10.1073/pnas.1712452115
7. Elli EM, Barate C, Mendicino F, Palandri F, Palumbo GA. Mechanisms underlying the anti-inflammatory and immunosuppressive activity of ruxolitinib. Front Oncol. 2019;9:1186. doi:10.3389/fonc.2019.01186
8. Choi J, Cooper ML, Alahmari B, et al. Pharmacologic blockade of JAK1/JAK2 reduces GvHD and preserves the graft-versus-leukemia effect. PLoS One. 2014;9(10):e109799. doi:10.1371/journal.pone.0109799
9. Carniti C, Gimondi S, Vendramin A, et al. Pharmacologic Inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin Cancer Res. 2015;21(16):3740–3749. doi:10.1158/1078-0432.CCR-14-2758
10. Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062–2068. doi:10.1038/leu.2015.212
11. Khandelwal P, Teusink-Cross A, Davies SM, et al. Ruxolitinib as salvage therapy in steroid-refractory acute graft-versus-host disease in pediatric hematopoietic stem cell transplant patients. Biol Blood Marrow Transplant. 2017;23(7):1122–1127. doi:10.1016/j.bbmt.2017.03.029
12. Ferreira AM, Pontes da Silva CA, Pereira AD, et al. Ruxolitinib in steroid-refractory chronic graft-versus-host disease: experience of a single center. Bone Marrow Transplant. 2018;53(4):503–506. doi:10.1038/s41409-017-0068-2
13. Khoury HJ, Langston AA, Kota VK, et al. Ruxolitinib: a steroid sparing agent in chronic graft-versus-host disease. Bone Marrow Transplant. 2018;53(7):826–831. doi:10.1038/s41409-017-0081-5
14. Modi B, Hernandez-Henderson M, Yang D, et al. Ruxolitinib as salvage therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2019;25(2):265–269. doi:10.1016/j.bbmt.2018.09.003
15. Escamilla Gomez V, Garcia-Gutierrez V, Lopez Corral L, et al. Ruxolitinib in refractory acute and chronic graft-versus-host disease: a multicenter survey study. Bone Marrow Transplant. 2020;55(3):641–648. doi:10.1038/s41409-019-0731-x
16. Przepiorka D, Luo L, Subramaniam S, et al. FDA approval summary: ruxolitinib for treatment of steroid-refractory acute graft-versus-host disease. Oncologist. 2020;25(2):e328–e334. doi:10.1634/theoncologist.2019-0627
17. Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135(20):1739–1749. doi:10.1182/blood.2020004823
18. Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800–1810. doi:10.1056/NEJMoa1917635
19. Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828.
20. Jagasia MH, Greinix HT, Arora M, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: i. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e381. doi:10.1016/j.bbmt.2014.12.001
21. Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21(6):984–999. doi:10.1016/j.bbmt.2015.02.025
22. Zhao JY, Liu SN, Xu LP, et al. Ruxolitinib is an effective salvage treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation without posttransplant cyclophosphamide. Ann Hematol. 2021;100(1):169–180. doi:10.1007/s00277-020-04273-2
23. Gonzalez Vicent M, Molina B, Gonzalez de Pablo J, Castillo A, Diaz MA. Ruxolitinib treatment for steroid refractory acute and chronic graft vs host disease in children: clinical and immunological results. Am J Hematol. 2019;94(3):319–326. doi:10.1002/ajh.25376
24. Neumann T, Schneidewind L, Weigel M, et al. Ruxolitinib for therapy of graft-versus-host disease. Biomed Res Int. 2019;2019:8163780. doi:10.1155/2019/8163780
25. Dang SH, Liu Q, Xie R, et al. Ruxolitinib add-on in corticosteroid-refractory graft-vs-host disease after allogeneic stem cell transplantation: results from a retrospective study on 38 Chinese patients. World J Clin Cases. 2020;8(6):1065–1073. doi:10.12998/wjcc.v8.i6.1065
26. Moiseev IS, Morozova EV, Bykova TA, et al. Long-term outcomes of ruxolitinib therapy in steroid-refractory graft-versus-host disease in children and adults. Bone Marrow Transplant. 2020;55(7):1379–1387. doi:10.1038/s41409-020-0834-4
27. Uygun V, Karasu G, Daloglu H, et al. Ruxolitinib salvage therapy is effective for steroid-refractory graft-versus-host disease in children: a single-center experience. Pediatr Blood Cancer. 2020;67(4):e28190. doi:10.1002/pbc.28190
28. Yang W, Zhu G, Qin M, et al. The effectiveness of ruxolitinib for acute/chronic graft-versus-host disease in children: a retrospective study. Drug Des Devel Ther. 2021;15:743–752. doi:10.2147/DDDT.S287218
29. Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385(3):228–238. doi:10.1056/NEJMoa2033122
30. Isberner N, Kraus S, Grigoleit GU, et al. Ruxolitinib exposure in patients with acute and chronic graft versus host disease in routine clinical practice-a prospective single-center trial. Cancer Chemother Pharmacol. 2021;88(6):973–983. doi:10.1007/s00280-021-04351-w
31. Lin R, Liu Q. Diagnosis and treatment of viral diseases in recipients of allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2013;6:94. doi:10.1186/1756-8722-6-94
32. Wu H, Shi J, Luo Y, et al. Evaluation of ruxolitinib for steroid-refractory chronic graft-vs-host disease after allogeneic hematopoietic stem cell transplantation. JAMA Netw Open. 2021;4(1):e2034750. doi:10.1001/jamanetworkopen.2020.34750
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

