Back to Journals » International Journal of General Medicine » Volume 15
RUNX1 and CCL3 in Diabetes Mellitus-Related Coronary Artery Disease: A Bioinformatics Analysis
Authors Zhong Y , Du G , Liu J, Li S, Lin J, Deng G, Wei J, Huang J
Received 26 November 2021
Accepted for publication 14 January 2022
Published 28 January 2022 Volume 2022:15 Pages 955—963
DOI https://doi.org/10.2147/IJGM.S350732
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yi Zhong,1,2,* Guoyong Du,1,2,* Jie Liu,1,2,* Shaohua Li,1 Junhua Lin,1 Guoxiong Deng,1,2 Jinru Wei,1,2 Jun Huang1,2
1Department of Cardiology, The Fifth Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530022, People’s Republic of China; 2Department of Cardiology, The First People’s Hospital of Nanning, Nanning, Guangxi, 530022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinru Wei; Jun Huang, Tel +867712636193, Email [email protected]; [email protected]
Background: Cardiovascular complications are a major cause of death and disability in patients with diabetes mellitus, but how such complications arise is unclear.
Methods: Weighted gene correlation network analysis (WGCNA) was performed on gene expression profiles from healthy controls, individuals with diabetes mellitus, and individuals with diabetes mellitus-associated coronary artery disease (DMCAD). Phenotypically related module genes were analyzed for enrichment in Gene Ontology (GO) terms and Kyoto Gene and Genome Encyclopedia (KEGG) pathways. Predicted biological functions were validated using gene set enrichment analysis (GSEA) and ClueGo analysis. Based on the TRRUST v2 database and hypergeometric tests, a global network was built to identify transcription factors (TFs) and downstream target genes potentially involved in DMCAD.
Results: WGCNA identified three modules associated with progression from diabetes mellitus to DMCAD. The module genes were significantly involved in biological processes related to interferon and viral infection, while GSEA of DMCAD samples suggested involvement in viral myocarditis, chemokine signaling and phagosomes. RUNX1 was identified as a potential TF regulating these module genes. Analysis of the global regulatory network of TFs and their targets suggested that CCL3 may be a key regulator in DMCAD.
Conclusion: We found bioinformatic evidence that CCL3 may be a key regulator and RUNX1 a key TF in DMCAD.
Keywords: diabetes mellitus, coronary artery disease, transcription factors
Introduction
Diabetes mellitus is a common metabolic disease that can lead to macro- and microvascular complications, such as coronary artery disease.1 Individuals with diabetes mellitus are at higher risk of coronary artery disease than the general population, and they are nearly twice as likely to be hospitalized for myocardial infarction or to die from cardiovascular problems as those without diabetes.2 Coronary angiography has become the standard diagnostic method for coronary artery disease (CAD) and can improve the early detection of clinical disease.3 However, coronary angiography is expensive and invasive, so there is a need for better diagnostic methods, such as finding a new biomarker.
In the previous studies, the key genes of diabetes mellitus-related coronary heart disease4 and ischemic stroke related to diabetes were acquired.5 In that work, a classifier that can diagnose coronary artery disease was developed,6 but it is not applicable to diabetes mellitus-associated coronary artery disease (DMCAD). How DMCAD arises is poorly understood, although it seems to involve the ubiquitin-proteasome system,7 inflammatory factors (fibrinogen, C-reactive protein, galectin-3) and metabolic factors such as lipoproteins.8–10 We wondered whether the transcription factor (TF) RUNX1 might also be involved. RUNX1 is involved in vertebrate hematopoiesis, and it is one of the genes most frequently mutated in hematological malignancies.11 There is also growing evidence that RUNX1 plays a role in non-hematopoietic tissues of various epithelial origin.12,13 Consistent with the present study, RUNX1 may also contribute to atherosclerotic plaque formation,14 and the downregulation of RUNX1 in diabetes mellitus leads to defects in macrophage maturation.15
We also wondered whether chemokines might be involved in DMCAD. These factors recruit leukocytes to sites of injury and activate proliferation of vascular smooth muscle cells, neovascularization and platelets.16,17 CLL3 is two-sided in that it not only has anticancer properties but also promotes cancer development. CC chemokines, including CLL3, are important in the initiation of immune responses.18 They induce the recruitment of dendritic cells, neutrophils, monocytes, macrophages, NK cells and T cells to sites of inflammation.18 In tumors, tumor-associated macrophages19 and MSCs20 secrete CLL3, which promotes tumor growth.21 CCL3 causes migration and invasion of cancer cells via CCR5.22 In particular, CC chemokine ligand (CCL) 3 has been detected in human atherosclerotic lesions.23
Here we performed gene expression profiling of healthy individuals, individuals with diabetes mellitus, and those with DMCAD. Our analyses implicate RUNX1 in the progression from diabetes mellitus to DMCAD, and they implicate CCL3 as a key regulator of DMCAD pathology.
Materials and Methods
Data Collection and Processing
Gene expression profiles were downloaded from the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/).24 The GSE90074 dataset, based on the GPL6480 platform,25 contains gene expression profiles from mononuclear cells in whole blood from 55 patients with coronary artery disease, 17 with diabetes mellitus, 38 with DMCAD and 33 healthy controls. For the present study, we excluded the data from patients with coronary artery disease alone. The normalizeBetweenArrays function in the “limma” package26 was used to normalize gene expression profiles. When the same gene was detected using multiple probes, the expression level was defined as the average across all probes. Probes were excluded if they hybridized to multiple genes. The workflow of the present study is shown in Figure 1.
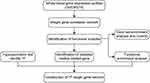 |
Figure 1 Study workflow. Abbreviation: TF, transcription factor. |
Weighted Correlation Network Analysis (WGCNA)
Using the “WGCNA” package27 in R, we examined the correlations between genes in GSE90074 and phenotype, which was categorized as normal (1), diabetes mellitus (2) or DMCAD (3). We focused on genes that strongly correlated with phenotype and were associated with a significant p value. Candidate power (1 to 20) was used to test average connectivity degrees of different modules and their independence. If the degree of independence was >0.85, the power value was selected. Hierarchical clustering analysis was performed using the hclust function in the WGCNA package. Furthermore, we explored the correlation between modules and phenotype, and focused on modules that correlated with progression from a healthy state through diabetes mellitus to DMCAD.
Functional Enrichment Analysis of Gene Modules
To explore the signaling pathways and biological characteristics of genes in modules of interest, we analyzed the genes for enrichment in Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways using the “clusterProfiler” package28 in R and the Clue GO plug-in29 in Cytoscape.30 We also performed gene set enrichment analysis (GSEA)31 on the entire gene expression profiles using the software GSEA (www.gsea-msigdb.org/gsea/downloads.jsp). The reference gene sets were c5.bp.v7.0.symbols.gmt and c2.cp.kegg.v7.0.symbols.gmt, downloaded from the Molecular Signature Database.32 Results associated with P < 0.05 were considered significant.
Prediction of TFs in Modules and Construction of a Global Regulatory Network
Based on the interaction of human TFs with their target genes in the TRRUST v2 database,33 the hypergeometric test within the “igraph” package in R (https://igraph.org/r/) was used to predict TFs that may regulate functional modules. Only TFs within modules were retained. Then the genes targeted by those TFs were extracted, and a map of TFs and target genes was constructed. Results associated with P < 0.05 were considered significant.
Results
Gene Modules Related to DMCAD
WGCNA was performed on 996 genes, and soft-thresholding was determined to be 6 based on the scale-free fit index and average connectivity (Figure 2A). Average linkage hierarchical clustering and module merging divided the genes into four modules (Figure 2B). Three modules were considered of interest (Figure 2C): turquoise, which showed a negative correlation with phenotype (r = −0.39, p = 2e-02); and blue and brown modules, which showed a positive correlation with phenotype (r = 0.37, p = 4e-04). The grey module was reserved for unassigned genes and was not analyzed further.
DMCAD is Associated with Dysfunction in Multiple Modules
Gene expression across the three groups was analyzed by clustering analysis (Figure 3A). Genes in the three modules were involved in GO biological processes including responses to virus, to type I interferon, and to interferon-gamma (Figure 3B). Genes in the three modules were also significantly involved in pathways related to viral myocarditis, phagosomes, tight junctions, type 1 diabetes mellitus and chemokine signaling (Figure 3C). GSEA identified several pathways enriched in DMCAD samples: leukocyte transendothelial migration, RNA destabilization, the proteasome and type 1 diabetes mellitus (Figure 3D). In contrast, GSEA identified pathways related to cellular defense responses as well as antigen processing and presentation in individuals with diabetes mellitus (Figure 3E). ClueGo analysis indicated that genes in the three modules were involved in granulocyte migration and responses to type 1 interferon and interferon-gamma (Figure 3F).
TFs Potentially Involved in DMCAD
Hypergeometric tests identified the TFs IRF9, PML and RUNX1 in the turquoise, blue, and brown modules as potentially involved in DMCAD. These TFs strongly correlated with their respective target genes CCL3, FLT3, PML and SP100 (Figure 4A). In GSE90074, the three TFs were expressed at higher levels in DMCAD patients than in individuals with diabetes mellitus or in healthy controls (Figure 4B).
Global Regulatory Landscape in DMCAD
A network of TFs and their target genes in DMCAD was constructed (Figure 5A), providing a first glimpse into the global transcriptional regulatory landscape. CCL3 was significantly related to pathways involving chemokine signaling, cytokine-receptor interactions and Toll-like receptor signaling (Figure 5B), which therefore may be involved in DMCAD (Figure 5C).
Discussion
Diabetes mellitus is a chronic disease that endangers human health and poses a severe socioeconomic burden. DMCAD is associated with greater risk of adverse events than diabetes on its own,34,35 but how DMCAD occurs is poorly understood. In the present study, we performed WGCNA on whole-blood gene expression profiles in the GSE90074 database in order to identify gene modules involved in DMCAD pathogenesis. Leukocyte transendothelial migration has already been linked to DMCAD,36 suggesting that our analysis is reliable and that LDB2, a previously identified inhibitor of leukocyte transendothelial migration, may be a useful therapeutic target.37 We also found evidence that RUNX1 is up-regulated during progression from a healthy state to diabetes mellitus and then to DMCAD. This suggests that RUNX1 is a key TF in DMCAD. It has been proposed that RUNX1 mutations cause cardiac developmental defects and coronary vascular dysplasia, and a direct role of RUNX1 in upregulating mesenchymal markers in epicardial cells and promoting EMT cannot be excluded,38 which endocardial cells play an important role in coronary artery development.39
Our global transcriptional regulatory network in DMCAD suggests that CCL3 may be a marker of the condition. In fact, chemokines and chemokine receptors are important mediators in coronary artery disease.40 CCL3, for example, can indirectly affect cytokine production, cellular growth and differentiation, apoptosis and migration along a chemokine gradient.41 Indeed, monocytes from individuals with diabetes mellitus show a defect in chemotaxis.42,43 CCL3 acts as an inflammatory cytokine through Toll-like receptor signaling pathways,44 and diabetes mellitus involves systemic low-grade inflammation, which may increase risk of coronary artery disease. Our observation of a link between CCL3 and DMCAD provides testable hypotheses about which signaling pathways are involved in the condition, since CCL3 is known to interact with the receptors CCR1 and CCR5.45 CCR1 mediates pro-fibrotic effects in hematopoietic cells,46 while CCR5 regulates obesity, insulin resistance and glucose homeostasis.47,48 Notably, it has been proposed that CCL3 causes migration and invasion of cancer cells through CCR5,22 associated with activation of PI3K-Akt/PKB and ERK MAPK pathways.19 New signaling pathways were obtained in the present study, so this deserves further deeper investigation as potential therapeutic targets to prevent or mitigate DMCAD.
Our findings should be interpreted with caution in light of several limitations. First, our analyses were based entirely on bioinformatics, so our results should be verified and extended in cellular and biochemical experiments. Second, our sample was relatively small, so larger studies are needed to determine whether RUNX1 and CCL3 or their downstream targets can be considered DMCAD biomarkers.
Conclusion
Our work links DMCAD to the TF RUNX1 and the regulatory factor CCL3. Further work should explore these molecules and their downstream pathways in DMCAD, which may elucidate pathogenesis and suggest therapeutic strategies.
Data Sharing Statement
The raw data GSE90074 was download from GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE90074), analyses and codes in this study can be obtained from the corresponding author Dr Wei and Dr Huang upon reasonable request.
Ethics Approval and Informed Consent
Not applicable because GEO belongs to public databases, the patients involved in the database have obtained ethical approval, users can download relevant data for free for research and publish relevant articles, and our study is based on open-source data. Ethics committee from the Fifth Affiliated Hospital of Guangxi Medical University specifically do not require research using publicly available data to be submitted for review to their ethics committee, so this study does not require approval.
Acknowledgments
This study was supported by the Project of Nanning Scientific Research and Technology Development Plan (ZC20203010), the Project of Qingxiu District of Nanning Scientific Research and Technology Development Plan (2020059), the Scientific Research Project of Guangxi Health Commission (Z20201226), and the Guangxi Medical and Health Key Discipline Construction Project (Department of Cardiology, The First People’s Hospital of Nanning).
Disclosure
The authors have no potential conflicts of interest to declare.
References
1. Zhang P, Gregg E. Global economic burden of diabetes and its implications. Lancet Diabetes Endocrinol. 2017;5(6):404–405. doi:10.1016/S2213-8587(17)30100-6
2. Ali MK, McKeever Bullard K, Imperatore G, et al. Characteristics associated with poor glycemic control among adults with self-reported diagnosed diabetes–National Health and Nutrition Examination Survey, United States, 2007–2010. MMWR Suppl. 2012;61(2):32–37.
3. McCullough PA. Coronary artery disease. Clin J Am Soc Nephrol. 2007;2(3):611–616. doi:10.2215/CJN.03871106
4. Huang Q, Deng G, Wei R, Wang Q, Zou D, Wei J. Comprehensive identification of key genes involved in development of diabetes mellitus-related atherogenesis using weighted gene correlation network analysis. Front Cardiovasc Med. 2020;7:580573. doi:10.3389/fcvm.2020.580573
5. Zhou H, Huang L, Liang L, et al. Identification of an miRNA regulatory network and candidate markers for ischemic stroke related to diabetes. Int J Gen Med. 2021;14:3213–3223. doi:10.2147/IJGM.S319503
6. Liu J, Wang X, Lin J, Li S, Deng G, Wei J. Classifiers for predicting coronary artery disease based on gene expression profiles in peripheral blood mononuclear cells. Int J Gen Med. 2021;14:5651–5663. doi:10.2147/IJGM.S329005
7. Marfella R, D’Amico M, Di filippo C, et al. The possible role of the ubiquitin proteasome system in the development of atherosclerosis in diabetes. Cardiovasc Diabetol. 2007;6:35. doi:10.1186/1475-2840-6-35
8. Mazzone T. Strategies in ongoing clinical trials to reduce cardiovascular disease in patients with diabetes mellitus and insulin resistance. Am J Cardiol. 2004;93(11A):27C–31C. doi:10.1016/j.amjcard.2004.02.003
9. Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–1146. doi:10.1161/01.cir.100.10.1134
10. Seferovic JP, Lalic NM, Floridi F, et al. Structural myocardial alterations in diabetes and hypertension: the role of galectin-3. Clin Chem Lab Med. 2014;52(10):1499–1505. doi:10.1515/cclm-2014-0265
11. Sood R, Kamikubo Y, Liu P. Role of RUNX1 in hematological malignancies. Blood. 2017;129(15):2070–2082. doi:10.1182/blood-2017-12-819789
12. Mevel R, Draper JE, Lie ALM, Kouskoff V, Lacaud G. RUNX transcription factors: orchestrators of development. Development. 2019;146(17). doi:10.1242/dev.148296
13. Blyth K, Cameron ER, Neil JC. The RUNX genes: gain or loss of function in cancer. Nat Rev Cancer. 2005;5(5):376–387. doi:10.1038/nrc1607
14. Murakami N, Hashidate T, Harayama T, Yokomizo T, Shimizu T, Nakamura M. Transcriptional regulation of human G2A in monocytes/ macrophages: involvement of c/EBPs, Runx and Pu.1. Genes Cells. 2009;14(12):1441–1455. doi:10.1111/j.1365-2443.2009.01360.x
15. Alrdahe S, Al Sadoun H, Torbica T, et al. Dysregulation of macrophage development and phenotype in diabetic human macrophages can be rescued by Hoxa3 protein transduction. PLoS One. 2019;14(10):e0223980. doi:10.1371/journal.pone.0223980
16. Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95(9):858–866. doi:10.1161/01.RES.0000146672.10582.17
17. Weber C. Platelets and chemokines in atherosclerosis: partners in crime. Circ Res. 2005;96(6):612–616. doi:10.1161/01.RES.0000160077.17427.57
18. Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13(6):455–481. doi:10.1016/s1359-6101(02)00045-x
19. Kodama T, Koma YI, Arai N, et al. CCL3-CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab Invest. 2020;100(9):1140–1157. doi:10.1038/s41374-020-0441-4
20. Nishikawa G, Kawada K, Nakagawa J, et al. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death Dis. 2019;10(4):264. doi:10.1038/s41419-019-1508-2
21. Luo A, Meng M, Wang G, et al. Myeloid-derived suppressor cells recruited by chemokine (C-C Motif) ligand 3 promote the progression of breast cancer via phosphoinositide 3-kinase-protein kinase b-mammalian target of rapamycin signaling. J Breast Cancer. 2020;23(2):141–161. doi:10.4048/jbc.2020.23.e26
22. Baghel KS, Tewari BN, Shrivastava R, et al. Macrophages promote matrix protrusive and invasive function of breast cancer cells via MIP-1beta dependent upregulation of MYO3A gene in breast cancer cells. Oncoimmunology. 2016;5(7):e1196299. doi:10.1080/2162402X.2016.1196299
23. Ardigo D, Assimes TL, Fortmann SP, et al. Circulating chemokines accurately identify individuals with clinically significant atherosclerotic heart disease. Physiol Genomics. 2007;31(3):402–409. doi:10.1152/physiolgenomics.00104.2007
24. Keret D, Reis ND, Reijman EK. Malignant fibrous histiocytoma of bone. Harefuah. 1986;110(5):240–242.
25. Ravi S, Schuck RN, Hilliard E, et al. Clinical evidence supports a protective role for cxcl5 in coronary artery disease. Am J Pathol. 2017;187(12):2895–2911. doi:10.1016/j.ajpath.2017.08.006
26. Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi:10.1093/nar/gkv007
27. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 2008;9:559. doi:10.1186/1471-2105-9-559
28. Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi:10.1089/omi.2011.0118
29. Bindea G, Mlecnik B, Hackl H, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25(8):1091–1093. doi:10.1093/bioinformatics/btp101
30. Kohl M, Wiese S, Warscheid B. Cytoscape: software for visualization and analysis of biological networks. Methods Mol Biol. 2011;696:291–303. doi:10.1007/978-1-60761-987-1_18
31. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi:10.1073/pnas.0506580102
32. Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1(6):417–425. doi:10.1016/j.cels.2015.12.004
33. Han H, Cho JW, Lee S, et al. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2018;46(D1):D380–D386. doi:10.1093/nar/gkx1013
34. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi:10.1016/S0140-6736(10)60484-9
35. Mohammedi K, Woodward M, Marre M, et al. Comparative effects of microvascular and macrovascular disease on the risk of major outcomes in patients with type 2 diabetes. Cardiovasc Diabetol. 2017;16(1):95. doi:10.1186/s12933-017-0574-y
36. Hong LF, Li XL, Luo SH, et al. Relation of leukocytes and its subsets counts with the severity of stable coronary artery disease in patients with diabetic mellitus. PLoS One. 2014;9(3):e90663. doi:10.1371/journal.pone.0090663
37. Shang MM, Talukdar HA, Hofmann JJ, et al. Lim domain binding 2: a key driver of transendothelial migration of leukocytes and atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(9):2068–2077. doi:10.1161/ATVBAHA.113.302709
38. Lluri G, Huang V, Touma M, Liu X, Harmon AW, Nakano A. Hematopoietic progenitors are required for proper development of coronary vasculature. J Mol Cell Cardiol. 2015;86:199–207. doi:10.1016/j.yjmcc.2015.07.021
39. Wu B, Zhang Z, Lui W, et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151(5):1083–1096. doi:10.1016/j.cell.2012.10.023
40. Waehre T, Damas JK, Gullestad L, et al. Hydroxymethylglutaryl coenzyme a reductase inhibitors down-regulate chemokines and chemokine receptors in patients with coronary artery disease. J Am Coll Cardiol. 2003;41(9):1460–1467. doi:10.1016/s0735-1097(03)00263-8
41. Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–197. doi:10.1146/annurev.pharmtox.48.121806.154841
42. Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: a potential predictor for the individual capacity to develop collaterals. Circulation. 2000;102(2):185–190. doi:10.1161/01.cir.102.2.185
43. Bouma G, Coppens JM, Lam-Tse WK, et al. An increased MRP8/14 expression and adhesion, but a decreased migration towards proinflammatory chemokines of type 1 diabetes monocytes. Clin Exp Immunol. 2005;141(3):509–517. doi:10.1111/j.1365-2249.2005.02865.x
44. Bustamante J, Tamayo E, Herreros J. Genomics in cardiovascular diseases: analysis of the importance of the toll-like receptor signaling pathway. Int J Gen Med. 2012;5:849–859. doi:10.2147/IJGM.S33416
45. Ajuebor MN, Hogaboam CM, Le T, Proudfoot AE, Swain MG. CCL3/MIP-1alpha is pro-inflammatory in murine T cell-mediated hepatitis by recruiting CCR1-expressing CD4(+) T cells to the liver. Eur J Immunol. 2004;34(10):2907–2918. doi:10.1002/eji.200425071
46. Seki E, De Minicis S, Gwak GY, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119(7):1858–1870. doi:10.1172/jci37444
47. Gonzalez P, Alvarez R, Batalla A, et al. Genetic variation at the chemokine receptors CCR5/CCR2 in myocardial infarction. Genes Immun. 2001;2(4):191–195. doi:10.1038/sj.gene.6363760
48. Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115(8):1029–1038. doi:10.1161/CIRCULATIONAHA.106.638379
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.