Back to Journals » OncoTargets and Therapy » Volume 13
Ropivacaine Inhibits the Growth, Migration and Invasion of Gastric Cancer Through Attenuation of WEE1 and PI3K/AKT Signaling via miR-520a-3p
Authors Zhang N, Xing X, Gu F, Zhou G , Liu X, Li B
Received 2 January 2020
Accepted for publication 21 April 2020
Published 9 June 2020 Volume 2020:13 Pages 5309—5321
DOI https://doi.org/10.2147/OTT.S244550
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Nianliang Zhang,1 Xiangji Xing,2 Fengcai Gu,1 Gang Zhou,1 Xianglan Liu,3 Baoqiang Li1
1Department of Anesthesiology, Rizhao People’s Hospital, Rizhao 276826, Shandong, People’s Republic of China; 2Department of Anesthesiology, Rizhao Women and Children Hospital, Rizhao 276800, Shandong, People’s Republic of China; 3ECG Room, Rizhao People’s Hospital, Rizhao 276826, Shandong, People’s Republic of China
Correspondence: Gang Zhou
Department of Anesthesiology, Rizhao People’s Hospital, No. 126 Taian Road, Rizhao 276826, Shandong, People’s Republic of China
Tel +86 06333365069
Email [email protected]
Background: Metastasis remains one of the greatest challenges involved in treating gastric cancer (GC). Ropivacaine (Rop) is not only a well-documented local anesthetic medicament but also has been reported to exert an antitumor role in cancer development. This study explored the effects of ropivacaine on the growth, migration and invasion of gastric cancer and the underlying mechanisms.
Methods: Cell Counting Kit-8 (CCK8) assay was conducted to test the effect of Rop on the proliferation of AGS and BGC-823 GC cells. Moreover, cell apoptosis, migration and invasion were examined by flow cytometry and transwell assay, respectively. The expression of miR-520a-3p was determined by qRT-PCR. miRNA targeting sites were analyzed using bioinformatics analysis and dual-luciferase reporter assay. Protein levels of WEE1 and PI3K/AKT were detected by Western blot. Furthermore, the tumor-forming experiment of nude mice was used to detect the growth of cells in vivo.
Results: Rop inhibited proliferation but promoted apoptosis of GC cells. Besides, the migration and invasion of GC cells were also inhibited by Rop. Moreover, miR-520a-3p expression was enhanced by Rop, and transfection with miR-520a-3p mimic decreased cell proliferation, migration and invasion. The upregulation of miR-520a-3p was partly contributed to the inhibitory effect of ropivacaine on GC cell lines. Finally, Rop inactivated WEE1 and PI3K/AKT pathway via upregulation of miR-520a-3p.
Conclusion: Our results suggested that Rop decreased growth, migration and invasion of GC cells via regulating miR-520a-3p expression and further inactivated WEE1 and PI3K/AKT signaling pathways.
Keywords: gastric cancer, ropivacaine, miR-520a-3p, WEE1, PI3K/AKT
Introduction
Gastric cancer (GC) is one of the most common malignant tumors worldwide. The latest data shows that the global incidence of GC ranks fifth among malignant tumors, and it is the third leading cause of cancer-related deaths globally.1 At present, surgery is the most important treatment for GC, but the rate of recurrence remains high due to its high invasiveness and metastasis. What’s more, the specific etiology and pathogenesis of GC have not been fully elucidated,2 and its targeted molecular drugs are lacking.3 Therefore, exploring the molecular mechanism of GC proliferation and metastasis is the key to improve the diagnosis and treatment of GC.
Ropivacaine (Ropivacaine, Rop) is S-(-)-1-propyl-N-(2,6-xylyl)-2-piperidinecarboxamide hydrochloride monohydrate and its anhydride, which is a new type long-acting amide local anesthetic with levorotatory form, and has been widely used in anesthesia, postoperative analgesia and other fields.4 In recent years, ropivacaine has attracted increasing attention in cancer research. For example, lidocaine and Rop have shown antiproliferative effects on colon cancer cell lines after high concentration and long-term exposure to local anesthetics.5 In addition, ropivacaine inhibits the proliferation and promotes apoptosis of liver cancer cells in a dose- and time-dependent manner.6 Rop also significantly reduces the viability of GTPases, including RhoA, Rac1, and Ras, and inhibits isoprenylation in esophageal cancer cells.7 However, the effect of Rop on GC cells needs further exploration.
MicroRNAs (miRNAs) are a class of endogenous noncoding RNA that are about 20 nucleotides in length and considered to be gene expression regulators post-transcriptionally. In recent years, increasing researches have revealed that miRNAs play key roles in the occurrence and development of malignant tumors.8 For example, miR-29 is downregulated in acute myeloid leukemia and liver cancer.9 Upregulated miR-21 is involved in the proliferation and migration of hepatocellular carcinoma by inhibiting SMAD7.10 Moreover, miR-362 induces GC cell proliferation by activating NF-κB pathway, while miR-181a inhibits the proliferation, invasion and metastasis of GC cells.11 As an important member of miRNAs, miR-520a-3p is located at 19q13.42 and is 85bp in length. Studies have shown that miR-520a-3p is abnormally expressed in a variety of tumors. For example, miR-520a-3p attenuates the the proliferation, migration and invasion of NSCLC cells via inhibition of HOXD8.12 DEX inhibits the proliferation and migration of osteosarcoma cells and promotes its apoptosis by upregulating miR-520a-3p.13 However, the role of miR-520a-3p in GC needs further exploration.
The protein of WEE1 contains 647 amino acids. Functionally, it inhibits the viability of CDK kinase by phosphorylating Thr14 and Tyr15 sites of CDK, thereby inhibiting cells from entering mitosis, regulating cell G2/M phase transformation, histone synthesis, and repair of DNA damage, thus maintaining genome stability.14,15 Besides, studies have shown that WEE1 is highly expressed in various cancers and has oncogenic functions. More importantly, the upregulation of WEE1 is also related to the malignant phenotype of GC, and also enhances cell proliferation and metastasis of GC.16
PI3K is an intracellular phosphatidylinositol kinase consisting of regulatory subunit p85 and catalytic subunit p110, while AKT is a serine/threonine-specific protein kinase. Both of them have been found to play key roles in the malignant processes of glucose metabolism, apoptosis, proliferation, transcription, and migration in various cells. For example, Tanshinone IIA (Tan IIA) inhibits EGF and TGF-β1-induced EMT in HepG2 cells by inactivating the PI3K/AKT/ERK pathway.17 In addition, studies have found that non-SMC condensin I complex subunit G (NCAPG) acts as an oncogene in liver cancer and promotes cell proliferation and reduces apoptosis by activating the PI3K/AKT/FOXO4 pathway.18 Nevertheless, whether Rop could affect the activation of PI3K/AKT in GC by regulating miR-520a-3p remains unclear.
In this study, we found that Rop dose-dependently inhibited the proliferation of GC cells both in vitro and in vivo, and also attenuated the migration and invasion of GC cells. In addition, Rop promoted the expression of miR-520a-3p but decreased the protein level of WEE1, phosphorylated PI3K (p-PI3K) and phosphorylated Akt (p-Akt). What’s more, miR-520a-3p inhibited the development of GC and enhanced the inhibitory effects of Rop on GC by affecting the proliferation, invasion and migration of GC cells. Functionally, miR-520a-3p was found to target WEE1 and inhibited it. Overall, this study revealed new molecular mechanisms in the progression of GC, and provided a new theoretical reference for the treatment of GC.
Materials and Methods
Cell Culture and Drug Action
AGS and BGC-823 cell lines were purchased from the Cell Center of Chinese Academy of Sciences, Shanghai, China. Cells were cultured in RPMI1640 medium (Thermo Fisher Scientific, MA, USA) containing 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, MA, USA) and 1% penicillin/streptomycin (Invitrogen, CA, USA) in an incubator in 5% CO2 at 37°C. Cells at the logarithmic growth stage were trypsinized by 0.25% trypsin (Thermo Fisher HyClone, Utah, USA) for cell passage. Rop (CAS NO. 84057-95-4, Sigma, Shanghai, China) was dissolved in DMSO and diluted with 0.9% physiological saline. Cells were treated with 0–40 μmol/L of Rop for 24h and then subjected to other experiments.
Cell Transfection
The GC cells of AGS and BGC-823 at the logarithmic growth phase were taken, and inoculated in a 6-well plate at a density of 5×106/well after trypsinization and passage. Subsequently, miR-520a-3p mimics or inhibitors and the matched negative controls were transfected into GC cells of AGS and BGC-823, respectively, according to the instructions of FuGENE® HD Transfection Reagent (Roche, Shanghai, China). Next, the cells were cultured in an incubator in 5% CO2 at 37°C. After 24 hours of transfection, total RNA of the cells was extracted and the real-time fluorescence quantitative PCR (RT-PCR) was performed to detect miRNAs expressive changes in the cells.
CCK8 Assay
AGS and BGC-823 GC cells were trypsinized and adjusted to reach a cell density of 2×103/mL, then inoculated in a 96-well plate with 100 μL of cell suspension per well. Afterwards, the 96-well plate was placed in an incubator for further culture. Twenty-four hours later, 10 μL of CCK8 solution (Hubei Bios Biotechnology Co., Ltd.) was added to each well and the cells were cultured for another 1 hour in the incubator. After the culture was terminated, the 96-well plate was placed in a microplate reader, and the absorbance (OD value) of each well at 450 nm was measured.
Transwell Assay
The cells were inoculated into a 24-well plate (5×104 cells/well) on the first day, and transfected according to the instructions of Lipofactamine ™ 2000 when the cell abundance reached about 80% on the second day. Twenty-four hours after transfection, the transfected cells were inoculated into the chamber of a Transwell (2.5×104 cells/well), and the cells in the chamber were resuspended in a serum-free medium, while the bottom of the chamber was a medium containing 20% serum. In migration experiments, no matrigel was coated with the interior chamber, while in invasion experiments, a layer of matrigel was pre-coated into the interior chamber to simulate extracellular matrix. After 24 hours of cell incubation, the chamber was taken out, and the cells in the lower well were fixed by mixture of formaldehyde and acetic acid for 15 minutes, then washed with PBS, stained with crystal violet. Finally, the number of invaded or migrated cells was counted.
Flow Cytometry
Apoptosis of GC cells was detected by flow cytometry using Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Sigma, Shanghai, China) according to the manufacturer’s instructions. After incubation for 48 h, AGS and BGC-823 cells were washed with PBS, resuspended in binding buffer and then double stained with Annexin V-FITC and PI. The apoptotic cells were analyzed by using a flow cytometer (Becton–Dickinson, Franklin Lakes, NJ, USA) with CellQuest software (BD Biosciences). Every sample was prepared in triplicate.
qRT-PCR
The expression of WEE1 mRNA and miR-520a-3p were detected by fluorescent quantitative PCR. The suspended cells were lysed with TRIzol (Invitrogen, Shanghai, China). RNAs in cell lysates were extracted by chloroform-isoamyl alcohol extraction, and an ultraviolet spectrophotometer was used to detect the purity and concentration of RNA samples. Then, 2 μg of RNA samples was taken to prepare 20 reverse transcription systems according to instructions of the reverse transcription kit (Thermo Scientific, Shanghai, China) and reverse transcribed into cDNAs under the catalysis of transcriptase. Next, 1 μL of cDNA sample was taken to prepare a 50μL-quantitative PCR reaction system which contained SYBR Green (Thermo Scientific, Shanghai, China) fluorescence and primers of miRNA-520-3p, and the procedures were as follows: initial denaturation (94°C, 1 min), denaturation (95 °C, 25s), primer annealing (62°C, 30s), and extension (72 °C, 20s), a total of 35 cycles were conducted. After the end of the cycles, the extension was performed at 72 °C for another 5min. The heating rate was 0.472 °C/time, and the acting time was 30s/time. A melting curve was made to evaluate the specificity of PCR amplification. The internal reference gene of miR-520a-3p was U6, and the relative expression was calculated using the 2−Δ∆Ct method. Primers for miR-520a-3p: Forward primers: 5ʹ-AACCTCCCAAAGTGCTTCCCTTT-3; Reverse primers: 5ʹ-CAGTGCAGGGTCCGAGGT-3ʹ.
Primers for WEE1: Forward primers: 5ʹ-GCTGCCTCTGAAGAAGGAGA-3; Reverse primers: 5ʹ-ACATACCACTGTGAGGGCAA-3ʹ.
Dual-Luciferase Reporter Assay
The targeted relationship between WEE1 and miR-520a-3p was verified by dual luciferase reporter assay. The sequence of the 3ʹUTR of WEE1 were cloned into the pmirGLO vectors (Promega, Madison, WI, USA) to generate WEE1-WT plasmids. Next, site-specific mutagenesis was performed to create WEE1-Mut plasmids. The pRL-TK renilla luciferase reporter vector (Promega, Madison, WI, USA) was used as the internal reference. AGS cells were cotransfected with the WEE1-WT or WEE1-MT and miR-520a-3p mimics or negative control. The cells were harvested 36 hours after transfection, and the luciferase viability of AGS and BGC-823 cells was detected according to instructions of the luciferase viability detection kit (Promega. Relative luciferase activity equaled to firefly luciferase viability value/renilla luciferase viability value.
Western Blot
AGS and BGC-823 cells were seeded in 6-well plates (6×105/well) respectively, and transfected with the miR-520a-3p control group. After 72 hours of culture, the total protein of AGS and BGC-823 cells and the transfected cells were extracted, and the protein concentration was determined by BCA method. Subsequently, the protein was separated by 10% polyacrylamide gel electrophoresis and transferred to the membrane, then the membranes were sealed with skimmed milk and incubated with primary antibodies of WEE1 (ab137377, 1: 1000, abcam), PI3K (ab32089, 1: 1000, abcam), Akt (ab8805, 1: 1000, abcam) at 4 °C overnight. After being washed by TBST, the membranes were then incubated with sheep anti-rabbit secondary antibody (ab150077, 1: 2500, abcam) at room temperature for 2 hours. Afterwards, the cells were developed with ECL chemiluminescence reagent, and gel imaging system was used for imaging, while ImageJ software was used for grayscale analysis. GAPDH was used as the endogenous control.
Tumor Formation in Nude Mice
Six-week-old BALB/c-nu nude mice were used to establish tumor models in vivo. AGS cells in the logarithmic growth phase were taken, trypsinized with 0.25% trypsin and collected, then washed and resuspend in a serum-free medium to make single cell suspension (concentration: 3 ×108/mL). Eighty nude mice were divided into 4 groups, then 0.1mL of cell suspension was injected subcutaneously into the left forelimb of each nude mouse. During the next 7 weeks, tumor volumes of each group were measured. The longest diameter (a) and the shortest diameter (b) perpendicular of the tumors were measured by vernier caliper. The tumor volume was calculated according to the formula: V (mm3) = 0.5×a×b2. Moreover, the mice were anesthetized at the 21th day, 28th day and 35th day, respectively and then the tumors in vivo of each group were collected and the size and weight of the tumors were measured. The mice of the ropivacaine group were injected with 20, 40, and 60 μmol/kg body weight of Rop once a day from the day of tumor formation to the end of the experiment. All experiments were performed following the guidelines and regulations of Ethics Committee of the Rizhao People’s Hospital.
Data Analysis
The data in this study were all analyzed using SPSS22.0 statistical software (SPSS Inc., Chicago, IL, USA). The experiments consisted of three repetitive wells in each group of cells, and were repeated at least 3 times. The mean ± standard deviation was used to show the measurement data with normal distribution. The student’s t test was used to compare the measurement data between the two groups. P <0.05 was considered statistically significant.
Results
Rop Inhibited the Proliferation, Migration and Invasion of GC Cells
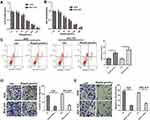
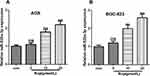
To preliminarily explore the effect of Rop on GC cells, Rop with different concentration gradients (2.5 μmol/L, 5 μmol/L, 10 μmol/L, 20 μmol/L, and 40 μmol/L) was used to act on GC cells, and CCK8 assay was adopted to detect cell viability. The results showed that the cell viability of AGS and BGC-823 both dose-dependently decreased significantly compared with that of the control group (P<0.05, Figure 1A). In addition, we found that Rop inhibited the proliferation of AGS and BGC-823 in a time-dependent manner (P<0.05, Figure 1B). Moreover, we detected the level of apoptosis of GC cells and found that Rop significantly increased the apoptotic rate of GC cells (Figure 1C). As migration and invasion were the key steps to metastasis of GC, we further used transwell assay to detect the effect of Rop on the migration and invasion of GC cells in this study. Interestingly, our results showed that the migration and invasion of GC cells AGS and BGC-823 were signally reduced after treated by Rop of 5 μmol/L (P<0.05, Figure 1D and E). Thus, those data showed that Rop inhibited the development of GC in vitro.
Rop Inhibited the WEE1 and PI3K/AKT Signaling Pathways
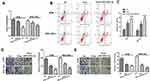
Previous studies indicated that both WEE1 and PI3K/AKT signaling pathway play an important role in the proliferation and metastasis of GC. We found through bioinformatics analysis in the GEPIA database (http://gepia.cancer-pku.cn/) that WEE1 was dramatically upregulated in GC (Figure 2A). Here, the mRNA and protein expression of WEE1 were detected by using RT-PCR and Western blot, respectively. The results revealed that Rop markedly inhibited the mRNA and protein level of WEE1 (P <0.05, Figure 2B–D). We also found through Western blot that Rop observably inhibited the expression of p-PI3K and p-AKT (P <0.05, Figure 2C, E, F). The above results indicated that Rop inhibited the WEE1 and PI3K/AKT signaling pathways.
 |
Figure 2 Rop inhibited WEE1 and PI3K/AKT in GC cells. (A) The WEE1 expression in GC was analyzed in GEPIA database (http://gepia.cancer-pku.cn/) and the results showed that WEE1 was upregulated in GC tissues compared with that of normal tissues. (B) qRT-PCR was used to detect the expression of WEE1 mRNA in GC cell lines (AGS, BGC-823). ***P <0.001 (vs control group). (C–F) Western blot was used to detect the levels of WEE1 (C and D) and PI3K, AKT (E and F) in GC cell lines (AGS, BGC-823), respectively. ***P <0.01 (vs control group). |
Rop Promoted the Expression of miR-520a-3p in GC Cells
It has been reported that miR-520a-3p inhibits cell migration, promotes apoptosis, and causes cycle arrest of colon cancer cells, and it may also have a similar role in GC, while its regulation in GC remains unclear. Therefore, we conducted qRT-PCR to detect the expression of miR-520a-3p in GC cells under the treatment of Rop with different concentrations (2.5 μmol/L, 5 μmol/L, 10 μmol/L, 20 μmol/L, 40 μmol/L) on the expression of miR-520a-3p. Our study showed that the expression of miR-520a-3p showed an upward trend as the concentration of Rop increased (Figure 3A and B), indicating that Rop dose-dependently promoted the expression of miR-520a-3p in GC cells.
MiR-520a-3p Targeted WEE1
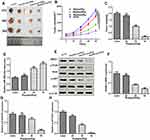
Whereas the expressions of miR-520a-3p and WEE1 were both regulated by Rop, we were curious whether there was a targeting relationship between the two molecules. Interestingly, through bioinformatics analysis, we found that miR-520a-3p targeted the 3ʹUTR site of WEE1 mRNA (Figure 4A). To further clarify the targeting relationship between miR-520a-3p and WEE1, we performed the dual-luciferase reporter assay, and it was found that miR-520a-3p notably reduced the luciferase viability of WEE1-WT, while had no significant effect on that of WEE1-MUT (Figure 4B). Next, we established a miR-520a-3p overexpressing cell model (Figure 4C) and used qRT-PCR to detect the expressive changes of WEE1 mRNA. It was found that miR-520a-3p significantly inhibited the expression of WEE1 mRNA compared with that of the control group (Figure 4D). Furthermore, bioinformatics analysis that WEE1 was positively correlated with AKT expression in GC (Figure 4E), and KEGG analysis also revealed that PI3K/Akt is an underlying signaling pathway of WEE1 (Figure 4F). Moreover, results of Western blot showed that overexpressing miR-520a-3p not only inhibited the WEE1 protein level compared with that of the control group (Figure 4G and H), but also inhibited the expression of p-PI3K and p-AKT (Figure 4I and J). The above results showed that miR-520a-3p had a targeting relationship with WEE1 and inhibited its downstream PI3K/AKT pathway in GC cells.
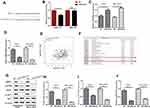 |
Figure 4 MiR-520a-3p targeted WEE1. (A) We analyzed the downstream molecules of miR-520a-3p through the StarBase database (http://starbase.sysu.edu.cn/), and noticed that miR-520a-3p contained a potential binding site for WEE1. (B) Dual-luciferase reporter assay was used to detect the targeting relationship between miR-520a-3p and WEE1. ns P> 0.05. **P <0.01 (vs miR-NC group). (C) qRT-PCR was used to determine the expression of miR-520a-3p in GC cell lines (AGS, BGC-823). **P <0.01, ***P <0.001. (D) qRT-PCR was used to detect WEE1 mRNA expression in GC cell lines (AGS, BGC-823) after transfection of miR-520a-3p mimics. **P <0.01, ***P <0.001 (vs miR-NC group). (E) Bioinformatics analysis through GEPIA (http://gepia.cancer-pku.cn/) revealed that the expression of WEE1 and AKT was positively correlated. (P <0.001, R2 = 0.22). (F) KEGG analysis revealed that PI3K/Akt is an underlying signaling pathway of WEE1. (G–J) Western blot was used to detect the expressions of WEE1, PI3K and AKT. ***P <0.001. |
Overexpression of miR-520a-3p Inhibited Proliferation, Migration and Invasion of GC Cells
To verify the role of miR-520a-3p in GC progression, first, we conducted bioinformatics analysis in the KM plotter database (http://kmplot.com/analysis/). The results suggested that downregulated miR-520a-3p predicts poorer survival of GC patients (Figure 5A). Next, we conducted experiments to explore the role of miR-520a-3p in GC, and our data revealed that overexpression of miR-520a-3p notably reduced the proliferation, but aggravated the apoptosis of GC cells compared with that of the control group (Figure 5B and C). What’s more, the migration and invasion of GC cells were also remarkedly inhibited by overexpressed miR-520a-3p (Figure 5D and E). As a result, our data suggested that miR-520a-3p is an antitumor regulator in GC.
Overexpression of miR-520a-3p Enhanced the Inhibitory Effect of Rop on the Proliferation, Migration and Invasion of GC Cells
In order to further explore the role of miR-520a-3p in Rop induced GC progression, we treated GC cells with Rop intervention on the basis of miR-520a-3p overexpression. Our data showed that the proliferation, migration and invasion of GC cells were significantly inhibited by miR-520a-3p, and upregulating miR-520a-3p further aggravated the apoptotic level of GC cells compared with that of the Rop group (P <0.05, Figure 6A–E). Collectively, these results indicated that miR-520a-3p promoted the inhibitory effect of Rop on GC.
Ropivacaine Inhibited the Progression of GC Cells in vivo
In order to further explore the inhibitory effect of ropivacaine on GC cells, we performed tumor formation experiment in nude mice in vivo to detect tumor volume and mass at different time (the 21th day, 28th day, and 35th day), and found that Rop dramatically reduced the volume and weight of tumors in vivo in a dose-dependent manner (Figure 7A–C). Moreover, qRT-PCR and Western-blot showed Rop promoted the expression of miR-520a-3p (P <0.05, Figures 7D) and signally inhibited the expression of WEE1, p-PI3K and p-AKT (P <0.05, Figure 7E–H). As a consequence, those resulted suggested that Rop attenuated the progression of GC in vivo through downregulating WEE1 mediated PI3K/Akt pathway by miR-520a-3p.
Discussion
With the continuous exploration of GC, an increasing number of drugs are being used for its treatment. Therefore, studying the mechanism of drugs in GC tissues plays an important role in the clinical treatment of GC. In this study, we found that Rop inhibited the malignant biological behaviors of GC in a dose- and time-dependent manner. Further experiments showed that Rop promoted the expression of miR-520a-3p and inhibited the WEE1 and PI3K/AKT signaling pathways, thus playing an inhibitory effect on the proliferation, migration and invasion of GC, which was of great significance for finding new therapeutic targets for GC drugs.
Accumulating studies have proved that narcotics are promising treatments in curing cancers. For example, lidocaine significantly inhibits the proliferation, migration, and invasion of cancer cells and induces apoptosis in a dose-dependent manner.19 Besides, lidocaine also reduces the growth, migration and invasion of GC cell MKN45 by regulating the expression of miR-145 and further inactivating the MEK/ERK and NF-κB signaling pathways.20 In addition, propofol plays an antitumor role through regulating the viability, migration and invasion of bladder cancer by targeting the microRNA-10b/HOXD10 signaling pathway.21 On the other hand, studies have confirmed that midazolam and Rop synergistically inhibit bone cancer pain in rats with different mechanisms.22 Moreover, Rop also plays an important role in tumors. For example, Rop regulates CD62E expression and reduces tumor cell arrest through NF-κB signaling pathway.23 In this study, we found that Rop inhibited the proliferation of GC cells in a dose- and time-dependent manner, besides, Rop promoted the apoptosis and attenuated the migration and invasion of GC cells, indicating that Rop shows good potentials in the treatment of GC.
The role of miRNAs in tumors has been extensively studied, and studies have confirmed that miRNAs are vital regulators in a variety of tumor. For example, miR-23b regulates epithelial-mesenchymal transition of colorectal cancer cells.24 MiR-346 inhibits the growth of glioma cells by targeting NFIB.25 Upregulation of miR-203a-3p increases cell viability and cell proliferation, and also inhibits apoptosis.26 In addition, miRNAs also play important roles in GC. For example, miR-6884-5p inhibits GC cell proliferation, invasion, and EMT by directly reducing S100A16 expression.27 miR-337-3p inhibits GC metastasis by targeting ARHGAP10.28 As one member of the miRNAs, miR-520a-3p has also been found in previous studies to regulate the progression of tumors. For example, miR-520a-3p attenuates the inhibition of HOXD-AS2-depletion on the proliferation, migration, and invasion of NSCLC cells.29 LINC01116 upregulates IL6R in OS by targeting miR-520a-3p, thereby activating the JAK-STAT signaling pathway and promoting OS procession.30 Moreover, it has also been reported as a tumor suppressor gene in lung cancer and breast cancer31 as well as GC.32 In this study, we found that miR-520a-3p was significantly upregulated by Rop in GC cells, and its overexpression observably inhibited cell proliferation, invasion and migration but increased cell apoptosis, suggesting that Rop exerted its tumor suppressive effect by promoting miR-520a-3p expression in GC.
WEE1 is a member of the serine/threonine protein kinase family and is a key regulator of cell cycle progression. It has been reported that WEE1 are overexpressed in several cancers, such as malignant melanoma, breast cancer, osteosarcoma, and gliomas.33–36 Studies have shown that knocking down WEE1 eliminated the combination-mediated effects on cell migration and proliferation in BRAF mutant BRAF inhibitor-sensitive cells, while WEE1 silencing alone inhibited cell migration in NRAS mutant cells.37 Similarly, ablation of WEE1 notably reduced the invasion and migration of GC cells, and its overexpression enhanced the viability, invasion, and migration of GC cells.16 More importantly, studies have revealed that WEE1 is an adaptive resistance gene activated after PI3K inhibition, and the inhibition of WEE1 enhances the effectiveness of PI3K targeted inhibition, indicating that WEE1 and PI3K have a joint inhibitory effect.38 In addition, PI3K/AKT is involved in the growth of various cells.39 And it is also found that the migration and invasion of GC cells are attenuated by inhibiting the PI3K/AKT signaling pathway.40 In addition, PI3K/AKT signaling pathway can also affect EMT. For example, breast cancer 1 (AIB1) enhances EMT by activating PI3K/AKT signaling.41 GINS2 promotes cell proliferation, migration, invasion, and EMT by regulating the PI3K/AKT and MEK/ERK signaling pathways.42 In this study, we found that WEE1 was markedly downregulated in GC tissues after Rop action, accompanied by a significant downregulation of PI3K/AKT phosphorylation expression. By detecting changes in the biological behavior of GC tissues, we found that Rop signally inhibited the proliferation, migration, and invasion of GC cells. Overall, Rop exerted its tumor suppressive effect by downregulating WEE1 and inhibiting the PI3K/AKT signaling pathway.
Accumulating studies have found that miRNAs inhibit the progression of GC by regulating the PI3K/AKT signaling pathway. For example, miR-520a-3p regulates the proliferation and glycolysis of GC cells by targeting the AKT1/mTOR/HIF1α pathway, and overexpressing miR-520a-3p inhibits the proliferation of GC cells by downregulating the AKT1/mTOR/HIF1α pathway.43 Besides, miR-520a-3p regulates the proliferation, migration and invasion of NSCLC through the PI3K/AKT/mTOR signal pathway, and overexpressing miR-520a-3p downregulates the PI3K/AKT/mTOR signal pathway to exert its inhibitory effect on NSCLC.44 In the present study, we have confirmed the expression of miR-520a-3p and WEE1 and their biological functions in GC, but the interaction between them remained unclear, so we performed a luciferase reporter experiment and the results showed that there was a binding site between miR-520a-3p and WEE1, and their expressions were negatively correlated. Namely, overexpression of miR-520a-3p dramatically inhibited the expression of WEE1 in GC cells and enhanced the tumor suppressive effect of Rop, indicating that miR-520a-3p targets the WEE1 and PI3K/AKT signaling pathways to affect the proliferation and invasion of GC cells.
In conclusion, our research confirms that Rop inhibits the proliferation, migration, invasion and increases apoptosis of GC cells by upregulating the expression of miR-520a-3p and further regulating the WEE1 and PI3K/AKT signaling pathways. This study better elaborates the molecular mechanism by which Rop regulates the occurrence and development of GC, and provides new theoretical supports for the treatment of GC.
Data Sharing Statement
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
Our study was approved by the Ethics Review Board of Rizhao People’s Hospital. All experiments were performed following the guidelines and regulations of Ethics Committee of the Rizhao People’s Hospital.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors.
Disclosure
The authors declare that they have no competing interests.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Zhang XY, Zhang PY. Gastric cancer: somatic genetics as a guide to therapy. J Med Genet. 2017;54:305–312. doi:10.1136/jmedgenet-2016-104171
3. Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. Clin Oncol. 2007;25:2107–2116. doi:10.1200/JCO.2006.09.5224
4. Li H, Yang L, Guo Z, et al. Successful treatment of refractory cancer pain with morphine and ropivacaine. Medicine (Baltimore). 2017;96(22):e7052. doi:10.1097/MD.0000000000007052
5. Siekmann W, Tina E, Von Sydow Anita K, et al. Effect of lidocaine and ropivacaine on primary (SW480) and metastatic (SW620) colon cancer cell lines. Oncol Lett. 2019;18:395–401. doi:10.3892/ol.2019.10332
6. Wang W, Zhu M, Xu Z, et al. Ropivacaine promotes apoptosis of hepatocellular carcinoma cells through damaging mitochondria and activating caspase-3 activity. Biol Res. 2019;52:36. doi:10.1186/s40659-019-0242-7
7. Zhang Y, Peng X, Zheng Q, et al. Ropivacaine inhibits the migration of esophageal cancer cells via sodium-channel-independent but prenylation-dependent inhibition of Rac1/JNK/paxillin/FAK. Biochem Biophys Res Commun. 2018;501:1074–1079. doi:10.1016/j.bbrc.2018.05.110
8. Liu W-L, Wang H-X, Shi C-X, et al. MicroRNA-1269 promotes cell proliferation via the AKT signaling pathway by targeting RASSF9 in human gastric cancer. Cancer Cell Int. 2019;19:308. doi:10.1186/s12935-019-1026-4
9. Xiong Y, Fang JH, Yun JP, et al. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology. 2010;51(3):836–845. doi:10.1002/hep.23380
10. Wang Y, Zhang P, Yuan M, et al. Overexpression of miRNA-21 promotes the proliferation and invasion in hepatocellular carcinoma cells via suppressing SMAD7. Technol Cancer Res Treat. 2019;18:1533033819878686. doi:10.1177/1533033819878686
11. Motoyama K, Inoue H, Mimori K, et al. Clinicopathological and prognostic significance of PDCD4 and microRNA-21 in human gastric cancer. Int J Oncol. 2010;36(5):1089–1095. doi:10.3892/ijo_00000590
12. Liu Y, Miao L, Ni R, et al. microRNA-520a-3p inhibits proliferation and cancer stem cell phenotype by targeting HOXD8 in non-small cell lung cancer. Oncol Rep. 2016;36(6):3529–3535. doi:10.3892/or.2016.5149
13. Wang X, Xu Y, Chen X, et al. Dexmedetomidine inhibits osteosarcoma cell proliferation and migration, and promotes apoptosis by regulating miR-520a-3p. Oncol Res. 2018;26:495–502. doi:10.3727/096504017X14982578608217
14. Krajewska M, Heijink AM, Bisselink YJ, et al. Forced activation of Cdk1 via wee1 inhibition impairs homologous recombination. Oncogene. 2013;32(24):3001–3008. doi:10.1038/onc.2012.296
15. Xu D, Liang SQ, Yang H, et al. CRISPR screening identifies WEE1 as a combination target for standard chemotherapy in malignant pleural mesothelioma. Mol Cancer Ther. 2020;19(2):661–672. doi:10.1158/1535-7163.MCT-19-0724
16. Kim HY, Cho Y, Kang H, et al. Targeting the WEE1 kinase as a molecular targeted therapy for gastric cancer. Oncotarget. 2016;7(31):49902–49916. doi:10.18632/oncotarget.10231
17. Zhang L, Lin W, Chen X, Wei G, Zhu H, Xing S. Tanshinone IIA reverses EGF- and TGF-β1-mediated epithelial-mesenchymal transition in HepG2 cells via the PI3K/Akt/ERK signaling pathway. Oncol Lett. 2019;18(6):6554–6562. doi:10.3892/ol.2019.11032
18. Gong C, Ai J, Fan Y, et al. NCAPG promotes the proliferation of hepatocellular carcinoma through PI3K/AKT signaling. Onco Targets Ther. 2019;12:8537–8552. doi:10.2147/OTT.S217916
19. Ye L, Zhang Y, Chen YJ, Liu Q. Anti-tumor effects of lidocaine on human gastric cancer cells in vitro. Bratisl Lek Listy. 2019;120(3):212–217. doi:10.4149/BLL_2019_036
20. Sui H, Lou A, Li Z, Yang J. Lidocaine inhibits growth, migration and invasion of gastric carcinoma cells by up-regulation of miR-145. BMC Cancer. 2019;19(1):233. doi:10.1186/s12885-019-5431-9
21. Qi Z, Yuan L, Sun N. Propofol exhibits a tumor-suppressive effect and regulates cell viability, migration and invasion in bladder carcinoma by targeting the microRNA-10b/HOXD10 signaling pathway. Oncol Lett. 2019;18:6228–6236. doi:10.3892/ol.2019.10968
22. Guo C, Bai L, Wu H, et al. Midazolam and ropivacaine act synergistically to inhibit bone cancer pain with different mechanisms in rats. Oncol Rep. 2017;37(1):249–258. doi:10.3892/or.2016.5241
23. Su Z, Huang P, Ye X, et al. Ropivacaine via nuclear factor kappa B signalling modulates CD62E expression and diminishes tumour cell arrest. J Anesth. 2019;33:685–693. doi:10.1007/s00540-019-02699-1
24. Viswanathan V, Damle S, Zhang T, et al. An miRNA expression signature for the human colonic stem cell niche distinguishes malignant from normal epithelia. Cancer Res. 2017;77(14):3778–3790. doi:10.1158/0008-5472.CAN-16-2388
25. Li Y, Xu J, Zhang J, et al. MicroRNA-346 inhibits the growth of glioma by directly targeting NFIB. Cancer Cell Int. 2019;19:294. doi:10.1186/s12935-019-1017-5
26. Zhang Y, Zheng Y, Zhu G. MiR-203a-3p targets PTEN to promote hepatocyte proliferation by regulating PI3K/Akt pathway in BRL-3A cells. Biosci Biotechnol Biochem. 2020;84(4):725–733. doi:10.1080/09168451.2019.1694860
27. Lv H, Hou H, Lei H, et al. MicroRNA-6884-5p regulates the proliferation, invasion and EMT of gastric cancer cells by directly targeting S100A16. Oncol Res. 2019. doi:10.3727/096504019X15753718797664
28. Wang Z, Yao L, Li Y, et al. miR-337-3p inhibits gastric tumor metastasis by targeting ARHGAP10. Mol Med Rep. 2020;21(2):705–719. doi:10.3892/mmr.2019.10856
29. Liu Y, Lin X, Zhou S, Zhang P, Shao G, Yang Z. Long noncoding RNA HOXA-AS2 promotes non-small cell lung cancer progression by regulating miR-520a-3p. Biosci Rep. 2019;39(5):BSR20190283. doi:10.1042/BSR20190283
30. Zhang B, Yu L, Han N, et al. LINC01116 targets miR-520a-3p and affects IL6R to promote the proliferation and migration of osteosarcoma cells through the Jak-stat signaling pathway. Biomed Pharmacother. 2018;107:270–282. doi:10.1016/j.biopha.2018.07.119
31. Su H, Ren F, Jiang H, et al. Upregulation of microRNA-520a-3p inhibits the proliferation, migration and invasion via spindle and kinetochore associated 2 in gastric cancer. Oncol Lett. 2019;18:3323–3330. doi:10.3892/ol.2019.10663
32. Li R, Yuan W, Mei W, et al. MicroRNA520d-3p cut EphA2 expression through inhibiting gastric cancer cell proliferation, migration and invasion. Mol Cell Biochem. 2014;396:295–305. doi:10.1007/s11010-014-2164-6
33. Magnussen GI, Holm R, Emilsen E, Rosnes AK, Slipicevic A, Flørenes VA. High expression of Wee1 is associated with poor disease-free survival in malignant melanoma: potential for targeted therapy. PLoS One. 2012;7(6):e38254. doi:10.1371/journal.pone.0038254
34. Aarts M, Bajrami I, Herrera-Abreu MT, et al. Functional genetic screen identifies increased sensitivity to WEE1 inhibition in cells with defects in fanconi anemia and HR pathways. Mol Cancer Ther. 2015;14(4):865–876. doi:10.1158/1535-7163.MCT-14-0845
35. Garimella SV, Rocca A, Lipkowitz S. WEE1 inhibition sensitizes basal breast cancer cells to TRAIL-induced apoptosis. Mol Cancer Res. 2012;10(1):75–85. doi:10.1158/1541-7786.MCR-11-0500
36. Mueller S, Hashizume R, Yang X, et al. Targeted Wee1 treatment children with high grade glioma. Neural Oncol. 2014;16(3):352–360.
37. Das I, Wilhelm M, Höiom V, et al. Combining ERBB family and MET inhibitors is an effective therapeutic strategy in cutaneous malignant melanoma independent of BRAF/NRAS mutation status. Cell Death Dis. 2019;10(9):663. doi:10.1038/s41419-019-1875-8
38. Wu S, Wang S, Gao F, et al. Activation of WEE1 confers resistance to PI3K inhibition in glioblastoma. Neuro Oncol. 2018;20(1):78–91. doi:10.1093/neuonc/nox128
39. Pei Q, G-S L, Li H-P, et al. Long noncoding RNA SNHG14 accelerates cell proliferation, migration, invasion and suppresses apoptosis in colorectal cancer cells by targeting miR-944/KRAS axis through PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2019;23:9871–9881. doi:10.26355/eurrev_201911_19551
40. Qiao J, Li M, Sun D, Li W, Xin Y. Knockdown of ROS proto-oncogene 1 inhibits migration and invasion in gastric cancer cells by targeting the PI3K/Akt signaling pathway. Onco Targets Ther. 2019;12:8569–8582. doi:10.2147/OTT.S213421
41. Diao L, Li Y, Mei Q, Han W, Hu J. AIB1 induces epithelial-mesenchymal transition in gastric cancer via the PI3K/AKT signaling. J Cell Biochem. 2019. doi:10.1002/jcb.29530
42. Liu X, Sun L, Zhang S, et al. GINS2 facilitates epithelial-to-mesenchymal transition in non-small-cell lung cancer through modulating PI3K/Akt and MEK/ERK signaling [published online ahead of print, 2019 Nov 4]. J Cell Physiol. 2019. doi:10.1002/jcp.29381
43. Pan C, Liu Q, Wu X, et al. HIF1α/miR-520a-3p/AKT1/mTOR feedback promotes the proliferation and glycolysis of gastric cancer cells. Cancer Manag Res. 2019;11:10145–10156. doi:10.2147/CMAR.S223473
44. Lv X, C-Y L, Han P, et al. MicroRNA-520a-3p inhibits cell growth and metastasis of non-small cell lung cancer through PI3K/AKT/mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:2321–2327. doi:10.26355/eurrev_201804_14822
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.