Back to Journals » OncoTargets and Therapy » Volume 12
Roles Of EAAT1, DHFR, And Fetuin-A In The Pathogenesis, Progression And Prognosis Of Chondrosarcoma
Authors He L , Shi X, Liu Z, Ren X, Zhang C, Yang Z , Li Z
Received 9 July 2019
Accepted for publication 20 September 2019
Published 14 October 2019 Volume 2019:12 Pages 8411—8420
DOI https://doi.org/10.2147/OTT.S222426
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Lile He,1,2 Xiangyu Shi,3 Zhongyue Liu,1 Xiaolei Ren,1 Chenghao Zhang,1 Zhulin Yang,4 Zhihong Li1,2
1Department of Orthopedics, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, People’s Republic of China; 2Hunan Key Laboratory of Tumor Models and Individualized Medicine, Changsa, Hunan 410011, People’s Republic of China; 3Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, People’s Republic of China; 4Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, People’s Republic of China
Correspondence: Zhihong Li
Department of Orthopedics, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, People’s Republic of China
Tel +86 139 7511 2458
Email [email protected]
Aims: Chondrosarcoma (CS) is a high-morbidity, relatively common bone malignancy without well-established biomarkers. The proteins EAAT1, DHFR, and fetuin-A have been investigated in many cancers, but their specific relationship to CS has not been reported. The present study examined EAAT1, DHFR, and fetuin-A expression in CS and the clinicopathological significance of these proteins in CS pathogenesis, progression, and prognosis.
Methods: EAAT1, DHFR, and fetuin-A protein levels in 80 CS and 25 chondroma specimens were measured by immunohistochemistry and related to histological and clinical factors with chi-squared tests. Following univariate survival analysis, ROC curves calculation, and multivariate analysis.
Results: EAAT1, DHFR, and fetuin-A expression levels were significantly higher in the CS group than in the chondroma group (p < 0.05). Their immunopositivity rates were significantly greater in tissues with moderate or poor tumor differentiation, AJCC stage III or IV, Enneking stage II or III, and metastasis (p<0.05 or p<0.01 or p<0.001). Kaplan–Meier survival analysis showed significantly shorter survival in patients with moderately or poorly differentiated tumors, AJCC stage III or IV CS, Enneking stage II or III CS, metastasis, invasion, or EAAT1, DHFR, and fetuin-A immunopositivity (p < 0.05 or p < 0.001). Cox regression analysis showed that moderate or poor tumor differentiation, AJCC stage III or IV, Enneking stage II or III, metastasis, invasion, and EAAT1, DHFR, or fetuin-A immunopositivity correlated negatively with postoperative survival and positively with mortality (p < 0.05). The AUCs for EAAT1, DHFR, and fetuin-A were 0.654 (95% CI: 0.532–0.776, p = 0.025), 0.638 (95% CI: 0.519–0.756, p = 0.039), and 0.670 (95% CI: 0.556–0.784, p = 0.011), respectively.
Conclusion: EAAT1, DHFR, and fetuin-A may be important biomarkers of the pathogenesis and progression of CS and predictors of its prognosis.
Keywords: chondrosarcoma, chondroma, EAAT1, DHFR, fetuin-A, immunohistochemistry
Introduction
Chondrosarcoma (CS) is the second most common primary solid malignant tumor in bone after osteosarcoma. There are approximately three new cases per million people per year.1 Due to its resistance to chemotherapy and radiotherapy, surgical resection remains the standard treatment of CS, and the prognosis for patients is generally poor.2 Reliable biomarkers related to its pathogenesis, progression, and prognosis are critical to improving treatment possibilities, but have yet to be established for clinical practice.3
It has been widely reported that the glutamatergic (Glu) system acts as a primary regulator in bone tissue.4 After activating its cognate receptors, released glutamate is taken up into astrocytes and neurons by cell membrane excitatory amino acid transporters (EAATs), resulting in termination of the glutamatergic signal. Recently, it was demonstrated that non-neuronal glutamatergic transmission occurs outside the central nervous system.5 Cancer cells, including melanoma, colorectal carcinoma, hepatocellular carcinoma, and prostate carcinoma, have been shown to be modulated by a transmission system in which glutamate acted as an intercellular signaling factor.6 Moreover, alterations in glutamate transport were found in malignant cells; repression of GLT-1/EAAT27 or mis-localization of excitatory amino acid transporter 1 (EAAT1) prevented cancer cells from taking up glutamate. The expression of EAATs was also found to be inversely correlated with tumor grade and restoring the function of EAATs decreased cancer cell proliferation and induced apoptosis in glioma cell lines.8 In addition, EAAT1 expression was observed in osteosarcoma cell line MG-63 and shown to play an important role in bone pathophysiology.9 In bone tissue, initial evidence for the presence of the Glu system was based on the expression of EAAT1 after mechanical loading on rat osteocytes.10 Functional molecules of the Glu system have also been identified in T leukemia cells, thyroid carcinoma, melanoma and several other cancers.11 However, the role of glutamatergic signaling in CS development and progression is still not well understood.
Dihydrofolate reductase (DHFR) is a folate metabolism enzyme critical to the processes of DNA synthesis, repair, and methylation.12 Altered cellular folate levels are associated with aberrant DNA repair and methylation, including elevated hypo- and hypermethylation of tumor suppressor gene promotors.13 DHFR is a known target of chemotherapeutic agents such as methotrexate (MTX), which reduces DNA synthesis and cell proliferation rates in cancer cells. Increasing cellular expression of DHFR results in tumor resistance to MTX. Possible mechanisms underlying MTX resistance include DHFR mutations that decrease the affinity of DHFR protein to MTX, and reduced uptake of MTX due to impaired transport.14 These events have been reported to play a role in carcinogenesis in the colon.13 However, reported associations between DHFR and cancers are often conflicting. Several studies have revealed, in various diseases, that deletion of DHFR is associated with higher morbidity.15,16 DHFR expression was found to affect breast cancer cell proliferation and MTX sensitivity.17 On the contrary, Xu et al16 reported that there was no correlation between breast cancer susceptibility and DHFR genotype. The discrepancy between these studies may be the result of differences in the studied populations and their exposure to diverse carcinogens. Regardless, DHFR has not been well studied in CS.
Fetuin-A is a 63-kDa glycoprotein that is synthesized mainly by the liver and secreted into the serum. Of its multiple functions,18 the most widely accepted one is bone remodeling and inhibiting excess systemic ectopic calcification. The role of fetuin-A in tumor progression is that of mediator of tumor cell adhesion, which is an important step in tumor growth and development of metastases.19 Moreover, activation of resident osteoclasts to break down bone enables tumor cell colonization. The mineral component of bone, hydroxyapatite, has a strong affinity for fetuin-A. Breast cancer20 and multiple myeloma21 are regarded as osteoclastic tumors because they are associated with greater osteoclast activity relative to osteoblast activity. Being a chemoattractant,22 fetuin-A could play a role in attracting tumor cells to the bone metastatic niche. Indeed, prostate cancer cells that colonize the bone have been shown to synthesize and secrete ectopic fetuin-A,23 and fetuin-A has been shown to promote breast cancer and lung carcinoma progression.24,25 Additionally, Thompson et al26 suggested that fetuin-A plays a role in tumorigenesis of head and neck cancer. To our knowledge, there are no reports about the role of fetuin-A in CS.
In the present study, we used immunohistochemistry that investigated the expression of EAAT1, DHFR, and fetuin-A in CS and chondroma tissues, a common benign bone tumor. The clinical and pathological significance of these proteins in CS and their roles in the pathogenesis, progression, and prognosis of this malignancy were analyzed.
Materials And Methods
Specimens And Clinical Data
This study was conducted with resected tumor specimens from 80 patients with CS and 25 patients with chondroma, collected between January 2011 and June 2015 at the Second and Third Xiangya Hospitals, Central South University in Changsha, China. Diagnoses were confirmed by histopathology. Of the 80 CS tissues, 73 tissues were conventional CS (91.2%), 1 tissue was clear cell CS (1.3%), 2 tissues were mesenchymal CS (2.5%), and 4 tissues were dedifferentiated CS (5.0%). Tissues were formalin-fixed and paraffin-embedded using standard procedures.
Clinicopathological data collected included patient age and sex, extent of tumor differentiation, tumor size, American Joint Committee on Cancer (AJCC) and Enneking stages, and the presence of metastasis and/or invasion. Survival information for patients with CS was obtained by phone or email.
Ethics Statement
This study was approved by the Medical Ethics Committee of the Second Xiangya Hospital, Central South University. Written, informed consent was obtained from each participant or their legal custodian, in accordance with the Declaration of Helsinki.
Immunohistochemistry
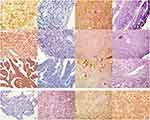
Four-micrometer-thick sections were cut from paraffin-embedded tissues with a cryostat. EAAT1, DHFR, and fetuin-A were labeled with the EnVisionTM detection kit (Dako Laboratories, Carpinteria, CA) according to the manufacturer’s protocol. Briefly, sections were deparaffinized and endogenous peroxidase was blocked by incubation in 3% H2O2 in the dark for 15 mins. Following a 20-min sitting in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) at room temperature for 20 mins, the sections were incubated with rabbit anti-human EAAT1 [1:50], DHFR [1:50], or fetuin-A [1:50] antibodies (Boster Biological Technology Co., Ltd., California, USA) for 2 hrs and then rinsed with 1× phosphate buffered saline three times for 5 mins each. Sections were then incubated at room temperature for 30 mins in Solution A, which contained horseradish peroxidase-conjugated anti-rabbit secondary antibody (400 µg/mL) (Santa Cruz Biotechnology). After rinsing with 1× PBS three times, for 5 mins each, sections were stained with 3,3′-diaminobenzidine, counterstained with hematoxylin, dehydrated with alcohol, soaked in xylene, and mounted with neutral balsam. Positivity for EAAT1, DHFR, or fetuin-A was defined as ≥20% of cells with a staining intensity ≥2 or 10–20% of cells with a staining intensity of 3. The staining intensity was graded as follows: 0, no staining; 1, yellow; 2, brownish yellow; and 3, brown.27(Figure 1).
Statistical Analysis
Data were analyzed in the Statistical Package for the Social Sciences version 18.0 (SPSS Inc., Chicago, IL). Protein expression levels in CS and chondroma specimens and their relationships with histological and clinical factors were analyzed with chi-squared tests. Kaplan–Meier and log rank tests were used for univariate survival analysis, with calculation of receiver operating characteristic (ROC) curves and areas under the curves (AUCs). A Cox proportional-hazard model was used for multivariate analysis and determination of 95% confidence intervals (CIs). A p-value less than 0.05 was considered significant.
Results
EAAT1, DHFR, And Fetuin-A Protein Expression In CS And Chondroma Tissues
EAAT1, DHFR, and fetuin-A immunopositivity was located mainly in the cytoplasm and/or plasma membrane and/or nuclear of tumor cells (Figure 1). EAAT1, DHFR, and fetuin-A immunopositivity was observed in 42/80 (52.5%), 38/80 (47.5%), and 40/80 (50.0%) CS specimens, respectively. EAAT1, DHFR, and fetuin-A immunopositivity was observed in 5/25 (20.0%), 5/25 (20.0%), and 4/25 (16.0%) chondroma specimens. For all three proteins, levels were significantly higher in CS than in chondroma specimens (EAAT1 p = 0.004, DHFR p = 0.015, fetuin-A p = 0.003; Table 1).
 |
Table 1 EAAT1, DHFR, And Fetuin-A Expression In CS And Chondroma |
Association Of EAAT1, DHFR, And Fetuin-A Expression With CS Clinicopathological Features
EAAT1, DHFR, and fetuin-A expression in CS samples was not significantly associated with patient age, sex, or tumor size (Table 2). Immunopositivity percentages were higher in tissues with moderate or poor tumor differentiation, AJCC stage III or IV, Enneking stage II or III, and metastasis than in those with good differentiation, AJCC stage I or II, Enneking stage I, and no metastasis (p<0.05 or p<0.01 or p<0.001). In addition, EAAT1 immunopositivity was higher in CS tissues with invasion than in those without (p = 0.020).
 |
Table 2 Association Of EAAT1, DHFR, And Fetuin-A Expression With Clinicopathological Characteristics Of CS |
Correlations Of EAAT1, DHFR, And Fetuin-A Expression In CS
Of the 42 CS samples positive for EAAT1, 30 were also DHFR-positive and 27 were fetuin-A-positive. Of the 38 tissues negative for EAAT1 expression, 30 were DHFR-negative and 25 were fetuin-A-negative. Of the 38 samples positive for DHFR expression, 28 were fetuin-A-positive. Of the 42 samples negative for DHFR expression, 30 were fetuin-A-negative. There was a significant positive correlation between the expression of EAAT1 and DHFR (χ2 = 20.302, p < 0.001), EAAT1 and fetuin-A (χ2 = 7.218, p = 0.007), and DHFR and fetuin-A (χ2 = 16.241, p < 0.001).
Correlations Of Clinicopathological Parameters, EAAT1, DHFR, And Fetuin-A Expression With The Mean Survival Of Patients With CS
In an 84-month follow-up period, 53/80 (66.3%) patients with CS died. Patients who had died of other causes or who had disenrollment or who were alive at the time of the last follow-up were censored. Kaplan–Meier survival analysis revealed significantly shorter survival in patients with moderately or poorly differentiated tumors, AJCC stage III or IV CS, Enneking stage II or III CS, metastasis, invasion, or EAAT1, DHFR, and fetuin-A immunopositivity (p < 0.05 or p < 0.001). Mean survival was not associated with patient age, sex, or tumor size (Figure 2, Table 3).
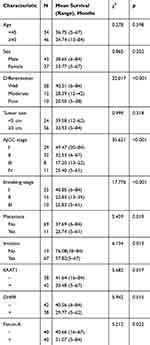 |
Table 3 Relationship Of Clinicopathological Characteristics With Survival In Patients With CS |
Multivariate Analysis
Multivariate Cox regression analysis illustrated that moderate or poor tumor differentiation, AJCC stage III or IV, Enneking stage II or III, metastasis, invasion, or positive staining for EAAT1, DHFR, and fetuin-A correlated negatively with postoperative survival rate and positively with mortality. Expressions of EAAT1, DHFR, and fetuin-A were found to be independent predictors of CS (p < 0.05; Table 4). AUCs for EAAT1, DHFR, and fetuin-A were 0.654 (95% CI: 0.532–0.776, p = 0.025), 0.638 (95% CI: 0.519–0.756, p = 0.039), and 0.670 (95% CI: 0.556–0.784,p = 0.011), respectively (Figure 3).
 |
Table 4 Multivariate Cox Regression Analysis Of Clinicopathologic Characteristics With Overall Survival In Patients With CS |
Discussion And Conclusion
In the present study, EAAT1, DHFR, and fetuin-A expression levels revealed by immunohistochemistry were found to be higher in CS tumors than in chondromas, indicating that these proteins might play an important role in the pathogenesis of CS. These findings are consistent with other studies showing that EAAT1,9 DHFR,28 and fetuin-A19 are over-expressed in malignant tissues. Moreover, expression of these proteins was associated with CS severity, progression, and poor prognosis. These findings suggest that EAAT1, DHFR, and fetuin-A may be useful biomarkers for this malignancy. Notably, our finding showing that EAAT1 expression was significantly higher in CS tissues with invasion than in those without suggests that EAAT1 may serve as a specific marker of invasive CS. We also detected significant positive correlations between the expression of EAAT1 and DHFR, EAAT1 and fetuin-A, and DHFR and fetuin-A, suggesting that these proteins may mutually regulate each other or that their expression is regulated through the same pathway. Finally, our AUC analyses indicated that EAAT1, DHFR, or fetuin-A immunopositivity are associated with a higher risk of CS.
The present EAAT1 data fit with prior studies,8,9 implicating this protein in the pathogenesis, progression, and prognosis of CS. There are multiple mechanisms by which EAAT1 may come to be over-expressed in malignant tumors. First, the expression and activity of EAATs are modulated by caspase-3, which have been shown to be responsible for impairment of glutamate uptake in amyotrophic lateral sclerosis.29 Second, the promoter that regulates EAAT expression has four binding sits for the transcription factor NF-κB.30 NF-κB also regulates the expression of growth factors that are overexpressed in malignant tumors. Karki et al31 demonstrated that upregulating EAAT1 may in turn activate NF-κB via ERK and PI3K/Akt signaling pathways. Finally, overexpression of the transcription factor Ying Yang 1, which can up- or down-regulate transcription depending on the cellular context, reduces EAAT1 mRNA levels and glutamate uptake activity.32 Further studies are required to find out the individual contributions and significance of these pathways.
DHFR is important for DNA synthesis and repair because the product of its enzymatic action, tetrahydrofolate, is essential for de novo purine and thymidylate synthesis. Therefore, DHFR has become a target of interest for cancer drug development.33 For example, MTX was approved by the US Food and Drug Administration in 1985, raltitrexed in 1998, pemetrexed in 2001, and pralatrexate in 2009. In agreement with our results, other studies have also described a role for DHFR as a potential biomarker in the development and progression of cancer.34 Zaiwiah et al35 found that the presence of DHFR predicted drug response and early relapse in colorectal cancer, and likely operated via the 5-fluorouracil pathway. Ovarian cancer cell resistance to cisplatin was found to be increased with higher expression of DHFR.36 However, DHFR expression was found to be not related to breast cancer.37 The above studies showed that the role of DHFR in cancers is complex, and the seemingly contradictory conclusions may be a result of cancer heterogeneity.
DHFR expression is regulated by multiple mechanisms, including gene amplification,38 transcriptional upregulation,39 and microRNA-mediated transcriptional repression.40 The chemotherapeutic agent fluorouracil reduces the ability of nascent DHFR mRNA to relocate to the cytoplasm, probably due to inhibition of mRNA processing or transport.41 A 19-bp deletion polymorphism (D-allele) in intron-1 of the DHFR gene leads to lowered levels of DHFR and reduced folate in the cell.42 Given the complexity of DHFR regulation, further studies are required to understand its role in cancer progression.
Fetuin-A affects insulin resistance, which is associated with increased risk of cancer. The progression of several cancers, such as pancreatic cancer,43 prostate cancer,23 and glioblastoma multiforme,44 is driven by synthesis of ectopic fetuin-A. Research has shown that a higher level of fetuin-A is associated with a modestly higher risk of colorectal cancer.45 Fetuin-A has also been shown to be a major driver of cell proliferation both in vitro and in vivo.46 Guillory et al24 reported that a lack of murine fetuin-A delays the growth of breast cancer. Additionally, fetuin-A plays a role in cell attachment,47 a key process in cancer growth and metastasis. All these findings are in consisteny with ours, indicating fetuin-A has an important role in pathogenesis, progression and prognosis of CS. However, contradictory results have also been reported. For instance, because fetuin-A is a competitive inhibitor of TGF-β, its increased expression in cancer is likely to neutralize TGF-β signaling, which has been shown to be stimulatory in advanced and metastatic tumors.48 A study demonstrated that fetuin-A knockout mice developed more intestinal tumors, due to a lack of fetuin-A allowing TGF-β to drive tumorigenicity.24 Therefore, the complex mechanisms by which fetuin-A promotes adhesion, motility, and cell proliferation required further study.19,24,46
It is noteworthy that the results of this study should be interpreted with caution since there are some limitations. They include its small sample size, its performance at only two academic medical centers and the lack of validation of the presented results in an independent patient cohort. However, we believe that the results of this study provide a useful reference for further research.
In conclusion, in the present study, EAAT1, DHFR, and fetuin-A were highly expressed in CS compared to chondroma. Over-expression of these proteins was observed in patients with moderate or poor differentiation, AJCC stage III or IV, Enneking stage II or III, and metastasis. Moreover, expression levels of EAAT1, DHFR, and fetuin-A were found to be associated with shorter patient survival. Demonstrated by AUC analyses, immunopositivity of these proteins is related to a higher risk of CS. Together these results indicate that EAAT1, DHFR, and fetuin-A proteins may serve as biomarkers of pathogenesis and progression, and predictors of prognosis, in CS.
Abbreviations
CS, chondrosarcoma; EAAT1, excitatory amino acid transporter 1; DHFR, Dihydrofolate reductase. AJCC, American Joint Committee on Cancer; ROC, receiver operating characteristic; AUC, areas under the curves.
Funding
This study was sponsored by the National Natural Science Foundation of China (No.81372180), the Hunan Provincial Science and Technology Association Program (2017TJ-Q19), the Hunan Provincial Science and Technology Association Key-Point Program (2017DK2013) and the Natural Science Foundation of Hunan Province, China (2018JJ3716).
Disclosure
The authors report no conflicts of interest in this work.
References
1. van Oosterwijk JG, Anninga JK, Gelderblom H, et al. Update on targets and novel treatment options for high-grade osteosarcoma and chondrosarcoma. Hematol Oncol Clin North Am. 2013;27(5):1021–1048. doi:10.1016/j.hoc.2013.07.012
2. Group ESESNW. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii113–iii123. doi:10.1093/annonc/mdu256
3. Jeong W, Kim HJ. Biomarkers of chondrosarcoma. J Clin Pathol. 2018;71(7):579–583. doi:10.1136/jclinpath-2018-205071
4. Skerry TM, Genever PG. Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci. 2001;22(4):174–181. doi:10.1016/s0165-6147(00)01642-4
5. Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002;3(9):748–755. doi:10.1038/nrn916
6. Li L, Hanahan D. Hijacking the neuronal NMDAR signaling circuit to promote tumor growth and invasion. Cell. 2013;153(1):86–100. doi:10.1016/j.cell.2013.02.051
7. Lee SG, Kim K, Kegelman TP, et al. Oncogene AEG-1 promotes glioma-induced neurodegeneration by increasing glutamate excitotoxicity. Cancer Res. 2011;71(20):6514–6523. doi:10.1158/0008-5472.CAN-11-0782
8. de Groot JF, Liu TJ, Fuller G, Yung WKA. The excitatory amino acid transporter-2 induces apoptosis and decreases glioma growth in vitro and in vivo. Cancer Res. 2005;65(5):1934–1940. doi:10.1158/0008-5472.CAN-04-3626
9. Kalariti N, Lembessis P, Papageorgiou E, Pissimissis N, Koutsilieris M. Regulation of the mGluR5, EAAT1 and GS expression by glucocorticoids in MG-63 osteoblast-like osteosarcoma cells. J Musculoskelet Neuronal Interact. 2007;7(2):113–118.
10. Mason DJ, Suva LJ, Genever PG, et al. Mechanically regulated expression of a neural glutamate transporter in bone: a role for excitatory amino acids as osteotropic agents? Bone. 1997;20(3):199–205. doi:10.1016/s8756-3282(96)00386-9
11. Kalariti N, Pissimissis N, Koutsilieris M. The glutamatergic system outside the CNS and in cancer biology. Expert Opin Investig Drugs. 2005;14(12):1487–1496. doi:10.1517/13543784.14.12.1487
12. Duthie SJ. Folate and cancer: how DNA damage, repair and methylation impact on colon carcinogenesis. J Inherit Metab Dis. 2011;34(1):101–109. doi:10.1007/s10545-010-9128-0
13. Ulrich CM. Nutrigenetics in cancer research–folate metabolism and colorectal cancer. J Nutr. 2005;135(11):2698–2702. doi:10.1093/jn/135.11.2698
14. Hill BT, Bailey BD, White JC, Goldman ID. Characteristics of transport of 4-amino antifolates and folate compounds by two lines of L5178Y lymphoblasts, one with impaired transport of methotrexate. Cancer Res. 1979;39(7 Pt 1):2440–2446.
15. Ongaro A, De Mattei M, Della Porta MG, et al. Gene polymorphisms in folate metabolizing enzymes in adult acute lymphoblastic leukemia: effects on methotrexate-related toxicity and survival. Haematologica. 2009;94(10):1391–1398. doi:10.3324/haematol.2009.008326
16. Xu X, Gammon MD, Wetmur JG, et al. A functional 19-base pair deletion polymorphism of dihydrofolate reductase (DHFR) and risk of breast cancer in multivitamin users. Am J Clin Nutr. 2007;85(4):1098–1102. doi:10.1093/ajcn/85.4.1098
17. Nakano M, Fukami T, Gotoh S, Nakajima M. A-to-I RNA editing up-regulates human dihydrofolate reductase in breast cancer. J Biol Chem. 2017;292(12):4873–4884. doi:10.1074/jbc.M117.775684
18. Mori K, Emoto M, Inaba M. Fetuin-A: a multifunctional protein. Recent Pat Endocr Metab Immune Drug Discov. 2011;5(2):124–146.
19. Watson K, Koumangoye R, Thompson P, et al. Fetuin-A triggers the secretion of a novel set of exosomes in detached tumor cells that mediate their adhesion and spreading. FEBS Lett. 2012;586(19):3458–3463. doi:10.1016/j.febslet.2012.07.071
20. Le Pape F, Vargas G, Clezardin P. The role of osteoclasts in breast cancer bone metastasis. J Bone Oncol. 2016;5(3):93–95. doi:10.1016/j.jbo.2016.02.008
21. Terpos E, Ntanasis-Stathopoulos I, Gavriatopoulou M, Dimopoulos MA. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 2018;8(1):7. doi:10.1038/s41408-017-0037-4
22. Nangami GN, Watson K, Parker-Johnson K, et al. Fetuin-A (alpha2HS-glycoprotein) is a serum chemo-attractant that also promotes invasion of tumor cells through matrigel. Biochem Biophys Res Commun. 2013;438(4):660–665. doi:10.1016/j.bbrc.2013.07.125
23. Mintz PJ, Rietz AC, Cardo-Vila M, et al. Discovery and horizontal follow-up of an autoantibody signature in human prostate cancer. Proc Natl Acad Sci U S A. 2015;112(8):2515–2520. doi:10.1073/pnas.1500097112
24. Guillory B, Sakwe AM, Saria M, et al. Lack of fetuin-A (alpha2-HS-glycoprotein) reduces mammary tumor incidence and prolongs tumor latency via the transforming growth factor-beta signaling pathway in a mouse model of breast cancer. Am J Pathol. 2010;177(5):2635–2644. doi:10.2353/ajpath.2010.100177
25. Kundranda MN, Henderson M, Carter KJ, et al. The serum glycoprotein fetuin-A promotes lewis lung carcinoma tumorigenesis via adhesive-dependent and adhesive-independent mechanisms. Cancer Res. 2005;65(2):499–506.
26. Thompson PD, Sakwe A, Koumangoye R, et al. Alpha-2 Heremans Schmid Glycoprotein (AHSG) modulates signaling pathways in head and neck squamous cell carcinoma cell line SQ20B. Exp Cell Res. 2014;321(2):123–132. doi:10.1016/j.yexcr.2013.12.003
27. Wang Y, Chen JJ, Wang XF, Wang Q. Clinical and prognostic significance of Raf kinase inhibitory protein expression in gastrointestinal stromal tumors. World J Gastroenterol. 2018;24(23):2508–2517. doi:10.3748/wjg.v24.i23.2508
28. Organista-Nava J, Gomez-Gomez Y, Illades-Aguiar B, et al. Overexpression of dihydrofolate reductase is a factor of poor survival in acute lymphoblastic leukemia. Oncol Lett. 2018;15(6):8405–8411. doi:10.3892/ol.2018.8429
29. Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH, Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006;281(20):14076–14084. doi:10.1074/jbc.M600653200
30. Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. Embo J. 2005;24(3):510–520. doi:10.1038/sj.emboj.7600555
31. Karki P, Hong P, Johnson J
32. Rosas S, Vargas MA, Lopez-Bayghen E, et al. Glutamate-dependent transcriptional regulation of GLAST/EAAT1: a role for YY1. J Neurochem. 2007;101(4):1134–1144. doi:10.1111/j.1471-4159.2007.04517.x
33. Costi MP, Ferrari S, Venturelli A, Calò S, Tondi D, Barlocco D. Thymidylate synthase structure, function and implication in drug discovery. Curr Med Chem. 2005;12(19):2241–2258. doi:10.2174/0929867054864868
34. Ceppi F, Gagne V, Douyon L, et al. DNA variants in DHFR gene and response to treatment in children with childhood B ALL: revisited in AIEOP-BFM protocol. Pharmacogenomics. 2018;19(2):105–112. doi:10.2217/pgs-2017-0153
35. Zawiah M, Yousef AM, Kadi T, et al. Early disease relapse in a patient with colorectal cancer who harbors genetic variants of DPYD, TYMS, MTHFR and DHFR after treatment with 5-fluorouracil-based chemotherapy. Drug Metab Pers Ther. 2018;33(4):201–205. doi:10.1515/dmpt-2018-0012
36. Li Z, Wang Q, Zhang W, Yang Z, Li L. [Cisplatin resistant effects of dihydrofolate reductase gene expression up-regulation in epithelial ovarian cancer]. Zhonghua Fu Chan Ke Za Zhi. 2015;50(11):854–860.
37. Eskandari-Nasab E, Hashemi M, Rezaei H, et al. Evaluation of UDP-glucuronosyltransferase 2B17 (UGT2B17) and dihydrofolate reductase (DHFR) genes deletion and the expression level of NGX6 mRNA in breast cancer. Mol Biol Rep. 2012;39(12):10531–10539. doi:10.1007/s11033-012-1938-8
38. Alt FW, Kellems RE, Bertino JR, Schimke RT. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978;253(5):1357–1370.
39. Dynan WS, Sazer S, Tjian R, Schimke RT. Transcription factor Sp1 recognizes a DNA sequence in the mouse dihydrofolate reductase promoter. Nature. 1986;319(6050):246–248. doi:10.1038/319246a0
40. Song B, Wang Y, Kudo K, Gavin EJ, Xi Y, Ju J. miR-192 regulates dihydrofolate reductase and cellular proliferation through the p53-microRNA circuit. Clin Cancer Res. 2008;14(24):8080–8086. doi:10.1158/1078-0432.CCR-08-1422
41. Armstrong RD, Lewis M, Stern SG, Cadman EC. Acute effect of 5-fluorouracil on cytoplasmic and nuclear dihydrofolate reductase messenger RNA metabolism. J Biol Chem. 1986;261(16):7366–7371.
42. Johnson WG, Stenroos ES, Spychala JR, Chatkupt S, Ming SX, Buyske S. New 19 bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR): a risk factor for spina bifida acting in mothers during pregnancy? Am J Med Genet A. 2004;124A(4):339–345. doi:10.1002/ajmg.a.20505
43. Chen J, Wu W, Chen L, et al. [Expression and clinical significance of AHSG and complement C3 in pancreatic ductal adenocarcinoma]. Zhonghua Yi Xue Za Zhi. 2014;94(28):2175–2179.
44. Nangami GN, Sakwe AM, Izban MG, et al. Fetuin-A (alpha 2HS glycoprotein) modulates growth, motility, invasion, and senescence in high-grade astrocytomas. Cancer Med. 2016;5(12):3532–3543. doi:10.1002/cam4.940
45. Nimptsch K, Aleksandrova K, Boeing H, et al. Plasma fetuin-A concentration, genetic variation in the AHSG gene and risk of colorectal cancer. Int J Cancer. 2015;137(4):911–920. doi:10.1002/ijc.29448
46. Sakwe AM, Koumangoye R, Goodwin SJ, Ochieng J. Fetuin-A ({alpha}2HS-glycoprotein) is a major serum adhesive protein that mediates growth signaling in breast tumor cells. J Biol Chem. 2010;285(53):41827–41835. doi:10.1074/jbc.M110.128926
47. Fisher HW, Puck TT, Sato G. Molecular growth requirements of single mammalian cells: the action of fetuin in promoting cell attachment to glass. Proc Natl Acad Sci U S A. 1958;44(1):4–10. doi:10.1073/pnas.44.1.4
48. Freudlsperger C, Bian Y, Contag Wise S, et al. TGF-beta and NF-kappaB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene. 2013;32(12):1549–1559. doi:10.1038/onc.2012.171
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.