Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 10
Role of pulse oximetry in detecting critical congenital heart disease among newborns delivered at a high altitude setting in Ethiopia
Authors Tekleab AM , Sewnet YC
Received 1 June 2019
Accepted for publication 26 July 2019
Published 15 August 2019 Volume 2019:10 Pages 83—88
DOI https://doi.org/10.2147/PHMT.S217987
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Atnafu Mekonnen Tekleab,1 Yilikal Chanie Sewnet2
1Pediatrics and Child Health, St Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 2Cardiac Center of Ethiopia, Addis Ababa, Ethiopia
Background: Congenital heart disease is one of the most common birth defects. It is not detected in some newborns until after their hospital discharge. Pulse oximetry (SpO2) screening for critical congenital heart disease (CCHD) is practiced in some settings, mainly based on evidence derived from studies done in lowland areas. This study aimed to assess the role of SpO2 screening performed before discharge in detecting CCHD in our setting (Addis Ababa) which is located at high altitude.
Methods: Oxygen saturation of 941 apparently healthy term newborns in the nursery unit of St Paul’s Hospital Millennium Medical College located in Addis Ababa, was measured before discharge during the period from January 2018 to July 2018. SpO2 reading ≥95% was taken as a negative screening result. Positive SPO2 was defined as SpO2 <90% in any extremity, or a persistent SpO2 of 90%–94% in both right arm and lower extremity sites on three measurements or a persistent right arm to lower extremity SpO2 difference of >3%. Subsequent confirmatory echocardiography examination was done for those who tested positive during the SpO2 screening test. Data were analyzed using Statistical Package for Social Sciences version 20.0.
Results: A total of 56/941 (6.0%) newborns tested positive during the screening test. Of those 56 cases, subsequent echocardiography examination detected persistent pulmonary hypertension of the newborn (PPHN) in ten (17.9%) cases (subsequently two of them were found to have sepsis), patent ductus arteriosus in eleven (19.6%) cases, and atrial septal defect in two (3.6%) cases. No case of CCHD was detected among the screened newborns.
Conclusion: SpO2 screening detected non-cardiac causes of hypoxemic illnesses (sepsis and PPHN) which otherwise would have been missed. However, we recommend a larger sample size study to assess the efficacy of SpO2 screening in detecting CCHD in our setting.
Keywords: pulse oximetry, screening, congenital heart disease, high altitude
Background
Approximately 1% of newborns have congenital heart disease which is responsible for 30%–50% of infant mortality due to birth defects.1,2 Of those infants with congenital heart disease, a quarter of them are assumed to have critical congenital heart disease (CCHD), which by definition is a lesion that needs corrective treatment within 1 year of life.3,4 But population-based studies in the US showed that CCHD was detected at a late stage in 29.5% of infants.5,6 Due to the persistence of fetal circulation in the first few days of life, physical findings like murmur, cyanosis, and tachypnea may not be detected before the newborn is discharged from the hospital, which may occur before 48 hours of life. This decreases the effectiveness of clinical examination as a screening method for CCHD in newborns.7,8 The decline of physical examination skill among the current trainees should also be taken into consideration.9 Because timely recognition of CCHD could improve outcomes, it is important to identify and evaluate strategies to enhance early detection. Pulse oximetry (SpO2) has been proposed as one such strategy, and in 2011 SpO2 screening for CCHD was added to the “Recommended Uniform Screening Panel for Newborns in the United States”.10 Subsequently, individual states in the US started to implement the legislation.11–13
Several studies reported SpO2 screening of CCHD at discharge to be feasible and cost-effective and to have comparable cost with the existing newborn screening tests.14–16
Implementing SpO2 screening in the newborn nursery prior to discharging newborns was found to reduce missed diagnosis of CCHD by 30%.4,17
Based on this evidence, it appears that CCHD represents a newborn condition that could be detected through screening in many settings. However, altitude affects oxygen saturation and high altitude is associated with low oxygen saturation.18 Hence, it is important to assess whether oximetry screening of CCHD is effective in a setting at high altitude (2,600 m above sea level). This study assessed the role of oximetry screening in detecting CCHD in a high altitude setting.
Methods
The study was conducted in the nursery unit of St Paul’s Hospital Millennium Medical College in Addis Ababa, Ethiopia, located at 2,600 m above sea level. Every month 600 newborns are delivered in the hospital. Data were collected prospectively from January 2018 to July 2018. As shown in Figure 1, 941 apparently healthy term neonates were included in the study. Consecutive cases who were asymptomatic at time of discharge from the nursery unit of the hospital were included. SpO2 screening was done for those who fulfilled the inclusion criteria as defined by: 1) normal vital signs (respiratory rate 30–60 breaths/min, pulse rate 100–160 beats/min, temperature 36.5–37.5°C), 2) no signs of respiratory distress, 3) baby was active and feeding normally, and 4) no murmur or diminished pulses. Newborns who were discharged from the hospital before 6 hours of age and those who had antenatal diagnoses of cardiac disease were excluded from the study. Gestational age was determined based on last menstrual period and Ballard Score was used in case of unreliable last menstrual period. Term gestation was defined as gestational age ≥37 completed weeks.
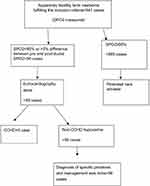 |
Figure 1 Schematic flow diagram of the study. Abbreviations: SpO2, pulse oximetry; CCHD, critical congenital heart disease. |
Two trained data collectors did the screening and SpO2 measurement. SpO2 measurement was done after 24 hours of delivery or before hospital discharge. The PC-66B Handheld Pulse Oximeter (Shenzhen Creative Industry Co., Ltd. Shenzhen GD, People's Republic of China) was used for the study using a neonatal probe. The probe was cleaned with an alcohol swab before each use to prevent cross-infection. The probe was placed on either foot and on the right hand finger for 30 seconds and reading was recorded when a good and stable waveform was observed. Second and/or third SPO2 measurement was done after 1 hour for those newborns who tested positive during the initial screening test.
SPO2 reading ≥95% was taken as a negative screening result. For those who had positive SPO2 screening test result, a certified pediatric cardiologist performed echocardiography examination on the same day. Echocardiography was done if the newborn met the following criteria: SPO2 <90% in any extremity, or a persistent SPO2 of 90%–94% in both preductal (right arm) and post-ductal (lower extremity) sites on three measurements or a persistent preductal to post-ductal SPO2 difference of >3%.10,19 Patent ductus arteriosus (PDA) was diagnosed when the defect size was >2 mm and had notable hemodynamic effect (like dilated left ventricle and left atrium). Atrial septal defect (ASD) was diagnosed when the defect size was >8 mm (since such defect size has less chance of spontaneous closure).20
Newborns with normal echocardiogram findings were evaluated and/or treated for non-cardiac causes of hypoxemia according to the hospital’s guideline.
Data were analyzed using Statistical Package for Social Sciences (SPSS) version 20.0 software. Data were reported as mean ± SD or median with interquartile range (IQR) for continuous variables and frequencies for categorical variables.
This study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Institutional Review Board of St Paul’s Hospital Millennium Medical College. Written informed consent was also obtained from the parent of each child.
Results
Nine hundred and forty-one asymptomatic term newborns were included in the study. The mean gestation age and birth weight of the study subjects were 39.4 weeks (SD 1.6) and 3,076.8 g (SD 490.5) respectively. Three quarters of the screened newborns (74.2%) were born at gestational age between 37–40 weeks and the remaining were born between 41–44 weeks. The birth weight of the study subjects ranged from 1,400 g to 4,600 g. Cesarean section was the mode of delivery for 484 (51.4%) of the study subjects. Age at screening was < 24 hours for 633 (67.3%) newborns with the median age being 8 hours (IQR 6, 24) (Table 1).
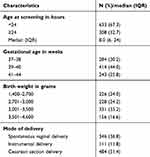 |
Table 1 Demographic and clinical characteristics of the newborns, Saint Paul’s Hospital Millennium Medical College, July 2018 |
Of the 941 study subjects, 123 (13.1%) and 70 (7.4%) of them had foot and right arm SPO2 readings <95% respectively, during the initial screening. The mean foot and right arm SPO2 readings of the study population were 95.8% (SD 2.3) and 96.0% (SD 2.2) respectively. One hundred and forty-two (15.1%) study subjects had either initial foot/right arm SPO2 reading <95% or foot to right arm SPO2 reading difference of >3%. Repeat second and/or third SPO2 measurement was done for those 142 study subjects and only 56 (39.4%) of them tested positive (having a persistent SPO2 of <95% or having arm to foot SPO2 difference >3% (Table 2).
 |
Table 2 Pulse oximeter reading characteristics of the study population, Saint Paul’s Hospital Millennium Medical College, July 2018 |
Echocardiography examination was done for those 56 newborns who had a positive SPO2 screening test result. The echocardiographic findings were: PDA: eleven (19.6%), PPHN: ten (17.9%), ASD: two (3.6%), and no abnormality was detected in 33 (58.9%). No case of CCHD was detected among the screened newborns. Those newborns who had a positive SPO2 screening test result and echocardiographic finding suggestive of PPHN were admitted to the neonatal intensive care unit of the hospital. Two of the ten cases with PPHN were later diagnosed to have sepsis and managed according to the hospital’s guideline. The remaining eight cases of PPHN were also treated according to the hospital’s treatment guideline and the PPHN resolved. Other causes of hypoxemia were ruled out among the cases with PDA and ASD, and patients were monitored until their SPO2 was normalized and then discharged with advice to parents to have them followed up.
Those newborns who tested positive based on SPO2 screening test but who were subsequently found to have a negative echocardiographic examination result (33/56) were carefully re-evaluated by a pediatrician. They all had unremarkable clinical findings and were sent home with advice to parents after the SPO2 reading was normalized.
Discussion
This study aimed to assess the role of SPO2 in detecting CCHD and other hypoxemic neonatal illnesses in a setting which is located at high altitude (2,600 m above sea level). Approximately 1% of newborns are expected to have congenital heart disease and cardiac lesion is responsible for 50% of mortality due to birth defects.1,2 The chance of detecting cases with cardiac disease through postnatal screening was reported to be higher in a setting where prenatal screening is not in place.21 However, implementing SPO2 screening in the newborn nursery prior to discharging newborns was reported to reduce missed diagnosis of CCHD by 30%, even in a setting where prenatal ultrasound screening is in place.4,17 In general, oximetry screening can reduce the chance of discharging apparently healthy infants with unknown CHD by 5.9 times.22 In the current study we found that among the 941 babies who underwent SPO2 screening, 56 (6.0%) of them failed the screening test. The fact that 6% of the screened newborns tested positive was an expectedly high figure as compared to the 1% expected prevalence of CHD, which could reflect the high false positive rate of SPO2 as a screening tool for CHD in our setting (2,600 m altitude). This was evidenced by the subsequent unremarkable echocardiography findings of the 33/56 (58.9%) cases who initially had a positive SPO2 screening test result. The high rate of false positive SPO2 screening result in our study can be explained by the high altitude where the setting is located. Altitude affects oxygen saturation and high altitude is associated with low oxygen saturation.18 The inherent limited sensitivity of SPO2 screening test, especially in detecting those cardiac lesions which do not affect oxygenation,22 could also have contributed to the high false positive rate.
On the other hand, in the current study, 10/56 (17.9%) cases initially tested positive with SPO2 screening but with subsequent echocardiography examination, they were found to have non-cardiac hypoxemic condition namely PPHN/sepsis. Basically, routine SPO2 screening targets primarily seven types of CCHD, namely common truncus, d-transposition of the great arteries, tetralogy of Fallot, pulmonary valve atresia, tricuspid valve atresia, hypoplastic left heart syndrome, and total anomalous pulmonary venous return.4,10 However, studies have shown that SPO2 screening which was applied for CCHD is also helpful in detecting life-threatening non-cardiac causes of hypoxemia like sepsis and PPHN.11,22 To this end, the fact that 10/56 (17.9%) of the cases who initially tested positive by SPO2 and were then found to have non-cardiac hypoxemic illness, can be taken as an additional benefit of the screening test and it can be useful in a setting like ours where sepsis is a common cause of neonatal morbidity and mortality.
Several other studies demonstrated that use of SPO2 for screening for CCHD at discharge was effective, feasible, and had comparable cost with the existing newborn screening tests. It has also been reported that it can easily be integrated with the existing referral system if community-based screening has to be implemented,14–16 with negligible increment of service burden to the hospital because of implementation.23 However, in our study no case of CCHD was detected by using the SPO2 screening test. The high false positive rate of the screening test leads to increased cost incurred due to the need for subsequent echocardiography imaging and increased service burden to the hospital. In our setting, a single echocardiography procedure was estimated to cost 500–700 Ethiopian birr (USD18–25 at the time of the data collection), which is expensive for ordinary citizens. The unnecessary parental anxiety because of false positive test results could also be a concern.
The mean post-ductal SPO2 and preductal SPO2 values of our study population were almost the same (95.8% and 96.0% respectively). Nevertheless, the mean SPO2 value of our study population was lower than the 97.4% mean SPO2 value reported by another study,24 and this can be explained by the high altitude location of our study setting.
The lower cut-off screening age in our study was 6 hours with the median screening age being 8 hours. This is lower than the 24 hours screening age recommended by the American Academy of Pediatrics. We took 6 hours postnatal age as the minimum age to be included in the study because our study setting allows discharge from the hospital 6 hours after delivery, as long as the mother and the baby are in good condition due to shortage of hospital beds. SPO2 tends to stabilize after 1–2 hours postnatal age at about 98%21 and hence, the 6 hours postnatal age that we took as a cut-of age for screening was acceptable.
A sizeable number of PPHN cases were detected in our study. This can be explained by the direct relationship of PPHN and altitude, since high altitude is associated with low oxygen saturation.18
The main limitation of our study was the inclusion of a smaller sample size of study subjects which was due to logistical reasons. The inclusion of cases <24 hours old, and the lack of follow-up for those study subjects who tested negative during the SPO2 screening were also a limitation of the study. The absence of follow-up may have caused failure to detect cases of CCHD which can manifest a few days after delivery.
Conclusion
In conclusion, we found a high false positive rate of SPO2 screening for CCHD that could lead to increased cost and service burden to the hospital. The unnecessary parental anxiety because of the false positive test result could be a concern. However, its ability to detect non-cardiac hypoxemic conditions like sepsis and PPHN can be taken as an advantage. Hence, further studies should be carried out before implementing SPO2 as a screening method to detect CCHD at high altitude settings like ours.
Availability of data and material
The datasets used during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank our data collectors and the participants of the study. This study was partially funded by St Paul’s Hospital Millennium Medical College. Nevertheless, the college did not have any role in the design of the study, data collection, analysis, and interpretation of the data and writing of the manuscript.
Author contributions
Atnafu Mekonnen Tekleab conceived the study. Both authors contributed to the study design, data acquisition, data analysis, and manuscript writing, have read and approved the final manuscript for publication, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Olney RS, Ailes EC, Sontag MK. Detection of critical congenital heart defects: review of contributions from prenatal and newborn screening. Semin Perinatol. 2015;39(3):230–237. doi:10.1053/j.semperi.2015.09.006
2. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi:10.1016/S0735-1097(02)01886-7
3. Talner CN. Report of the new england regional infant cardiac program, by Donald C. Fyler. Pediatrics, 1980;65(suppl):375–461. Pediatrics. 1998;102(pt 2):258–259. doi:10.1542/peds.102.1.e12
4. Mahle WT, Newburger JW, Matherne GP, et al.; On behalf of the American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research; and the American Academy of Pediatrics Section on Cardiology and Cardiac Surgery, and Committee on Fetus and Newborn. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120:447–458.
5. Peterson C, Ailes E, Riehle-Colarusso T, et al. Late detection of critical congenital heart disease among US infants: estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatr. 2014;168(4):361–370. doi:10.1001/jamapediatrics.2013.4779
6. Brown KL, Ridout DA, Hoskote A, Verhulst L, Ricci M, Bull C. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart. 2006;92:1298–1302. doi:10.1136/hrt.2005.077024
7. Danford DA. Sorting through the haystack: decision analysis and the search for heart disease among children with murmur. J Pediatr. 2002;141:465–467. doi:10.1067/mpd.2002.128657
8. Griebsch I, Knowles RL, Brown J, Bull C, Wren C, Dezateux CA. Comparing the clinical and economic effects of clinical examination, pulse oximetry, and echocardiography in newborn screening for congenital heart defects: a probabilistic cost-effectiveness model and value of information analysis. Int J Technol Assess Health Care. 2007;23:192–204. doi:10.1017/S0266462307070304
9. Gidding SS, Anisman P. What pediatric residents should learn (or what pediatricians should know) about congenital heart disease. Pediatr Cardiol. 2003;24:418–423. doi:10.1007/s00246-002-0405-z
10. Kemper AR, Mahle WT, Martin GR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128(5):e1259–e1267. doi:10.1542/peds.2010-3664
11. Lorraine FG, Kim Van NB, Mary MK, et al. Results from the New Jersey statewide critical congenital heart defects screening program. Pediatrics. 2013;132(2):e314–e323. doi:10.1542/peds.2013-0269
12. Kochilas LK, Lohr JL, Bruhn E, et al. Implementation of critical congenital heart disease screening in Minnesota. Pediatrics. 2013;132(3):e587–e594. doi:10.1542/peds.2013-0803
13. Kochilas LK, Menk JS, Saarinen A, Gaviglio A, Lohr JL. A comparison of retesting rates using alternative testing algorithms in the pilot implementation of critical congenital heart disease screening in Minnesota. Pediatr Cardiol. 2015;36(3):550–554. doi:10.1007/s00246-014-1048-6
14. Tsao P-C, Shiau Y-S, Chiang S-H, et al. Development of a newborn screening program for Critical Congenital Heart Disease (CCHD) in Taipei. PLoS One. 2016;11(4):e0153407. doi:10.1371/journal.pone.0153407
15. Movahedian AH, Mosayebi Z, Sagheb S. Evaluation of pulse oximetry in the early detection of cyanotic congenital heart disease in newborns. J Teh Univ Heart Ctr. 2016;11(2):73–78.
16. Cora Peterson SD, Grosse JG, Glidewell J, et al. A public health economic assessment of hospitals’ cost to screen newborns for critical congenital heart disease. Public Health Rep. 2014;129(1):86–93. doi:10.1177/003335491412900113
17. de Wahl Granelli A, Mellander M, Sunnegårdh J, Sandberg K, Ostman-Smith I. Screening for duct-dependant congenital heart disease with pulse oximetry: a critical evaluation of strategies to maximize sensitivity. Acta Paediatr. 2005;94(11):1590–1596. doi:10.1111/j.1651-2227.2005.tb01834.x
18. Lozano JM, Duque OR, Buitrago T, Behaine S. Pulse oximetry reference values at high altitude. Arch Dis Child. 1992;67:299–301. doi:10.1136/adc.67.3.299
19. de-Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338:a3037. doi:10.1136/bmj.b902
20. Allen HD, Driscoll DJ, Shaddy RE, Feltes TF. Atrial Septal Defects. In: Moss and Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young.
21. Koppel RI, Druschel CM, Carter T, et al. Effectiveness of pulse oximetry screening for congenital heart disease in asymptomatic newborns. Pediatrics. 2003;111(3):451–455. doi:10.1542/peds.111.3.451
22. Valmari P. Should pulse oximetry be used to screen for congenital heart disease? Arch Dis Child Fetal Neonatal Ed. 2007;92:F219–F224. doi:10.1136/adc.2005.090282
23. Jawin V, Ang H-L, Omar A, Thong M-K. Beyond critical congenital heart disease: newborn screening using pulse oximetry for neonatal sepsis and respiratory diseases in a middle-income country. PLoS One. 2015;10(9):e0137580. doi:10.1371/journal.pone.0137580
24. Bargava R, Mathur M, Patodia J. oxygen saturation trends in normal healthy term newborns: normal vaginal delivery vs. elective cesarean section. J Perinat Med. 2018;46(2):191–195. doi:10.1515/jpm-2016-0373
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
