Back to Journals » Journal of Pain Research » Volume 13
Role of Melatonin in the Regulation of Pain
Authors Xie S , Fan W, He H, Huang F
Received 24 August 2019
Accepted for publication 20 January 2020
Published 7 February 2020 Volume 2020:13 Pages 331—343
DOI https://doi.org/10.2147/JPR.S228577
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Michael Schatman
Shanshan Xie,1,2 Wenguo Fan,2,3 Hongwen He,2,4 Fang Huang1,2
1Department of Pediatric Dentistry, Guanghua School of Stomatology, Hospital of Stomatology, Sun Yat-sen University, Guangzhou, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Stomatology, Guangzhou, People’s Republic of China; 3Department of Anesthesiology, Guanghua School of Stomatology, Hospital of Stomatology, Sun Yat-sen University, Guangzhou, People’s Republic of China; 4Department of Oral Anatomy and Physiology, Guanghua School of Stomatology, Hospital of Stomatology, Sun Yat-sen University, Guangzhou, People’s Republic of China
Correspondence: Fang Huang; Hongwen He
Guanghua School of Stomatology, Hospital of Stomatology, Sun Yat-sen University, 74 Zhongshan Road 2, Guangzhou 510080, People’s Republic of China
Tel +86 20 87330570
Fax +86 20 87330709
Email [email protected]; [email protected]
Abstract: Melatonin is a pleiotropic hormone synthesized and secreted mainly by the pineal gland in vertebrates. Melatonin is an endogenous regulator of circadian and seasonal rhythms. Melatonin is involved in many physiological and pathophysiological processes demonstrating antioxidant, antineoplastic, anti-inflammatory, and immunomodulatory properties. Accumulating evidence has revealed that melatonin plays an important role in pain modulation through multiple mechanisms. In this review, we examine recent evidence for melatonin on pain regulation in various animal models and patients with pain syndromes, and the potential cellular mechanisms.
Keywords: melatonin, pain, cellular mechanisms
Introduction
Melatonin (N-acetyl-5-methoxytryptamine), a derivative of serotonin, is an endogenous neurohormone synthesized and secreted mainly by the pineal gland. Secretion increases at night and decreases during the day, following a rhythm of diurnal and nocturnal fluctuation.1 Melatonin is produced with tryptophan as a precursor.2 In addition, melatonin is considered to be synthesized locally.3 Traditionally, melatonin is known for its neurobiological role in sleep.4,5 However, melatonin has antioxidant and anti-inflammatory properties, acting as a free radical scavenger during inflammation and injury.6,7 For example, melatonin reduced the elevated expression of nuclear factor-kappa B (NF-κB) and inhibited the enhanced level of proinflammatory cytokines IL-6 or TNF-α to modulate neuroinflammation in a model of diabetic neuropathy.8 Some evidence suggests that melatonin also has immunomodulatory properties.9 Study shows that melatonin decreases peripheral and central Th1/Th17 cells responses protecting against experimental autoimmune encephalomyelitis.10
The efficacy of melatonin as an analgesic and anxiolytic agent has been demonstrated in animals and humans.11–13 It has been suggested that melatonin regulates pain via membrane receptors, nuclear receptors, and simple diffusion.14–17 Given these properties with few adverse side effects, melatonin has potential as a painkiller. The aim of this review is to discuss and analyze different lines of evidence for the effects of melatonin on pain modulation as well as to describe the cellular mechanisms of melatonin as a potential analgesic.
Melatonin Synthesis and Metabolism
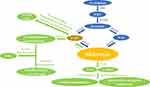
Synthesized and secreted by the pineal gland, melatonin follows a circadian rhythm controlled by the hypothalamic suprachiasmatic nucleus (SCN).18 In vertebrates, the precursor of melatonin synthesis is the essential amino acid tryptophan.2 The classical pathway of melatonin synthesis in mammals is a four-step enzyme-catalyzed reaction.19 The first step is catalyzed by tryptophan hydroxylase (TPH) and synthesizes 5-hydroxytryptophan (5-HT).20,21 Next, the aromatic amino acid decarboxylase (AAAD) synthesizes serotonin.22 At this point, the melatonin synthesis pathway divides. Under one branch, N-acetylserotonin (NAS) is produced under the catalysis of serotonin N-acetyltransferase (SNAT).23 In the other branch, 5-methoxytryptamine(5-MT) is synthesized by acetylserotonin O-methyltransferase (ASMT).24 In the final step, melatonin is synthesized either by the catalysis of ASMT with NAS as a substrate or by SNAT with 5-MT as a substrate25 (Figure 1). Research has revealed that SNAT is the rate-limiting enzyme for controlling the amount of melatonin synthesis.26
Melatonin is an indoleamine with two functional groups, a 5-methoxy group and a 3-amide group.27 Due to the hydrophilicity and lipophilicity conferred by these functional groups, melatonin can travel throughout the body. Once secreted by the pineal, melatonin crosses the blood-brain barrier and enters the circulation system, through which it reaches various tissues and cells of the body. In addition to the pineal gland, melatonin can be synthesized locally by the skin,28 bone marrow,29 oocytes,30 macrophages,31 gastrointestinal tract,32 and retina3 exerting specific intracrine, autocrine, and paracrine effects.
In vertebrates, hepatic cytochromes are the primary enzymes responsible for melatonin catabolism. The hepatic cytochromes (primarily CYP1A1, CYP1A2) catalyze melatonin to form 6-hydroxymelatonin(6-HMT).33,34 CYP1B1, another important enzyme, can catalyze melatonin to produce NAS.35 6-HMT and NAS are further degraded to form sulfate- or glucuronide-conjugated compounds that are subsequently excreted with urine.36 In the pineal gland and retina, melatonin is deacetylated to 5-MT, which contains a pyrrole ring that is further cleaved by either myeloperoxidase, indoleamine 2,3-dioxygenase, or reactive oxygen particles to form the metabolites N1-acetyl-N2-formyl-5-methoxykynurenamine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK).37 AFMK and AMK are considered major catabolic products of melatonin in the central nervous system. AFMK and AMK act as free radical scavengers and have a synergistic effect with melatonin that further enhances the antioxidant capacity of melatonin in the brain.38,39
The indolic and kynuric pathways are the main metabolic pathways of melatonin in skin; melatonin metabolites 6-HMT, AFMK, and 5-MT are detected in different skin cells.40 Furthermore, researchers have revealed that aryl acylamidases (AAAs) catalyze melatonin to produce 5-MT. In vertebrates, 5-MT is further catabolized by monoamine oxidase-A (MAO-A) to form 5-methoxyindole-3-acetaldehyde. 5-methoxyindole-3-acetaldehyde is then converted to 5-methoxychromitol (5-ML) by alcohol dehydrogenase or 5-methoxyindole-3-acetic acid by aldehyde dehydrogenase41 (Figure 1).
Melatonin Receptors and Transduction Systems
Melatonin-mediated effects occur through receptor-dependent and -independent pathways. In the receptor-dependent mechanism, melatonin receptors are primarily divided into cell membrane receptors or nuclear orphan receptors from the superfamily RZR/ROR. Membrane receptors (MT1 and MT2) belong to the G-protein-coupled receptor (GPCR) family containing seven transmembrane receptors.42 MT3 receptor once existed in theory, and then was proved to be quinone reductase II enzyme.43,44 MT1 and MT2 receptors are formed by 350 and 362 amino acids, respectively, and shows 60% homology. The nuclear orphan receptor GPR50, also known as the melatonin-related receptor, has high sequence homology to membrane receptors. However, melatonin or any other known ligand does not bind to GPR50.45 Membrane receptors have been identified and cloned in a great number of tissues in humans and rodents, such as the retina,46 brain, pituitary,47 gastrointestinal tract,48 oocytes,49 and pancreatic islet.50 Changes in MT1/MT2 and RZR/ROR receptor density fluctuate in relation to serum and intracellular melatonin levels following the circadian rhythm of melatonin secretion.48,51
Variation of sunshine exposure owns a selective pressure in melatonin receptors.52 MTNR1a is the gene for MT1 and 1b for MT2, whose genes mutation and expression variation may contribute to cancer susceptibility.53,54 In the central and peripheral nervous systems, MT1 and MT2 receptors both are localized on neuronal membranes.55 The two subtypes of membrane receptors rarely co-exist in the same cell. When they do, one of them dominates the cell membrane.56 MT1 may play an important role in the signaling pathway transduction of the nervous system. Recent research indicates that MT1 receptor is involved in neural pathways modulating depression and diurnal rhythms.57–59 Interestingly, little study demonstrated the involvement of MT1 in nociception modulation, and whether MT1 is involved in the transduction of nociceptive signals requires more research to validate.
Melatonin Effects on Nociception
Melatonin has been demonstrated to attenuate nociceptive responses to various noxious stimulus and is considered as a potential analgesic drug in the clinic. Administration of melatonin or its analogs through peripheral or central pathways has dose-dependent long-term antinociceptive effects in models of acute, neuropathic, and inflammatory pain. (Table 1)
 |
Table 1 Antinociceptive Activity of Melatonin and Its Analogs |
Acute Pain
It has been shown that melatonin (25–100mg/kg, i.p.) administration dose-dependently attenuates the hyperalgesic response and has ameliorative potential in reducing inflammation in a well-established model of hyperalgesia associated with inflammation.11 In addition, melatonin was shown to reduce the flinching response during Phase 1 and Phase 2 of formalin-evoked acute pain.60 Melatonin has also been found to play an important role in neuroprotection in acute pain caused by complete Freund’s adjuvant (CFA).61 Interestingly, other data suggest that dental pulp damage could cause acute pulpitis and reduce serum melatonin levels. Supplementation with exogenous melatonin via intraperitoneal injection induced pain relief.62 In morphine-exposed rodents, melatonin counteracted the resulting hyperalgesia and tolerance through inhibition of microglia activation and protein kinase Cγ (PKCγ) activities.63–65
In the past 10 years, researchers have conducted an increasing number of studies on the antinociceptive effects of melatonin. In addition to animal experiments, clinical trials have been carried out in this field. A meta-analysis of current trials of pharmacotherapy for cluster headache suggests that 10 mg of melatonin daily could be given for both acute treatment and preventive therapy.66 Melatonin displays a definite dose-dependent antinociceptive effect, which may be correlated with changes in pain threshold.67 Melatonin can also effectively relieve pain induced by anodal stimulation applied over the primary motor cortex.68 However, if the level of melatonin in the body is disordered, it may cause post-traumatic stress disorder.69,70 Interestingly, another clinical trial shows that the treatment effect on pain of melatonin is not observed in patients undergoing abdominal hysterectomy with mildly anxiety.71 Whether melatonin owns analgesic effect on acute pain seems to be controversial and needs further study.
Chronic Inflammatory Pain
In the last decade, an increasing number of clinical trials on the analgesic effect of melatonin have been carried due to the minor side effects and sequelae of melatonin. For instance, chronic musculoskeletal pain and generalized tenderness including allodynia or hyperalgesia from fibromyalgia syndrome are alleviated by melatonin treatment. Melatonin administration (3 mg or 5 mg/day) alone or combined with fluoxetine (20 mg/day) shows a significantly therapeutic effect in patients with fibromyalgia syndrome.12 Melatonin also attenuates inflammation and oxidative stress and is reported to be effective in repairing morpho-functional damage in a fibromyalgia syndrome model.72,73 A clinic trial suggests that reduction of melatonin synthesis and significant increase in 6-sulfatoxymelatonin secretion are positively correlated with clinical symptoms of fibromyalgia syndrome.74,75 Melatonin treatment also causes moderately increased expression of mitofusin2 and proliferator-activated receptor gamma coactivator-1alpha (PGC-1α) in reserpine-induced myalgic (RIM) rodents meant to mimic mitochondrial function.73
In the orofacial pain test, acute melatonin administration alters mechanical and thermal hyperalgesia with long-term effects.76 Post-hoc analysis also shows that melatonin treatment increases the mechanical pain threshold and improves sleep quality in chronic inflammatory pain patients.77–79 Another study provides evidence that melatonin could reduce pain scores, lower analgesic use, and improve sleep quality.78 Interestingly, melatonin achieved complete pain alleviation in the first post-traumatic/secondary case of long-lasting autonomic symptoms with hemicrania (LASH) syndrome.80 Moreover, exogenous melatonin supplementation can significantly relieve abdominal pain caused by irritable bowel syndrome (IBS).81 Furthermore, melatonin reduces indomethacin dosage during the treatment period of hemicrania continua and shows better pain relief effect.82
In sub-chronic arsenic-induced animals, exogenous melatonin administration exerts properties of scavenging oxidative and nitrosative radicals, inhibiting pro-inflammatory cytokines and repairing neuropharmacological disturbance.83 The hyperalgesic and inflammatory responses induced by CFA could be effectively attenuated by melatonin.84 In an animal model of oxaliplatin-induced pain, melatonin alleviates nociceptive response via repression of glial fibrillary acidic protein (GFAP) and inflammatory cytokines such as IL-1 and TNF-α, and neuropathic deficits via reduction of the loss of mitochondrial membrane potential.85,86 Moreover, melatonin derivatives such as benzoyl-melatonin (BMT) and acetyl-melatonin (AMT) perform the anti-inflammatory activities in lipopolysaccharide (LPS)-stimulated macrophage cells and exert antinociceptive effects, which result in the reduction of nitric oxide (NO) and prostaglandin E2 (PGE2).87
Neuropathic Pain
Thermal hyperalgesia, cold allodynia, and oxidative stress induced by chronic constriction injury (CCI) of the sciatic nerve are significantly attenuated by administration of melatonin (2.5 or 5 mg/kg, i.p.). L-arginine pretreatment can reverse the melatonin-induced protective effect suggesting the nitric oxide pathway is involved.88 Other researchers have found that melatonin could increase the mechanical pain threshold and slightly increase thermal hyperalgesia threshold. However, naloxone pretreatment abolishes the mechanical antinociceptive but not the thermal protect effect of melatonin.89 In addition, melatonin also increases the withdrawal latency during plantar tests in CCI rodents.90 Interestingly, agomelatine, a melatonin analog, administration alone had no effect on mechanical allodynia induced by chronic constriction (ligation) injury to the sciatic nerve (CCI-SN) or the infraorbital nerve (CCI-ION) rats but produced an anti-allodynic effect when combined with gabapentin.91 However, in another study, agomelatine dose-dependently decreased mechanical hypersensitivity in three neuropathic pain models (oxaliplatin, streptozocin, and CCI).92 The analgesic effect of agomelatine remains controversial and needs to be validated. While, piromelatine, another melatonin analog, is reported to significantly prolong thermal and mechanical latency and improve sleep of partial sciatic nerve ligation (PSL) mice.93 Furthermore, neuropathic pain is worse due to the reduction of endogenous melatonin from sleep deprivation or pinealectomy, while exogenous supplement of melatonin can alleviate the behavioral hypersensitivity.94,95 Otherwise, adjuvant therapy with melatonin has a superior anti-hyperalgesia effect. For instance, melatonin combined with an extracorporeal shock wave has a synergistic effect with short- and long-term improvement of neuropathic pain.96,97
Misaligned diet and sleep deprivation during the peri-CCI surgery and post-CCI distinctly decrease the paw withdrawal mechanical threshold, whereas melatonin pretreatment ameliorates the hypersensitivity and reverses the disturbed sleep rhythm.94,98 In other neuropathic pain models, such as cuff implantation, valproic acid, and paclitaxel, melatonin ameliorates mechanical and thermal allodynia by preventing the increases in NO levels, down-regulating c-fos, and increasing C-fiber activity.17,99,100 Growing evidence suggests that melatonin administration may reverse the nociceptive threshold in spinal nerve ligation (SNL) rodents.101,102 Meanwhile, MT2 receptor-selective antagonist treatment reverses the effect caused by melatonin, suggesting that MT2 receptors may be a novel target in treating neuropathic pain.15,103,104
Mechanisms of Action on Animal Models
Melatonin Receptors
Melatonin receptors in both central and peripheral nervous system have been considered antinociceptive, due to mounting evidence in many rodent models of neuropathic pain.89,93,105,106 In rat L5–L6 SNL and spared nerve injury models, a selective MT2 partial agonist, UCM924, exerted anti-allodynic effects by modulating the ON/OFF cells of the antinociceptive system, suggesting that MT2 receptor may be an important target in analgesic drug development.15 Meanwhile, in the hot-plate and formalin tests, UCM765 (another selective MT2 partial agonist) and UCM924 also exert an antinociceptive effect.14 Another study shows that MT2 receptor agonist, IIK-7, can relieve neuropathic pain through the inhibition of glial activation and downregulation of proteins involved in inflammation such as inducible nitric oxide synthase (iNOS) and caspase-3.107 In addition, MT2 receptor agonists are considered to be effective in the treatment of neuropathic pain and have several advantages over melatonin.108 MT receptors could transmit signals through the pertussis toxin-sensitive Gi/o protein and delivered to second messenger systems or through Gq/11-phospholipase C (PLC) and PKC-dependent mechanism to modulate Ca2+ signaling.109,110 Conversely, melatonin is considered to exert protective effects by suppressing PKC.63 The potential mechanisms remain controversial and require further investigation.
Interestingly, melatonin induces a reduction in T-type Ca2+ channel currents via the MT2 receptor coupled to Gβγ -mediated PKCη signal pathway. This subsequently reduces neuronal excitability and ameliorates CFA-induced mechanical hypersensitivity.111 Melatonin is able to suppress the mitogen-activated protein kinase (MAPK) and calcium signaling pathways via the MT2 receptor, which suppresses mechanical allodynia and thermal hyperalgesia induced by cuff-implanted.17 The membrane receptors of melatonin are one of the most important mechanisms of its antinociception effect, especially MT2 receptor. Thus, it is more critical to make extensive efforts to explore the downstream pathways of melatonin membrane receptors. Interestingly, accumulated evidence shows that ROR2 is activated and upregulated after CCI, while inhibition of ROR2 reverses the nociceptive effect.16 Therefore, we speculate that melatonin may exert pain-promoting effects through activation of ROR instead of MT receptors, which needs further study.
Ion Channels and Membrane Potential
Abnormal ion channel expression and physiology have been demonstrated in a variety of pain models.112,113 Some groups show that melatonin inhibits abdominal pain caused by psychological stress via interacting with Ca2+ channels.114 Melatonin modulates against Ca2+ influx via desensitization of transient receptor potential vanilloid type 1 and melastatin type 2 (TRPV1 and TRPM2).115 Moreover, melatonin exerts anti-thermal hypersensitivity and anti-mechanical allodynia effect by inhibiting the activities of voltage-gated sodium channels Nav1.8 and Nav1.9. The thermal stimuli is transmitted by small unmyelinated C-fiber and thinly myelinated A-δ fiber, while the mechanical stimuli is transmitted by large myelinated A-β fiber.97
In addition, melatonin reverses the inhibition activities of synaptosomal integral enzymes such as Na+, K+-ATPase, and acetylcholinesterase (AChE) in neuropathic pain induced by valproic acid.99 However, in medial lateral habenula (MLHb) neurons, experiments shows that melatonin significantly augments the amplitude of glutamate-mediated evoked excitatory post-synaptic currents (EPSC), thus increasing glutamatergic synaptic transmission, which promotes the release of glutamate and increases neuronal excitability.116 In contrast, another study shows that melatonin inhibits excitatory synaptic transmission and reduces norepinephrine release in hippocampus.117 Therefore, melatonin may have a dual effect on neuronal excitability in the central nervous system. Thus, future molecular studies are required to determine the main effect of melatonin on neuronal excitability and neuropathic pain due to the complexity of central nervous network and duality of melatonin action.
NO/NOS System
NO is a physiological gas molecule, which is synthesized intracellularly directly by nitric oxide synthase (NOS) using L-arginine as substrate. NOS exists as a family of three distinct isoforms: neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). NO/NOS system exerts a broad spectrum of physiological and pathophysiological activities in humans. Accumulating evidence demonstrates that the NO/NOS system plays an important role in the initiation and maintenance of nociceptive response in animal models.118 The enhanced levels of NO production and NOS expression are inhibited by melatonin administration in various nociceptive states.90,99 However, the protective effect is significantly reversed by L-arginine pretreatment.88 Interestingly, the addition of luzindole does not distinctly influence the expression of nNOS, suggesting that the antinociceptive effect of melatonin in this pathway is not mediated by MT receptors.17 Moreover, another study reveals that NO propagates the hypersensitive potentiation induced by hind-paw ischemia possibly mediated by group II metabotropic glutamate receptors (mGluRs) as this effect was blocked by group II mGluRs agonist LY354740.119 Substantial evidence supports that melatonin partially but effectively reduces both cyclooxygenase-2 (COX-2) and iNOS expression, thus inhibiting the production of PGE2 and NO, respectively, which alleviates the hyperalgesia with inflammation.11 Inhibition of NO production leads to decrease in PKC-dependent N-Methyl-D-aspartate (NMDA) receptor GluN1 subunit and ultimately contributes to improving the mechanical allodynia following peripheral nerve injury.120,121 In addition, another study suggests that reduction of NO production could mitigate allodynic and hypersensitive activities through NO-cGMP-PKG-K+-ATPase pathways.122,123
Opiate System
Earlier studies revealed that melatonin exerts antinociception via the opiate system.124,125 In agreement with these results, the overexpression of opioid receptors is observed after hyperbaric oxygen treatment of neuropathic pain, suggesting that the opiate system participates in attenuation of allodynia.106 Piromelatine is effective in treating neuropathic pain and sleep disturbance in PSL rats mediated by opioid receptors.93 Melatonin not only increased the pain threshold of mechanical allodynia but also enhanced the threshold of thermal hypersensitivity.89 Naloxone, an opioid receptor antagonist, reversed the anti-allodynic and anti-hypersensitive effect of melatonin suggesting that melatonin affects mechanical allodynia and thermal hypersensitivity through activation of opiate system.89,126,127 In addition, co-activation of δ-opioid and melatonin receptors could induce much longer analgesia than either receptor individually.128 While, naltrindole, a selective δ-opioid receptor antagonist, can partly reverse melatonin-induced antinociception, suggesting the activation of δ-opioid receptors in the antinociceptive effect of melatonin in diabetic rats.129
Adrenergic Receptors
Previous studies have shown that melatonin can accelerate norepinephrine transmission and activation of α1- and β-adrenoceptors.130 Moreover, activation of the noradrenergic descending pathway inhibits the activities of the spinal cord nociceptive receptors, such as α2-adrenoceptors.131,132 It is documented that intrathecal melatonin alleviates mechanical allodynia response in the formalin test, which is mediated through α1-adrenoceptors, α2-adrenoceptors, muscarinic, and nicotinic receptors in the spinal cord.60 In addition, agomelatine exhibits anti-allodynia through noradrenergic neurotransmission mediated by α2-adrenoceptors and β2-adrenoceptors.91
NMDA Receptors
Recent findings suggest that NMDA receptors pathways participate in the transmission of pain.133 Melatonin is considered to attenuate morphine-induced hypersensitivity and tolerance by suppressing NMDA receptor subtype 1 (NR1) activities in the spinal cord.61 The up-regulation of NMDA receptor subtype 2B (NR2B), Ca2+/calmodulin-dependent protein kinase II (CaMKII), and cyclic adenosine monophosphate-response element-binding protein (CREB) is induced by nerve injury, which can be recovered by melatonin pretreatment.98 Furthermore, melatonin administration attenuates the NR1 expression and reduces NMDA-induced currents in dorsal horn neurons in rodents with unilateral temporomandibular joint (TMJ) inflammation in a dose-dependent manner.134 The treatment of neuropathic pain achieves more efficacy using a combination of melatonin and dextromethorphan (DM; a clinically available NMDA receptor antagonist).135
Epigenetic Modifications
Epigenetic modifications alter gene expression without changing the primary DNA sequence. Epigenetic modifications primarily include DNA methylation, histone acetylation, and non-coding RNA interference. In the past decade, a growing number of studies have implicated epigenetic modifications in the induction and maintenance of neuropathic pain or inflammatory pain.136–139 Accumulating evidence suggests that spinal ten-eleven translocation methyl-cytosine dioxygenase 1 (Tet1)-dependent epigenetic demethylation is associated with nociception hypersensitivity development.140 Melatonin has been reported to inhibit Tet1 expression, Tet1-metabolic glutamate receptor subtype 5 (mGluR5) promoter coupling, hence leading to mGluR5 promoter methylation enrichment and low expression of mGluR5 in dorsal horn neurons, subsequently mitigating neuropathic pain.103 Melatonin has been reported to alleviate allodynia via histone acetylation modification. The experiment shows that the antinociceptive effect of melatonin is conducted by enhancing spinal serine-/threonine-specific phosphatase 2A (PP2A) expression that couples PP2A with histone deacetylase 4 (HDAC4) to dephosphorylate HDAC4 as well as prompts nuclear import of HDAC4, herein HDAC4 binds to histone of hmgb1 gene and increases high-mobility group protein B1 (HMGB1) expression in neurons.101,141
Other Mechanisms
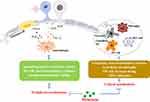
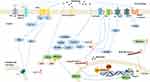
Melatonin also is reported to show an inhibition of the Toll-like receptor 4 (TLR4)/NF-κB pathway in the pulp of acute pulpitis rats to exert a protective effect. Moreover, in LPS-stimulated human dental pulp cells, melatonin could also influence the TLR4/NF-κB pathway.62 In the animal model of hyperalgesia associated with inflammation, the antinociceptive response of melatonin is mediated by inhibition of NF-κB signaling and MAPK.11 Conversely, in nerve injury-induced neuropathic pain, pinealectomy reverses the protective effect of melatonin due to phosphorylation of p38 MAPK, activation of microglia, and release of pro-inflammatory cytokines.95 (Figures 2 and 3)
Furthermore, the current data suggest that short-term administration of melatonin after acute pain may be associated with the pain regulation and neuroprotective effects of BDNF levels.61 In addition, melatonin therapy has been found to partially reverse morphine-induced hypersensitivity and tolerance by inhibiting microglia activation via the heat shock protein 27 (HSP27)-related pathway.65 Besides, it is reported that melatonin restored the antinociceptive effect of morphine through altering the expression of multiple genes.142 Thus, the molecular mechanism of melatonin exerting antinociceptive effect remains to be further studied.
Conclusion
Although melatonin and its analogs have been shown to attenuate hyperalgesia and allodynia in several animal models of acute, inflammatory, and neuropathic pain, conflicting evidence exists and the mechanisms are not fully understood. On the one hand, melatonin is a pleiotropic hormone with little side effects and has the potential to be used as an effective drug in antinociception activity. Therefore, an increasing number of clinical trials have been conducted to verify the analgesic effect of melatonin in humans. On the other hand, melatonin can travel throughout the body and act on a large number of targets due to its hydrophilicity and lipophilicity. At present, the main mechanism through which melatonin plays an antinociceptive role has not been determined. A comprehensive understanding of the underlying mechanisms for the observed effects of melatonin in nociception will be necessary before its use can be evaluated in clinical applications for the prevention and/or treatment of different pain states in humans. Thus, the exact mechanistic pathway by which melatonin exerts nociceptive effect remains to be elucidated.
Acknowledgments
This work was supported partly by the National Natural Science Foundation of China (No. 81870737 and 81771098) and Guangdong Financial Fund for High-Caliber Hospital Construction. We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Binkley SA. Circadian rhythms of pineal function in rats. Endocr Rev. 1983;4(3):255–270. doi:10.1210/edrv-4-3-255
2. Zagajewski J, Drozdowicz D, Brzozowska I, et al. Conversion L-tryptophan to melatonin in the gastrointestinal tract: the new high performance liquid chromatography method enabling simultaneous determination of six metabolites of L-tryptophan by native fluorescence and UV-VIS detection. J Physiol Pharmacol. 2012;63(6):613–621.
3. Gern WA, Ralph CL. Melatonin synthesis by the retina. Science. 1979;204(4389):183–184. doi:10.1126/science.432640
4. Gandhi AV, Mosser EA, Oikonomou G, Prober DA. Melatonin is required for the circadian regulation of sleep. Neuron. 2015;85(6):1193–1199. doi:10.1016/j.neuron.2015.02.016
5. Campos FL, da Silva FP
6. Lee JS, Cua DJ. Melatonin lulling Th17 cells to sleep. Cell. 2015;162(6):1212–1214. doi:10.1016/j.cell.2015.08.054
7. Tao J, Yang M, Wu H, et al. Effects of AANAT overexpression on the inflammatory responses and autophagy activity in the cellular and transgenic animal levels. Autophagy. 2018;14(11):1850–1869. doi:10.1080/15548627.2018.1490852
8. Negi G, Kumar A, Sharma SS. Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF-kappaB and Nrf2 cascades. J Pineal Res. 2011;50(2):124–131. doi:10.1111/j.1600-079X.2010.00821.x
9. Lissoni P, Mandala M, Brivio F. Abrogation of the negative influence of opioids on IL-2 immunotherapy of renal cell cancer by melatonin. Eur Urol. 2000;38(1):115–118. doi:10.1159/000020263
10. Alvarez-Sanchez N, Cruz-Chamorro I, Lopez-Gonzalez A, et al. Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance. Brain Behav Immun. 2015;50:
11. Esposito E, Paterniti I, Mazzon E, Bramanti P, Cuzzocrea S. Melatonin reduces hyperalgesia associated with inflammation. J Pineal Res. 2010;49(4):321–331. doi:10.1111/jpi.2010.49.issue-4
12. Hussain SA, Al K
13. Posa L, De Gregorio D, Gobbi G, Comai S. Targeting melatonin MT2 receptors: a novel pharmacological avenue for inflammatory and neuropathic pain. Curr Med Chem. 2018;25(32):3866–3882. doi:10.2174/0929867324666170209104926
14. Lopez-Canul M, Comai S, Dominguez-Lopez S, Granados-Soto V, Gobbi G. Antinociceptive properties of selective MT(2) melatonin receptor partial agonists. Eur J Pharmacol. 2015;764:
15. Lopez-Canul M, Palazzo E, Dominguez-Lopez S, et al. Selective melatonin MT2 receptor ligands relieve neuropathic pain through modulation of brainstem descending antinociceptive pathways. Pain. 2015;156(2):305–317. doi:10.1097/01.j.pain.0000460311.71572.5f
16. Zhou XL, Zhang CJ, Peng YN, Wang Y, Xu HJ, Liu CM. ROR2 modulates neuropathic pain via phosphorylation of NMDA receptor subunit GluN2B in rats. Br J Anaesth. 2019;123(2):e239–e248. doi:10.1016/j.bja.2018.08.025
17. Lin JJ, Lin Y, Zhao TZ, et al. Melatonin suppresses neuropathic pain via MT2-dependent and -independent pathways in dorsal root ganglia neurons of mice. Theranostics. 2017;7(7):2015–2032. doi:10.7150/thno.19500
18. Gooley JJ, Chamberlain K, Smith KA, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96(3):E463–E472. doi:10.1210/jc.2010-2098
19. Bonomini F, Borsani E, Favero G, Rodella LF, Rezzani R. Dietary melatonin supplementation could be a promising preventing/therapeutic approach for a variety of liver diseases. Nutrients. 2018;10(9):1135. doi:10.3390/nu10091135
20. Cornide-Petronio ME, Anadon R, Barreiro-Iglesias A, Rodicio MC. Tryptophan hydroxylase and serotonin receptor 1A expression in the retina of the sea lamprey. Exp Eye Res. 2015;135:81–87. doi:10.1016/j.exer.2015.04.017
21. Zhao D, Yu Y, Shen Y, et al. Melatonin synthesis and function: evolutionary history in animals and plants. Front Endocrinol (Lausanne). 2019;10:249. doi:10.3389/fendo.2019.00249
22. Li Y, Lv Y, Bian C, You X, Deng L, Shi Q. A comparative genomic survey provides novel insights into molecular evolution of l-aromatic amino acid decarboxylase in vertebrates. Molecules. 2018;23:4.
23. Chong NW, Bernard M, Klein DC. Characterization of the chicken serotonin N-acetyltransferase gene. Activation via clock gene heterodimer/E box interaction. J Biol Chem. 2000;275(42):32991–32998. doi:10.1074/jbc.M005671200
24. Rath MF, Coon SL, Amaral FG, Weller JL, Moller M, Klein DC. Melatonin synthesis: Acetylserotonin O-Methyltransferase (ASMT) is strongly expressed in a subpopulation of pinealocytes in the male rat pineal gland. Endocrinology. 2016;157(5):2028–2040. doi:10.1210/en.2015-1888
25. Tan DX, Hardeland R, Back K, Manchester LC, Alatorre-Jimenez MA, Reiter RJ. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species. J Pineal Res. 2016;61(1):27–40. doi:10.1111/jpi.12336
26. Byeon Y, Back K. Low melatonin production by suppression of either serotonin N-acetyltransferase or N-acetylserotonin methyltransferase in rice causes seedling growth retardation with yield penalty, abiotic stress susceptibility, and enhanced coleoptile growth under anoxic conditions. J Pineal Res. 2016;60(3):348–359. doi:10.1111/jpi.12317
27. Tan DX, Reiter RJ, Manchester LC, et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2(2):181–197. doi:10.2174/1568026023394443
28. Slominski AT, Zmijewski MA, Semak I, et al. Melatonin, mitochondria, and the skin. Cell Mol Life Sci. 2017;74(21):3913–3925. doi:10.1007/s00018-017-2617-7
29. Pires-Lapa MA, Carvalho-Sousa CE, Cecon E, Fernandes PA, Markus RP. Beta-adrenoceptors trigger melatonin synthesis in phagocytes. Int J Mol Sci. 2018;19(8):2182. doi:10.3390/ijms19082182
30. Xiao L, Hu J, Song L, et al. Profile of melatonin and its receptors and synthesizing enzymes in cumulus-oocyte complexes of the developing sheep antral follicle-a potential estradiol-mediated mechanism. Reprod Biol Endocrinol. 2019;17(1):1. doi:10.1186/s12958-018-0446-7
31. Xia Y, Chen S, Zeng S, et al. Melatonin in macrophage biology: current understanding and future perspectives. J Pineal Res. 2019;66(2):e12547. doi:10.1111/jpi.2019.66.issue-2
32. Matheus N, Mendoza C, Iceta R, Mesonero JE, Alcalde AI. Melatonin inhibits serotonin transporter activity in intestinal epithelial cells. J Pineal Res. 2010;48(4):332–339. doi:10.1111/jpi.2010.48.issue-4
33. Ma X, Idle JR, Krausz KW, Gonzalez FJ. Metabolism of melatonin by human cytochromes p450. Drug Metab Dispos. 2005;33(4):489–494. doi:10.1124/dmd.104.002410
34. Li C, Li G, Tan DX, Li F, Ma X. A novel enzyme-dependent melatonin metabolite in humans. J Pineal Res. 2013;54(1):100–106. doi:10.1111/jpi.12003
35. Yu Z, Tian X, Peng Y, et al. Mitochondrial cytochrome P450 (CYP) 1B1 is responsible for melatonin-induced apoptosis in neural cancer cells. J Pineal Res. 2018;65(1):e12478. doi:10.1111/jpi.2018.65.issue-1
36. Ma X, Chen C, Krausz KW, Idle JR, Gonzalez FJ. A metabolomic perspective of melatonin metabolism in the mouse. Endocrinology. 2008;149(4):1869–1879. doi:10.1210/en.2007-1412
37. Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J Pineal Res. 2013;54(3):245–257. doi:10.1111/jpi.2013.54.issue-3
38. Hardeland R. Melatonin metabolism in the central nervous system. Curr Neuropharmacol. 2010;8(3):168–181. doi:10.2174/157015910792246164
39. Galano A, Reiter RJ. Melatonin and its metabolites vs oxidative stress: from individual actions to collective protection. J Pineal Res. 2018;65(1):e12514.
40. Kim TK, Kleszczynski K, Janjetovic Z, et al. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013;27(7):2742–2755. doi:10.1096/fj.12-224691
41. Hardeland R. Melatonin, hormone of darkness and more: occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol Life Sci. 2008;65(13):2001–2018. doi:10.1007/s00018-008-8001-x
42. Zlotos DP, Jockers R, Cecon E, Rivara S, Witt-Enderby PA. MT1 and MT2 melatonin receptors: ligands, models, oligomers, and therapeutic potential. J Med Chem. 2014;57(8):3161–3185. doi:10.1021/jm401343c
43. Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13(5):1177–1185. doi:10.1016/0896-6273(94)90055-8
44. Nosjean O, Ferro M, Coge F, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275(40):31311–31317. doi:10.1074/jbc.M005141200
45. Batailler M, Mullier A, Sidibe A, et al. Neuroanatomical distribution of the orphan GPR50 receptor in adult sheep and rodent brains. J Neuroendocrinol. 2012;24(5):798–808. doi:10.1111/jne.2012.24.issue-5
46. Jones C, Helfer G, Brandstatter R. Melatonin receptor expression in the zebra finch brain and peripheral tissues. Chronobiol Int. 2012;29(2):189–202. doi:10.3109/07420528.2011.642912
47. Confente F, Rendon M, Besseau L, Falcon J, Munoz-Cueto JA. Melatonin receptors in a pleuronectiform species, solea senegalensis: cloning, tissue expression, day-night and seasonal variations. Gen Comp Endocrinol. 2010;167(2):202–214. doi:10.1016/j.ygcen.2010.03.006
48. Stebelova K, Anttila K, Manttari S, Saarela S, Zeman M. Immunohistochemical definition of MT(2) receptors and melatonin in the gastrointestinal tissues of rat. Acta Histochem. 2010;112(1):26–33. doi:10.1016/j.acthis.2008.03.004
49. El-Raey M, Geshi M, Somfai T, et al. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol Reprod Dev. 2011;78(4):250–262. doi:10.1002/mrd.21295
50. Nagorny CL, Sathanoori R, Voss U, Mulder H, Wierup N. Distribution of melatonin receptors in murine pancreatic islets. J Pineal Res. 2011;50(4):412–417. doi:10.1111/j.1600-079X.2011.00859.x
51. Venegas C, Garcia JA, Doerrier C, et al. Analysis of the daily changes of melatonin receptors in the rat liver. J Pineal Res. 2013;54(3):313–321. doi:10.1111/jpi.2013.54.issue-3
52. Ji LD, Xu J, Wu DD, Xie SD, Tang NL, Zhang YP. Association of disease-predisposition polymorphisms of the melatonin receptors and sunshine duration in the global human populations. J Pineal Res. 2010;48(2):133–141. doi:10.1111/j.1600-079X.2009.00736.x
53. Deming SL, Lu W, Beeghly-Fadiel A, et al. Melatonin pathway genes and breast cancer risk among Chinese women. Breast Cancer Res Treat. 2012;132(2):693–699. doi:10.1007/s10549-011-1884-5
54. Jablonska K, Pula B, Zemla A, et al. Expression of melatonin receptor MT1 in cells of human invasive ductal breast carcinoma. J Pineal Res. 2013;54(3):334–345. doi:10.1111/jpi.2013.54.issue-3
55. Lacoste B, Angeloni D, Dominguez-Lopez S, et al. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J Pineal Res. 2015;58(4):397–417. doi:10.1111/jpi.2015.58.issue-4
56. Klosen P, Lapmanee S, Schuster C, et al. MT1 and MT2 melatonin receptors are expressed in nonoverlapping neuronal populations. J Pineal Res. 2019;e12575. doi:10.1111/jpi.12575
57. Adamah-Biassi EB, Hudson RL, Dubocovich ML. Genetic deletion of MT1 melatonin receptors alters spontaneous behavioral rhythms in male and female C57BL/6 mice. Horm Behav. 2014;66(4):619–627. doi:10.1016/j.yhbeh.2014.08.012
58. Wu YH, Ursinus J, Zhou JN, et al. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord. 2013;148(2–3):357–367. doi:10.1016/j.jad.2012.12.025
59. Comai S, Ochoa-Sanchez R, Dominguez-Lopez S, Bambico FR, Gobbi G. Melancholic-like behaviors and circadian neurobiological abnormalities in melatonin MT1 receptor knockout mice. Int J Neuropsychopharmacol. 2015;18(3):pyu075–pyu075. doi:10.1093/ijnp/pyu075
60. Shin DJ, Jeong CW, Lee SH, Yoon MH. Receptors involved in the antinociception of intrathecal melatonin in formalin test of rats. Neurosci Lett. 2011;494(3):207–210. doi:10.1016/j.neulet.2011.03.014
61. Laste G, Ripoll Rozisky J, Caumo W, Lucena da Silva Torres I. Short- but not long-term melatonin administration reduces central levels of brain-derived neurotrophic factor in rats with inflammatory pain. Neuroimmunomodulation. 2015;22(6):358–364. doi:10.1159/000380912
62. Li JG, Lin JJ, Wang ZL, et al. Melatonin attenuates inflammation of acute pulpitis subjected to dental pulp injury. Am J Transl Res. 2015;7(1):66–78.
63. Song L, Wu C, Zuo Y. Melatonin prevents morphine-induced hyperalgesia and tolerance in rats: role of protein kinase C and N-methyl-D-aspartate receptors. BMC Anesthesiol. 2015;15:
64. Rozisky JR, Scarabelot VL, Oliveira C, et al. Melatonin as a potential counter-effect of hyperalgesia induced by neonatal morphine exposure. Neurosci Lett. 2016;633:
65. Lin SH, Huang YN, Kao JH, Tien LT, Tsai RY, Wong CS. Melatonin reverses morphine tolerance by inhibiting microglia activation and HSP27 expression. Life Sci. 2016;152:
66. Francis GJ, Becker WJ, Pringsheim TM. Acute and preventive pharmacologic treatment of cluster headache. Neurology. 2010;75(5):463–473. doi:10.1212/WNL.0b013e3181eb58c8
67. Stefani LC, Muller S, Torres IL, et al. A Phase II, randomized, double-blind, placebo controlled, dose-response trial of the melatonin effect on the pain threshold of healthy subjects. PLoS One. 2013;8(10):e74107. doi:10.1371/journal.pone.0074107
68. da Silva NR, Laste G, Deitos A, et al. Combined neuromodulatory interventions in acute experimental pain: assessment of melatonin and non-invasive brain stimulation. Front Behav Neurosci. 2015;9:
69. McFarlane AC, Barton CA, Briggs N, Kennaway DJ. The relationship between urinary melatonin metabolite excretion and posttraumatic symptoms following traumatic injury. J Affect Disord. 2010;127(1–3):365–369. doi:10.1016/j.jad.2010.05.002
70. Agorastos A, Linthorst AC. Potential pleiotropic beneficial effects of adjuvant melatonergic treatment in posttraumatic stress disorder. J Pineal Res. 2016;61(1):3–26.
71. Caumo W, Levandovski R, Hidalgo MP. Preoperative anxiolytic effect of melatonin and clonidine on postoperative pain and morphine consumption in patients undergoing abdominal hysterectomy: a double-blind, randomized, placebo-controlled study. J Pain. 2009;10(1):100–108. doi:10.1016/j.jpain.2008.08.007
72. Favero G, Trapletti V, Bonomini F, et al. Oral supplementation of melatonin protects against fibromyalgia-related skeletal muscle alterations in reserpine-induced myalgia rats. Int J Mol Sci. 2017;18(7):1389. doi:10.3390/ijms18071389
73. Favero G, Bonomini F, Franco C, Rezzani R. Mitochondrial dysfunction in skeletal muscle of a fibromyalgia model: the potential benefits of melatonin. Int J Mol Sci. 2019;20(3):765. doi:10.3390/ijms20030765
74. Bortolato B, Berk M, Maes M, McIntyre RS, Carvalho AF. Fibromyalgia and bipolar disorder: emerging epidemiological associations and shared pathophysiology. Curr Mol Med. 2016;16(2):119–136. doi:10.2174/1566524016666160126144027
75. Caumo W, Hidalgo MP, Souza A, Torres ILS, Antunes LC. Melatonin is a biomarker of circadian dysregulation and is correlated with major depression and fibromyalgia symptom severity. J Pain Res. 2019;12:
76. Scarabelot VL, Medeiros LF, de Oliveira C, et al. Melatonin alters the mechanical and thermal hyperalgesia induced by orofacial pain model in rats. Inflammation. 2016;39(5):1649–1659. doi:10.1007/s10753-016-0399-y
77. Vidor LP, Torres IL, Custodio de Souza IC, Fregni F, Caumo W. Analgesic and sedative effects of melatonin in temporomandibular disorders: a double-blind, randomized, parallel-group, placebo-controlled study. J Pain Symptom Manage. 2013;46(3):422–432. doi:10.1016/j.jpainsymman.2012.08.019
78. Schwertner A, Conceicao Dos Santos CC, Costa GD, et al. Efficacy of melatonin in the treatment of endometriosis: a phase II, randomized, double-blind, placebo-controlled trial. Pain. 2013;154(6):874–881. doi:10.1016/j.pain.2013.02.025
79. Ferini-Strambi L, Galbiati A, Combi R. Sleep disorder-related headaches. Neurol Sci. 2019;40(Suppl 1):107–113. doi:10.1007/s10072-019-03837-z
80. Rozen TD, Beams JL. A case of post-traumatic LASH syndrome responsive to indomethacin and melatonin (a man with a unique triad of indomethacin-responsive trigeminal autonomic cephalalgias). Cephalalgia. 2015;35(5):453–456. doi:10.1177/0333102414544980
81. Mozaffari S, Rahimi R, Abdollahi M. Implications of melatonin therapy in irritable bowel syndrome: a systematic review. Curr Pharm Des. 2010;16(33):3646–3655. doi:10.2174/138161210794079254
82. Rozen TD. How effective is melatonin as a preventive treatment for hemicrania continua? A clinic-based study. Headache. 2015;55(3):430–436. doi:10.1111/head.12489
83. Durappanavar PN, Nadoor P, Waghe P, Pavithra BH, Jayaramu GM. Melatonin ameliorates neuropharmacological and neurobiochemical alterations induced by subchronic exposure to arsenic in wistar rats. Biol Trace Elem Res. 2019;190(1):124–139. doi:10.1007/s12011-018-1537-1
84. Laste G, Vidor L, de Macedo IC, et al. Melatonin treatment entrains the rest-activity circadian rhythm in rats with chronic inflammation. Chronobiol Int. 2013;30(9):1077–1088. doi:10.3109/07420528.2013.800088
85. Wang YS, Li YY, Cui W, et al. Melatonin attenuates pain hypersensitivity and decreases astrocyte-mediated spinal neuroinflammation in a rat model of oxaliplatin-induced pain. Inflammation. 2017;40(6):2052–2061. doi:10.1007/s10753-017-0645-y
86. Areti A, Komirishetty P, Akuthota M, Malik RA, Kumar A. Melatonin prevents mitochondrial dysfunction and promotes neuroprotection by inducing autophagy during oxaliplatin-evoked peripheral neuropathy. J Pineal Res. 2017;62(3). doi:10.1111/jpi.12393
87. Phiphatwatcharaded C, Topark-Ngarm A, Puthongking P, Mahakunakorn P. Anti-inflammatory activities of melatonin derivatives in lipopolysaccharide-stimulated RAW 264.7 cells and antinociceptive effects in mice. Drug Dev Res. 2014;75(4):235–245. doi:10.1002/ddr.2014.75.issue-4
88. Kumar A, Meena S, Kalonia H, Gupta A, Kumar P. Effect of nitric oxide in protective effect of melatonin against chronic constriction sciatic nerve injury induced neuropathic pain in rats. Indian J Exp Biol. 2011;49(9):664–671.
89. Zurowski D, Nowak L, Machowska A, Wordliczek J, Thor PJ. Exogenous melatonin abolishes mechanical allodynia but not thermal hyperalgesia in neuropathic pain. The role of the opioid system and benzodiazepine-gabaergic mechanism. J Physiol Pharmacol. 2012;63(6):641–647.
90. Borsani E, Buffoli B, Bonazza V, Reiter RJ, Rezzani R, Rodella LF. Single administration of melatonin modulates the nitroxidergic system at the peripheral level and reduces thermal nociceptive hypersensitivity in neuropathic rats. Int J Mol Sci. 2017;18(10):2143. doi:10.3390/ijms18102143
91. M’Dahoma S, Poitevin M, Dabala E, et al. alpha2- and beta2-adrenoreceptor-mediated efficacy of the atypical antidepressant agomelatine combined with gabapentin to suppress allodynia in neuropathic rats with ligated infraorbital or sciatic nerve. Front Pharmacol. 2018;9:587. doi:10.3389/fphar.2018.00587
92. Chenaf C, Chapuy E, Libert F, et al. Agomelatine: a new opportunity to reduce neuropathic pain-preclinical evidence. Pain. 2017;158(1):149–160. doi:10.1097/j.pain.0000000000000738
93. Liu YY, Yin D, Chen L, et al. Piromelatine exerts antinociceptive effect via melatonin, opioid, and 5HT1A receptors and hypnotic effect via melatonin receptors in a mouse model of neuropathic pain. Psychopharmacology (Berl). 2014;231(20):3973–3985. doi:10.1007/s00213-014-3530-5
94. Huang CT, Chiang RP, Chen CL, Tsai YJ. Sleep deprivation aggravates median nerve injury-induced neuropathic pain and enhances microglial activation by suppressing melatonin secretion. Sleep. 2014;37(9):1513–1523. doi:10.5665/sleep.4002
95. Chiang RP, Huang CT, Tsai YJ. Melatonin reduces median nerve injury-induced mechanical hypersensitivity via inhibition of microglial p38 mitogen-activated protein kinase activation in rat cuneate nucleus. J Pineal Res. 2013;54(2):232–244. doi:10.1111/jpi.12029
96. Chen KH, Yang CH, Wallace CG, et al. Combination therapy with extracorporeal shock wave and melatonin markedly attenuated neuropathic pain in rat. Am J Transl Res. 2017;9(10):4593–4606.
97. Yang CH, Yip HK, Chen HF, et al. Long-term therapeutic effects of extracorporeal shock wave-assisted melatonin therapy on mononeuropathic pain in rats. Neurochem Res. 2019;44(4):796–810. doi:10.1007/s11064-018-02713-0
98. Xu F, Zhao X, Liu H, et al. Misaligned feeding may aggravate pain by disruption of sleep-awake rhythm. Anesth Analg. 2018;127(1):255–262. doi:10.1213/ANE.0000000000002727
99. Chaudhary S, Parvez S. Valproic acid induced neurotoxicological manifestations and its mitigation by melatonin in rat brain synaptosomes. Arch Med Res. 2018;49(7):441–450. doi:10.1016/j.arcmed.2019.01.004
100. Galley HF, McCormick B, Wilson KL, Lowes DA, Colvin L, Torsney C. Melatonin limits paclitaxel-induced mitochondrial dysfunction in vitro and protects against paclitaxel-induced neuropathic pain in the rat. J Pineal Res. 2017;63(4). doi:10.1111/jpi.12444
101. Lin TB, Hsieh MC, Lai CY, et al. Melatonin relieves neuropathic allodynia through spinal MT2-enhanced PP2Ac and downstream HDAC4 shuttling-dependent epigenetic modification of hmgb1 transcription. J Pineal Res. 2016;60(3):263–276. doi:10.1111/jpi.12307
102. Yang Z, Li C, Wang Y, et al. Melatonin attenuates chronic pain related myocardial ischemic susceptibility through inhibiting RIP3-MLKL/CaMKII dependent necroptosis. J Mol Cell Cardiol. 2018;125:185–194. doi:10.1016/j.yjmcc.2018.10.018
103. Hsieh MC, Ho YC, Lai CY, et al. Melatonin impedes Tet1-dependent mGluR5 promoter demethylation to relieve pain. J Pineal Res. 2017;63(4). doi:10.1111/jpi.12436
104. Ambriz-Tututi M, Granados-Soto V. Oral and spinal melatonin reduces tactile allodynia in rats via activation of MT2 and opioid receptors. Pain. 2007;132(3):273–280. doi:10.1016/j.pain.2007.01.025
105. Srinivasan V, Lauterbach EC, Ho KY, Acuna-Castroviejo D, Zakaria R, Brzezinski A. Melatonin in antinociception: its therapeutic applications. Curr Neuropharmacol. 2012;10(2):167–178. doi:10.2174/157015912800604489
106. Wu ZS, Wu SH, Lee SS, et al. Dose-dependent effect of hyperbaric oxygen treatment on burn-induced neuropathic pain in rats. Int J Mol Sci. 2019;20(8):1951.
107. Kuthati Y, Goutham Davuluri VN, Yang CP, Chang HC, Chang CP, Wong CS. Melatonin MT2 receptor agonist IIK-7 produces antinociception by modulation of ROS and suppression of spinal microglial activation in neuropathic pain rats. J Pain Res. 2019;12:2473–2485. doi:10.2147/JPR.S214671
108. Kuthati Y, Lin SH, Chen IJ, Wong CS. Melatonin and their analogs as a potential use in the management of Neuropathic pain. J Formos Med Assoc. 2018.
109. Serhatlioglu I, Bilgin B, Kacar E, et al. Agomelatine modulates calcium signaling through protein kinase C and phospholipase C-mediated mechanisms in rat sensory neurons. J Cell Physiol. 2019;234(7):10741–10746. doi:10.1002/jcp.v234.7
110. Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62(3):343–380. doi:10.1124/pr.110.002832
111. Zhang Y, Ji H, Wang J, et al. Melatonin-mediated inhibition of Cav3.2 T-type Ca(2+) channels induces sensory neuronal hypoexcitability through the novel protein kinase C-eta isoform. J Pineal Res. 2018. doi:10.1111/jpi.12476
112. Naziroglu M, Dikici DM, Dursun S. Role of oxidative stress and Ca(2)(+) signaling on molecular pathways of neuropathic pain in diabetes: focus on TRP channels. Neurochem Res. 2012;37(10):2065–2075. doi:10.1007/s11064-012-0850-x
113. Brittain JM, Duarte DB, Wilson SM, et al. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2)(+) channel complex. Nat Med. 2011;17(7):822–829. doi:10.1038/nm.2345
114. Tan W, Zhou W, Luo HS, Liang CB, Xia H. The inhibitory effect of melatonin on colonic motility disorders induced by water avoidance stress in rats. Eur Rev Med Pharmacol Sci. 2013;17(22):3060–3067.
115. Kahya MC, Naziroglu M, Ovey IS. Modulation of diabetes-induced oxidative stress, apoptosis, and Ca(2+) entry through TRPM2 and TRPV1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol Neurobiol. 2017;54(3):2345–2360. doi:10.1007/s12035-016-9727-3
116. Evely KM, Hudson RL, Dubocovich ML, Haj-Dahmane S. Melatonin receptor activation increases glutamatergic synaptic transmission in the rat medial lateral habenula. Synapse. 2016;70(5):181–186. doi:10.1002/syn.v70.5
117. Choi TY, Kwon JE, Durrance ES, Jo SH, Choi SY, Kim KT. Melatonin inhibits voltage-sensitive Ca(2+) channel-mediated neurotransmitter release. Brain Res. 2014;1557:
118. Sugiyama T, Shinoda M, Watase T, et al. Nitric oxide signaling contributes to ectopic orofacial neuropathic pain. J Dent Res. 2013;92(12):1113–1117. doi:10.1177/0022034513509280
119. Onishi T, Watanabe T, Sasaki M, et al. Acute spatial spread of NO-mediated potentiation during hindpaw ischaemia in mice. J Physiol. 2019. doi:10.1113/JP277615
120. Choi SR, Roh DH, Yoon SY, et al. Astrocyte D-serine modulates the activation of neuronal NOS leading to the development of mechanical allodynia in peripheral neuropathy. Mol Pain. 2019;15:1744806919843046. doi:10.1177/1744806919843046
121. Choi SR, Han HJ, Beitz AJ, Lee JH. nNOS-PSD95 interactions activate the PKC-epsilon isoform leading to increased GluN1 phosphorylation and the development of neuropathic mechanical allodynia in mice. Neurosci Lett. 2019;703:
122. Zulazmi NA, Gopalsamy B, Min JC, et al. Zerumbone alleviates neuropathic pain through the involvement of l-arginine-nitric oxide-cGMP-K(+) ATP channel pathways in chronic constriction injury in mice model. Molecules. 2017;22(4):555. doi:10.3390/molecules22040555
123. de Los Monteros-zuniga AE, Izquierdo T, Quinonez-Bastidas GN, Rocha-Gonzalez HI, Godinez-Chaparro B. Anti-allodynic effect of mangiferin in neuropathic rats: involvement of nitric oxide-cyclic GMP-ATP sensitive K(+) channels pathway and serotoninergic system. Pharmacol Biochem Behav. 2016;150–151:190–197). doi:10.1016/j.pbb.2016.10.007
124. Ebadi M, Govitrapong P, Phansuwan-Pujito P, Nelson F, Reiter RJ. Pineal opioid receptors and analgesic action of melatonin. J Pineal Res. 1998;24(4):193–200. doi:10.1111/j.1600-079X.1998.tb00532.x
125. Dai X, Cui SG, Li SR, Chen Q, Wang R. Melatonin attenuates the development of antinociceptive tolerance to delta-, but not to mu-opioid receptor agonist in mice. Behav Brain Res. 2007;182(1):21–27. doi:10.1016/j.bbr.2007.04.018
126. Chen C, Fichna J, Laudon M, Storr M. Antinociceptive effects of novel melatonin receptor agonists in mouse models of abdominal pain. World J Gastroenterol. 2014;20(5):1298–1304. doi:10.3748/wjg.v20.i5.1298
127. Deng YK, Ding JF, Liu J, Yang YY. Analgesic effects of melatonin on post-herpetic neuralgia. Int J Clin Exp Med. 2015;8(4):5004–5009.
128. Wang J, Wang L, Li M, Jin Q, Dong S. Preliminary analgesic properties of deltorphin-5-methoxytryptamine chimeric opioid peptides. Peptides. 2011;32(5):1055–1059. doi:10.1016/j.peptides.2011.01.032
129. Arreola-Espino R, Urquiza-Marin H, Ambriz-Tututi M, et al. Melatonin reduces formalin-induced nociception and tactile allodynia in diabetic rats. Eur J Pharmacol. 2007;577(1–3):203–210. doi:10.1016/j.ejphar.2007.09.006
130. Mantovani M, Kaster MP, Pertile R, Calixto JB, Rodrigues AL, Santos AR. Mechanisms involved in the antinociception caused by melatonin in mice. J Pineal Res. 2006;41(4):382–389. doi:10.1111/j.1600-079X.2006.00380.x
131. Bahari Z, Meftahi GH. Spinal alpha2 -adrenoceptors and neuropathic pain modulation; therapeutic target. Br J Pharmacol. 2019;176(14):2366–2381. doi:10.1111/bph.14580
132. Yoon SY, Lee JY, Roh DH, Oh SB. Pharmacopuncture with scolopendra subspinipes suppresses mechanical allodynia in oxaliplatin-induced neuropathic mice and potentiates clonidine-induced anti-allodynia without hypotension or motor impairment. J Pain. 2018;19(10):1157–1168. doi:10.1016/j.jpain.2018.04.015
133. Lawson K. Potential drug therapies for the treatment of fibromyalgia. Expert Opin Investig Drugs. 2016;25(9):1071–1081. doi:10.1080/13543784.2016.1197906
134. Wang S, Tian Y, Song L, et al. Exacerbated mechanical hyperalgesia in rats with genetically predisposed depressive behavior: role of melatonin and NMDA receptors. Pain. 2012;153(12):2448–2457. doi:10.1016/j.pain.2012.08.016
135. Wang S, Zhang L, Lim G, et al. A combined effect of dextromethorphan and melatonin on neuropathic pain behavior in rats. Brain Res. 2009;1288:
136. Zhao JY, Liang L, Gu X, et al. DNA methyltransferase DNMT3a contributes to neuropathic pain by repressing Kcna2 in primary afferent neurons. Nat Commun. 2017;8:
137. Penas C, Navarro X. Epigenetic modifications associated to neuroinflammation and neuropathic pain after neural trauma. Front Cell Neurosci. 2018;12:
138. Kami K, Taguchi S, Tajima F, Senba E. Histone acetylation in microglia contributes to exercise-induced hypoalgesia in neuropathic pain model mice. J Pain. 2016;17(5):588–599. doi:10.1016/j.jpain.2016.01.471
139. Liu C, Li C, Deng Z, Du E, Xu C. Long non-coding RNA BC168687 is involved in TRPV1-mediated diabetic neuropathic pain in rats. Neuroscience. 2018;374:
140. Hsieh MC, Lai CY, Ho YC, et al. Tet1-dependent epigenetic modification of BDNF expression in dorsal horn neurons mediates neuropathic pain in rats. Sci Rep. 2016;6:
141. Feldman P, Due MR, Ripsch MS, Khanna R, White FA. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J Neuroinflammation. 2012;9:
142. Cheng YC, Tsai RY, Sung YT, et al. Melatonin regulation of transcription in the reversal of morphine tolerance: microarray analysis of differential gene expression. Int J Mol Med. 2019;43(2):791–806. doi:10.3892/ijmm.2018.4030
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.