Back to Journals » Cancer Management and Research » Volume 15
Role of Human Monocarboxylate Transporter 1 (hMCT1) and 4 (hMCT4) in Tumor Cells and the Tumor Microenvironment
Authors Liu T , Han S , Yao Y, Zhang G
Received 17 May 2023
Accepted for publication 30 August 2023
Published 4 September 2023 Volume 2023:15 Pages 957—975
DOI https://doi.org/10.2147/CMAR.S421771
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Tian Liu,1,* Shangcong Han,2,* Yu Yao,1 Guiming Zhang1
1Department of Urology, The Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 2Department of Pharmaceutics, School of Pharmacy, Qingdao University, Qingdao, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guiming Zhang, Department of Urology, The Affiliated Hospital of Qingdao University, No. 16, Jiangsu Road, Qingdao, 266003, People’s Republic of China, Email [email protected]
Abstract: In recent years, the abnormal glucose metabolism of tumor cells has attracted increasing attention. Abnormal glucose metabolism is closely related to the occurrence and development of tumors. Monocarboxylate transporters (MCTs) transport the sugar metabolites lactic acid and pyruvate, which affect glucose metabolism and tumor progression in a variety of ways. Thus, research has recently focused on MCTs and their potential functions in cancer. The MCT superfamily consists of 14 members. MCT1 and MCT4 play a crucial role in the maintenance of intracellular pH in tumor cells by transporting monocarboxylic acids (such as lactate, pyruvate and butyrate). MCT1 and MCT4 are highly expressed in a variety of tumor cells and are involved the proliferation, invasion and migration of tumor cells, which are closely related to the prognosis of cancer. Because of their important functions in tumor cells, MCT1 and MCT4 have become potential targets for cancer treatment. In this review, we focus on the structure, function and regulation of MCT1 and MCT4 and discuss the developed inhibitors of MCT1 and MCT4 to provide more comprehensive information that might aid in the development of strategies targeting MCTs in cancer.
Keywords: monocarboxylate transporter, tumor, tumor microenvironment, function, regulation
Introduction
The monocarboxylate transporter (MCT) superfamily contains 14 members and is encoded by the solute carrier family 16 (SLC16A) gene family. The MCT proteins show sequence homology. Thus far, only the functions of MCT1–4 have been elucidated. These proteins mediate the bidirectional transport of monocarboxylic acids (such as lactate, pyruvate, ketone bodies and gamma-hydroxybutyrate) across the plasma membrane.1 A recent report also indicated that MCTs mediate succinate export in the retina.2 The concentration of H+ and monocarboxylic acid on both sides of the cell membrane determines the direction of transport.3 Some evidence has shown that MCTs facilitate the plasma membrane transport of certain drugs, such as salicylates and valproic acid.4 The transmembrane movement of monocarboxylic acids is essential for cells that rely on glycolysis for energy.5,6 Under physiological conditions, MCT1 and MCT4 cooperate to form a lactate shuttle system that maintains lactate homeostasis between glycolytic and oxidative cell.7 Lactate is an important metabolite in human health and disease.8
MCT1 (which is encoded by SLC16A1) is found in most tissues. MCT4 (SLC16A3) is strongly overexpressed in highly glycolytic and anaerobic tissues.9 MCT1 and MCT2 have very high affinity for pyruvate (Km ≈0.1–0.74 mmol/L) and stereoselective L-lactate (Km ≈1–3.5 mmol/L), and MCT4 has low affinity for pyruvate (Km ≈153 mmol/L) and lactate (Km ≈28 mmol/L).3,10–12 Consistent with their high affinity for pyruvate and lactate, MCT1 and MCT2 are mainly expressed in red skeletal muscle, heart and neurons, where they transport lactate into cells to provide fuel for oxidative phosphorylation.13 MCT3 is expressed only on the basolateral membrane of the choroid plexus and retinal pigment epithelium, with a moderate affinity for lactate (Km ≈6 mmol/L), and MCT4 is mainly expressed in highly glycolytic cells, such as white skeletal muscle fibers and astrocytes.14
Metabolic abnormalities are one of most important hallmarks of cancer. In cancer cells, glycolysis is amplified to meet the energy demands of rapid proliferation.15 Lactic acid produced by glycolysis must be transported to the extracellular environment; therefore, cancer cells require high levels of MCTs to maintain this metabolic phenotype.16,17 MCT1 and MCT4 play important roles in cancer metabolism and promote cancer development through multiple mechanisms.18 Some cancer cells still undergo glycolysis under aerobic conditions to produce energy to meet the high metabolic demand. This metabolic mode of aerobic glycolysis in cancer cells is called the “Warburg effect”.19 Lactic acid and other products generated by glycolysis require MCT-mediated transport to the outside of the cell to maintain intracellular pH homeostasis. The transported lactate also leads to a decrease in the pH of the microenvironment. In tumor tissue, the acidification of extracellular microenvironment caused by co-transporting protons is conducive to angiogenesis, tumor cell proliferation, invasion and metastasis.19 Therefore, targeting MCTs may represent a possible treatment strategy for cancer.
Structure and Function of MCT1 and MCT4
Structure
The crystal structures of MCTs have not been fully described. However, topological prediction indicates that all family members have 12 transmembrane helices (TMs), a C-terminus and N-terminus within the cell, and a large cytoplasmic loop between TM6 and TM7. In the MCT superfamily members, the TM regions are more conserved than the loop and C-terminus.11 Lysine 38, aspartic acid 302, and arginine 306 are particularly important for substrate binding and transporter activity, as shown by human MCT1 modeling, and MCT1 and MCT 4 have been confirmed as proton-linked MCTs.11 In the structure of hMCT1 and hMCT4, seven key residues are conserved and involved in the translocation cycle of L-lactate.20 Conserved helix 5 is essential for transport function and is involved in stereoselectivity.21 The loop between TM6 and TM7 contributes to the substrate affinity of hMCT.22 In MCT1, TM6 binds to basigin (an immune protein) mainly through hydrophobic interaction.7 An arginine residue in TM8 is conserved in most SLC16 family members due to its involvement in carboxyl binding of the carboxyl group of monocarboxylate esters.23,24 D302 and R306 on the inner surface of TM8 accept protons and lactate, respectively.25 F360 in helix 10 and R306 in TM8 play a key role in substrate selectivity by acting as steric hindrance protrusion channels, and residues around R306 are not always conserved, suggesting that these residues may be involved in substrate recognition.24,26 R278 in TM8 of hMCT4 is also a key residue for substrate recognition (Figure 1).27
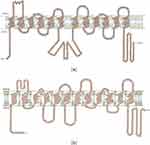 |
Figure 1 Prediction model of hMCT1 (a) and hMCT4 (b). Notes: Adapted from Sasaki S, Kobayashi M, Futagi Y, et al. Crucial residue involved in L-lactate recognition by human monocarboxylate transporter 4 (hMCT4). PLoS One. 2013;8(7):e67690. Creative Commons.28 |
After synthesis, MCTs need to be transported to the plasma membrane to function. The correct localization and function of MCT1 and MCT4 require the assistance of glycosylation chaperones.
CD147/basigin, a member of the immunoglobulin superfamily, is involved in immune response and immunosuppression.29 CD147/basigin is located on the cell surface and consists of an extracellular Ig-like domain, a single transmembrane fragment and a short intracellular cytoplasmic tail; it is widely distributed in vivo and promotes tumor migration by inducing matrix metalloproteinases (MMPs).30,31 CD147/basigin also regulates the expression of vascular endothelial growth factor (VEGF) and metalloproteinases in xenograft tumors and stimulates tumor angiogenic potential and growth rate.29
There are four isoforms of basigin (BSG) proteins, of which the BSG1 isoform is located in the retina and closely interacts with MCT3.32,33 BSG2 (hereafter, BSG), the most prevalent and studied isoform, contains two immunoglobulin-like domains that are chaperones of MCT1 and MCT4 and help them to fold properly. BSG thus maintains their stability, promotes their cellular expression and mediates proper localization on the cell membrane surface.31,34 Le Floch et al found that the expression of MCT1 and MCT4 was significantly reduced in cells under normoxia and hypoxia after knockdown of the CD147/BSG gene in colon adenocarcinoma cells.35 Thus, the interaction of MCT1 and MCT4 with BSG in the plasma membrane is important for their activity.36,37 In patients with bladder urothelial carcinoma, high expression of MCT1 and CD147 is associated with poor overall survival, while high expression of MCT4 is associated with poor recurrence-free survival; high expression of MCT1 and MCT4 is an independent prognostic indicator for poor overall survival and recurrence-free survival, respectively.38 One study showed that CD147 is upregulated in bladder tumor tissues and is significantly associated with tumor invasiveness and poor prognosis.39 Several studies have identified CD147 expression as an independent prognostic factor in bladder urothelial carcinoma.40,41 CD147 and MCT1 have also been associated with drug resistance of tumor cells. Afonso et al found that CD147 and MCT1 were associated with bladder cancer progression and resistance to cisplatin-based chemotherapy, revealing that targeting CD147 and MCT1 is helpful in treating cisplatin-resistant bladder cancer.39
Function
Lactic acid is mainly produced from glucose through a reaction catalyzed by a series of glycolytic enzymes. After glucose uptake by cells via glucose transporters, pyruvate is generated in the cytoplasm by the following steps: 1) glucose, catalyzed by hexokinase (HK), consumes one molecule of ATP to produce one molecule of glucose 6-phosphate (G-6-P); 2) G-6-P is converted to fructose-6-phosphate (F-6-P) catalyzed by phosphate hexose isomerase (PGI); 3) F-6-P, catalyzed by 6-phosphofructokinase 1 (PFK1), consumes one molecule of ATP to produce fructose-1,6-diphosphate (F-1,6-BP); 4) F-1,6-BP, catalyzed by aldolase (ALDO), produces glyceraldehyde 3-phosphate (G-3-P) and dihydroxyacetone phosphate (DHAP), which are isomers of each other; 5) G-3-P dehydrogenation is catalyzed by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) to 1,3-diphosphoglycerate (1,3-PGA); 6) 1,3-PGA is catalyzed by 3-phosphoglycerate kinase (PGK) to produce 3-phosphoglycerate (3-PG) and a molecule of ATP is produced; 7) 3-PG is converted to 2-phosphoglycerate (2-PG) under the catalysis of enolase, yielding phosphoenolpyruvate (PEP); and 8) PEP forms pyruvate under the catalysis of pyruvate kinase (PKM). Through this series of reactions, one molecule of glucose is converted to two molecules of pyruvate. In the case of sufficient oxygen supply, pyruvate enters the mitochondria to produce a large amount of energy through the tricarboxylic acid cycle (TCA). In the case of insufficient oxygen, pyruvate generates lactate under the catalysis of lactate dehydrogenase A. In normal tissue cells, such as red skeletal muscle, heart and neurons, MCT1 transports lactate into cells, where it is converted by lactate dehydrogenase B (LDHB) to pyruvate, which is supplied by oxidation through the TCA. In white skeletal muscle fibers and astrocytes, MCT4 transports lactate production out of cells and maintain intracellular acid-base balance (Figure 2).
Malignant tumors are characterized by heterogeneity; they contain both aerobic regions close to blood vessels and hypoxic regions far from blood vessels. Cancer cells in hypoxic areas obtain energy through glycolysis, which produces less energy; thus, cancer cells in hypoxic areas need more glucose to produce energy, which is called “glucose starvation”.42 Under hypoxic conditions, hypoxia-inducible factor-1 (HIF-1) is overexpressed, and HIF-1 induces the expression of glucose transporter 1 (GLUT1). GLUT1 is responsible for glucose uptake, and hypoxic cancer cells take up a large amount of glucose through overexpressed GLUT1. Glucose generates a large amount of energy to meet the needs of cell metabolism through the glycolytic pathway and simultaneously produce pyruvate and H+. Pyruvate is converted to lactate by LDHA, which leads to a decrease in intracellular pH.43 MCTs transport the produced lactate and pyruvate out of the cell and play an important role in the lactate shuttle between hypoxic and aerobic cancer cells.44 Similarly, oxidized cancer cells obtain an additional supply of lactate through MCT1/4-mediated lactate shuttle by promoting aerobic glycolysis in adjacent stromal fibroblasts.45,46 MCT4, which has low affinity for lactic acid, transports lactic acid produced by hypoxic cancer cells through glycolysis outside of cells. MCT1, which has a high affinity for lactic acid, can transport lactic acid to normoxic cancer cells. Lactate entering cancer cells is converted to pyruvate by lactate dehydrogenase catalyzed B (LDHB). After then, pyruvate enters the TCA as the substrate for cancer cell oxidation power (Figure 3). If lactate is the only energy source for tumor cells, inhibition of MCT1 leads to cell death.42,47 For aerobic cells, oxidative lactate metabolism may be more favorable than aerobic glycolysis because it produces ATP at most 7.5-fold.18 At the same time, aerobic cancer cells use lactate for energy, so more glucose can enter hypoxic cancer cells to produce energy to meet proliferation and metabolism needs. Studies have shown that glucose transporters and downstream glycolytic enzymes are overexpressed in more than 70% of cancers.48,49
Stromal cells in contact with tumor cells undergo metabolic reprogramming and transform into cancer-associated fibroblasts (CAFs). The lactic acid produced by CAFs through aerobic glycolysis can also be transported to the extracellular through MCT4, and lactic acid is taken up by aerobic cancer cells for oxidation energy.50 Additionally, the lactate shuttle between glycolytic cancer cells and vascular endothelial cells is also through MCTs.51 Lactate produced by tumor cells through high glycolysis is exported through MCT4, and vascular endothelial cells transport lactate into cells through MCT1.52,53 Therefore, MCTs play a crucial role in lactate transport between tumor cells, between tumor cells and stromal cells as well as between tumor cells and vascular endothelial cells (Figure 3). Within the same tumor cell, MCT1 and MCT4 are expressed in different amounts. Sonveaux et al found that aerobic cancer cells in human neck and colon xenograft tissues expressed high levels of MCT1 compared with hypoxic regions that showed almost no detectable MCT1 expression, but this phenomenon was not observed in other tumor types.47 MCT4 expression is increased in hypoxic and poorly angiogenic tumor regions.54,55 Another study found that MCT4 is associated with the sarcolemma of fast twitch fibers in muscle.56 In addition to the ability of MCT1 to transport lactate into cells, MCT1 also releases lactate into the medium.57 Mathupala et al showed that targeted inhibition of MCT1 and MCT2 expression significantly reduced lactate efflux from glioblastoma multiform cells.58 Thus, MCT1 can transport lactate in both directions. MCT1 and 4 also have a certain ability to transport ketone bodies, and the affinity of MCT1 for ketone bodies is higher than that of MCT4. Therefore, MCT1 participates in the development of tumors by mediating the transport of ketone bodies.1 These suggest that MCTs may be a potential target for anti-cancer therapy by knocking down MCTs (by inhibitors or siRNA) or by combining MCT knockdown with chemotherapy or radiotherapy for anti-tumor treatment.59
Lactic acid produced by tumor cells is not only taken up by cells for oxidative energy but it also inhibits the killing effect of immune cells on tumor cells, giving cancer cells immune tolerance.60,61 Lactate produced by tumors is internalized by cytotoxic T cells (CTLs) and inhibits cytotoxic T cell proliferation and anticancer function by inhibiting p38 and JNK/c-Jun activation.62 Lactate polarizes macrophages to promote the M2 phenotype of tumors and exerts an immunosuppressive function by activating G protein-coupled receptor 132 (GPR132). Lactate also inhibits the antitumor immune response of dendritic cells (DCs) by inhibiting the differentiation of monocytes into DCs.62 One study showed that the elevated lactate level in the tumor microenvironment is a by-product of glycolysis in breast cancer that both weakens the anticancer immune response in a concentration-dependent manner and plays a key role in the regulation of the tumor immune microenvironment.63 Another study reported that the down-regulation of MCT4 promoted the cytotoxicity of NK cells in breast cancer, indicating that MCT4 is involved in the suppression of the tumor immune microenvironment.64 Sun et al found that MCT4 may act as an immune suppressor by reducing macrophage maturation or perturbing T cell metabolism.65
Role of MCTs in the Proliferation, Migration and Progression of Tumor Cells
MCTs play an important role in tumor cell proliferation, invasion and migration and tumor progression. MCT4, together with proton cotransporter, transports lactate produced by cancer cells through glycolysis out of the cell and transports lactate into vascular endothelial cells through MCT1. Lactate and pyruvate stimulate the expression of HIF-1.66,67 HIF-1 further promotes the expression of vascular endothelial growth factor (VEGF), which promotes the generation of blood vessels, providing more nutrients for tumor cells.68 Gloire et al found that lactic acid promotes the phosphorylation and degradation of IkBα in endothelial cells, and then promotes the activation and transfer of free NF-κB to the nucleus for transcriptional regulation to promote angiogenesis.69 Other studies have shown that lactate promotes the secretion of IL-8 in endothelial cells, which in turn promotes the expression of NF-κB and angiogenesis. Inhibition of MCT1 inhibits the formation of the tumor microvascular network and tumor proliferation.70 Zhao et al found that MCT1 regulates tumor growth and metastasis by regulating the NF-κB pathway, and inhibition of MCT1 reduced the growth and metastasis of osteosarcoma and improved the effect of chemotherapy in osteosarcoma.71 Additionally, lactate exported by MCT4 is the fuel for cancer cell proliferation, and cancer cells transport lactate into cells through overexpression of MCT1.47 Hong et al showed that inhibition of MCT1 prevents breast cancer cell proliferation by preventing pyruvate transport to the outside of cells; however, it did not increase cell apoptosis.72 A previous study showed that silencing of MCT1 and MCT2 inhibits tumor growth and leads to apoptosis and necrosis of tumor cells.58 Pertega et al reported that both MCT1 and MCT4 expression were increased and positively correlated with prostate cancer progression.73
CAFs are important components affecting cancer cell invasion and metastasis. Giannoni et al found that CAFs trigger a pro-oxidative environment in cancer cells, profoundly affecting tumor progression and metastatic spread.74 In another study by the same group, CAFs were found to promote epithelial-mesenchymal transition in human prostate cancer cells and promote tumor growth, increase the development of stem cell markers and induce spontaneous metastasis.75 Studies have shown that MCT4 is involved in the metabolic reprogramming process of prostate cancer cells and CAFs, and a higher expression of MCT4 in prostate cancer cells corresponds with a worse clinical outcome.76 Other studies reported that CAFs interact with bladder cancer cells to promote tumor progression, and the expression of MCT1 and MCT4 is up-regulated in fibroblasts.77 Therefore, MCTs may affect cancer invasion and metastasis by affecting the metabolic reprogramming process of cancer cells and CAFs. MCT4 also discharges lactate produced by CAFs from cells and transports it into cancer cells through MCT1 to provide energy for cancer cell proliferation (Figure 3). Zhang et al found that inhibition of MCT1 reduced bladder cancer cell proliferation, migration and invasion.78 Another study found that inhibition of MCT4 by siRNA significantly inhibited the trans-pore migration of MDA-MB-231 cells by 85%.79 This suggests that MCTs can enhance the ability of cancer cells to migrate and invade not only by affecting stromal cells in the tumor microenvironment but also by directly influencing the tumor cells.
Under hypoxic conditions, tumor cells secrete a variety of stimuli to recruit macrophages to the hypoxic site.80 A large amount of lactic acid produced by glycolysis of tumor cells is absorbed into cells by macrophages through MCT1. Some reports showed that lactate entering macrophages activates GPR132 to induce M2 polarization of macrophages by increasing the expression of Arg1 and Mrc1. M2 macrophages can enhance the adhesion, migration and invasion of cancer cells.81,82
Regulation of MCT1 and MCT4
The expression levels of MCTs vary among different tissues. MCT levels are regulated at both pre- and post-transcriptional levels (Figure 4).83
HIF-1
Tumor cells are exposed to hypoxia, which induces the expression of hypoxia-inducible factor (HIF-1), a transcription factor that initiates a series of responses, including angiogenesis and various pro-survival mechanisms.84 HIF-1 is stably expressed in a hypoxic environment and upregulates the activity of GLUTs, glycolysis-related enzymes and other hypoxia-related genes, reducing the dependence of cells on the oxidative pathway and transferring cell metabolism to the glycolytic pathway.85 Elevated HIF-1 in rapidly growing cells, such as embryos and tumors, not only stimulates glycolysis but also reduces pyruvate flow to the TCA by inhibiting LDH in mitochondria.86,87 This HIF-1-mediated inhibition of LDH reprogramming in glucose metabolism is the main basis of the “Warburg effect”.13
In the hypoxic environment, HIF-1 binds to two hypoxia response elements upstream of the transcription start site to up-regulate the expression of MCT4. The MCT1 promoter lacks the hypoxia response elements that are bound by HIF-1, so HIF-1 does not induce the increase of MCT1 expression.88 Previous studies have shown that HIF-1 and specific protein 1 (SP1) jointly mediate the expression of CD147 in the hypoxic microenvironment of epithelial solid tumors.89 This up-regulation of CD147 expression indirectly plays a role in the regulation of MCT. Additionally, several investigators have shown that hypoxia does not lead to upregulation of MCT1 and MCT4 in MCF-7 cells, but it does lead to upregulation of MCT1-associated carbonic anhydrase IX (CAIX), which promotes the rapid secretion of lactate and H+ from breast cancer cells in uncatalyzed reactions.90
p53
The p53 tumor suppressor has been the subject of intensive investigation. The occurrence of cancer is closely related to mutation of the p53 gene, and approximately 50% of human cancers lose p53 function because of gene mutation.91 p53 regulates the expression of genes related to cell cycle progression, apoptosis, cell differentiation and senescence.92 p53 inhibits cell proliferation and mediates homeostatic regulation of immunity and inflammation, homeostatic regulation of cell competition and homeostatic regulation of stem cell self-renewal and differentiation.91 During stress, this activates p53 transcriptional activity and promotes senescence or apoptosis of damaged cells, contributing to the protection of tissue integrity under severe stress conditions.91 p53 is also involved in the regulation of metabolic pathways in tumor cells, and loss of p53 stimulates glycolysis and impairs the mitochondrial respiratory chain, thereby promoting the switch of ATP production from oxidative phosphorylation to glycolysis.93 Boidot et al found that p53 deficiency promoted the expression of MCT1 under hypoxic conditions, thereby promoting lactate release from elevated glycolytic flux.94 Under hypoxic conditions, loss of p53 is associated with the stabilization of MCT1 mRNA, and p53 inhibits MCT1 expression. NF-κB plays a crucial role in the hypoxia-induced increase of MCT1 expression in p53-deficient cells.
Myc
The Myc oncogene is activated in many malignant tumors and closely associated with tumorigenesis. Approximately 100,000 cancer-related deaths each year are associated with dysregulated Myc expression.95 Upregulation of Myc expression is observed in 50% to 60% of tumors and directly contributes to malignant transformation through its pathogenic role in tumorigenesis, development and maintenance.96 Myc transforms normal cells into tumor cells by increasing the transcription level of high-affinity target genes and even saturating them. Myc also up-and down-regulates low-affinity target genes.97 Myc oncoproteins belong to a family of so-called “hypertranscription factors” that may regulate at least 15% of genetic transcription across the genome, including multiple targets involved in cell growth, metabolism and division.98,99 Prominent targets of Myc regulation include genes that encode enzymes that drive aerobic glycolysis, a hallmark of most tumors.100,101
The MCT1 promoter region has a typical Myc binding site, and Myc regulates the expression of MCT1 through this site.102 MCT1 expression is increased in MCF10 breast epithelial cells expressing Myc and in certain tumors.103 Other studies have shown that c-Myc regulates MCT1 expression by regulating the activity of the MCT1 promoter.95 Myc also transcriptionally represses miR29A and miR29c, resulting in enhanced expression of MCT1.104
Butyrate
Butyrate, a short-chain fatty acid (SCFA), is produced by bacterial fermentation of undigested carbohydrates in the colon and is the main fuel for colon cells. In the gut, SCFAs are transported across the cell membrane by pH-dependent, H+-coupled MCT and sodium-coupled monocyclic acid transporter (SMCT).105 The main consequence of reduced intracellular SCFA oxidation is metabolic starvation and mucosal atrophy.106 After entering cells, butyrate is metabolized to provide energy for cells and also has a regulatory effect on gene expression. Butyrate regulates gene expression at several levels, including through transcription, mRNA stability and elongation mechanisms.107
One study showed that butyrate upregulates MCT1 expression and activity through transcriptional and post-transcriptional mechanisms.108 Borthakur et al demonstrated that butyrate upregulates MCT1 promoter activity, thereby promoting the expression of MCT1, and the authors showed that this regulatory process involves the NF-κB pathway.109 In another study by the same group, butyrate and niacin were shown to reduce intracellular cAMP levels, which in turn promoted MCT1 expression.110
G-Protein-Coupled Receptors (GPRs)
G protein-coupled receptor 81 (GPR81), also known as hydroxycarboxylate receptor 1 (HCAR1), is a lactate receptor that is highly expressed in adipocytes and present at low levels in a variety of normal cells.111–113 GPR81 is also expressed in a variety of cancer cells, such as colon, breast, lung, hepatocellular carcinoma, cervical cancer and salivary gland cancer cells.114 GPR81 has multiple functions in vivo, including in cell development and survival, lipid metabolism, lactate transport, promotion of tumor angiogenesis, tumor growth and metastasis.114–117 Roland et al showed that GPR81 regulates lactate uptake and MCT1 gene expression, and silencing GPR81 reduces tumor cell activity and tumor cell proliferation when lactate is the only available energy source.114
GPR109A, a nicotinic acid receptor, is highly expressed in neutrophils, macrophages, monocytes, dendritic cells, skin, liver cells, retinal cells, bone and adipocytes.118,119 GPR109A is activated by monomethyl fumarate (MMF) and niacin (niacin or vitamin B3).120 Promoter methylation inhibits GPR109A expression in human colon cancer cells, and IFN-γ reverses the DNA methylation-mediated silencing of GPR109A.121 Butyrate and niacin activate the expression of GPR109A and promote the expression of MCT1 in colon cancer cells. In cells silenced for GPR1089A with siRNA, butyrate showed no effect on the expression of MCT1.110
Metastasis-Associated in Colon Cancer-1 (MACC1)
MACC1 is an oncoprotein that regulates the hepatocyte growth factor/methionine kinase receptor epidermal growth factor (HGF/c-Met) pathway, which promotes carcinogenesis and tumor progression by promoting the migration and invasion of cancer cells and inhibiting the apoptosis of cancer cells.122 MACC1 plays a key role and functions as a biomarker for the progression and metastasis of a variety of solid tumors, such as gastrointestinal tumors and gynecological tumors. MACC1 has been demonstrated as a clinically useful prognostic and predictive biomarker in multiple types of solid tumors.123 MACC1 plays an important role in tumor cells and tumor microenvironment to promote glucose metabolism of tumor cells and provide energy for cells. MACC1 regulates the sensitivity of tumor cells to chemotherapeutic drugs.
Wang et al found that MACC1 regulates the expression of MCT1 in gastric cancer. The overexpression of MACC1 increased MCT1 protein expression in the gastric cancer cell line MKN45, while its silencing decreased MCT1 expression.124 Furthermore, increasing MCT1 expression in gastric cancer cells enhanced chemosensitivity to 5-FU and cisplatin. However, which pathway MACC1 regulates MCT1 expression is still unclear.
Phorbol 12-Myristate 13-Acetate (PMA) and Luminal Leptin
PMA, a protein kinase C agonist, significantly increased apical butyrate uptake and MCT1 protein expression in Caco-2 cells.125 Intestinal MCT1 gene expression is regulated by PMA at the transcriptional level, which is dependent on PKC activation, and the up-regulation of MCT1 gene expression by PMA involves atypical PKC-ζ isoforms and the activator protein 2 (AP2) transcription factor.126
Leptin is a hormone with multiple functions that is synthesized in different peripheral tissues, such as stomach, salivary glands, placenta and kidney.127 Leptin is associated with food intake and energy expenditure.128 Studies have shown that luminal leptin can regulate intestinal butyrate uptake by increasing cellular MCT1 mRNA expression.129 The pathway by which leptin regulates MCT1 expression requires further investigation.
Promoter Methylation
DNA methylation is a key epigenetic process that involves the addition of methyl groups to a cytosine adjacent to a guanidine, a CpG dinucleotide.130 Alterations in DNA methylation status within genes can lead to differences in gene transcription and mRNA expression levels without changing the nucleotide sequence. While hypermethylation is associated with gene silencing, hypomethylation is associated with activating gene expression.131 Many MCTs have been reported to contain CpG islands in their promoter sequences. In renal clear cell carcinoma, MCT4 is regulated by promoter methylation; the degree of promoter methylation is decreased and MCT4 mRNA expression is significantly increased in renal clear cell carcinoma tissues compared with adjacent non-tumor tissues.132 Asada et al found that the MDA-MB-231 human breast cancer cell line treated with 5-aza-2′-deoxycytidine, a demethylating agent, restored MCT1 mRNA expression, suggesting that MCT1 is similarly regulated by promoter methylation.133
Post-Transcriptional Regulation via miRNAs
MCTs are regulated not only before transcription, but also after transcription. Increasing studies have shown that MCTs are post-transcriptionally regulated by miRNAs, which bind to MCT mRNAs, leading to the degradation of mRNA. Ng et al found that miR-124 bound to the 3′-untranslated region of MCT1 mRNA in medulloblastoma, resulting in decreased MCT1 expression, suggesting that miR-124 is a negative regulator of MCT1.134 Liang et al demonstrated that miR-495 binds to the 3′ untranslated region of human MCT1 and down-regulates MCT1 mRNA and protein expression in HeLa cells.135 Additionally, miR-342-3p directly targets MCT1 in triple-negative breast cancer, and loss of miR-342-3p leads to MCT1 overexpression and metabolic reprogramming.136
While several studies have evaluated the regulation of MCT1 by miRNA, few reports are available on miRNA-mediated regulation of MCT4. Xu et al found that miR-1 mimics and inhibitors down-regulate the mRNA and protein expression of MCT4 and this miRNA may indirectly regulate the expression of MCT4 by regulating HIF-1.137 In hepatocellular carcinoma, miR-145 inhibited lactate efflux by targeting MCT4, thereby reducing the intracellular pH of tumor cells; the therapeutic effect of miR-148 was demonstrated in a HepG2 tumor-bearing mouse model.138 In diabetic vascular complications, miR-425-5p down-regulates the expression of MCT4 In vascular endothelial cells, which becomes a target for the treatment of diabetic complications.139
In addition to the mechanisms described above, there are a number of other regulatory modalities. Fan et al found that autophagy promotes MCT1 expression and induces glycolysis in liver cancer cells by activating Wnt/β-catenin signaling to promote transcription.140 In addition, and Sprowl-Tanio et al found that MCT1 is also a direct Wnt target gene in colon cancer cell lines.141 Transcription factors upstream stimulators 1 and 2 (urf1/2) suppress MCT1 expression in the human gut by inhibiting the transcription of MCT1.142 In colon cancer cells and stromal cells, the antioxidant transcription factor nuclear factor E2-related factor-2 (Nrf2) promotes metabolic symbiosis between colorectal cancer cells and stromal cells by regulating MCT1 and MCT4.143 In L6 cells, Hashimoto et al found that lactic acid stimulates the generation of reactive oxygen species, which activates the NF-κB and Nrf2 pathways and in turn leads to MCT1 expression.144 The authors also showed through microarray analysis that AP-1 may be involved in the regulation of MCT1. Increasing carbonic anhydrase affects MCT activity on the plasma membrane and regulates MCT. Carbonic anhydrase II (CAII) enhances the transport activity of MCT1.145 The activities of MCT1 and MCT4 were enhanced by carbonic anhydrases IV (CAIV) and IX (CAIX).146,147 Aspatwar et al found that co-expression of catalytically inactive carbonic anhydrase–related proteins VIII, X and XI in Xenopus oocytes enhanced the transporter activity of MCT1.148 Some studies have indicated the regulation of MCT4 by AMPK, PKC, FBI-1 and interleukin 1β.149–153
The adjustment of MCTs is summarized in Table 1.
 |
Table 1 Summary of MCTs Regulation |
Inhibitors of MCTs
MCTs transport monocarboxylic acids, especially lactic acid, provide energy for tumor cells, promote the proliferation, invasion and migration of tumor cells and are closely related to the degree of malignancy in a variety of tumors. In solid tumor cells, such as in non-small cell lung cancer, increased MCT expression indicates poor prognosis and high expression of MCT4 predicts poor prognosis.154 MCT1 is an independent prognostic marker for survival in non-small cell lung cancer.155 Drug resistance of tumor cells is also related to the up-regulation of MCT expression. Therefore, inhibition of MCT may be a therapeutic strategy for a variety of tumors. For example, inhibition of MCT1 has an antimetabolic effect on oxidized tumor cells, while targeting MCT1 in endothelial cells has a strong antiangiogenic effect.51 Aerobic cancer cells mainly transport lactate produced by hypoxic cancer cells into cells through MCT1 to generate energy for cell metabolism. After MCT1 inhibition, aerobic cancer cells switch from lactate metabolism to glucose metabolism to generate energy, forcing aerobic cells to take up more glucose from nearby blood vessels. Because of the high glucose consumption of aerobic cells, hypoxic cells are deprived of glucose, which is the only energy source for these cells, leading to glucose starvation and subsequent apoptosis induction in hypoxic cells, indirectly inhibiting hypoxic cancer cell survival.42 After MCT4 inhibition, hypoxic cancer cells are unable to transport lactate out of the cell, leading to cytosolic acidification, which in turn leads to cell death. Therefore, MCT inhibitors may play a crucial role in the treatment of a variety of cancer cells. Compared with inhibitors against MCT1, MCT4 inhibitors are relatively rare.
AZD3965 and AR-C155858
AZD3965, an analogue of AR-C155858, is a pyrrolidine derivative that is a first-rate potent inhibitor of MCT1 with high affinity for MCT1. AZD3965 has been shown to have promising tumor inhibitory effects in a variety of preclinical xenograft tumor models.156–159 Through in vitro cell experiments, AZD3965 was demonstrated to inhibit cell proliferation.160 Upon co-expression of MCT1 and MCT4, tumor cells showed relative tolerance to AZD3965 single-agent activity, suggesting that MCT4 may continue to drive lactate transport even in the presence of AZD3955.104 Clinical evaluation of AZD3965 is also ongoing.161 AZD3965 not only targets MCT1-mediated lactate transport, but it has also been reported to be involved in enhancing pyruvate/mitochondrial metabolism.72,162 Moreover, mitochondrial complex I inhibitor, metformin and mitochondrial pyruvate carrier inhibitor UK5099 increased the sensitivity to AZD3965.162
AR-C155858, a potent MCT1 inhibitor, does not inhibit MCT4. AR-C155858 exerts its inhibitory effect by binding to the TM7–10 of MCT1.163 AR-C155858 selectively blocks MCT1 and MCT2 activities in activated T lymphocytes and prevents lactate efflux.57 AR-C155858 inhibits the lactate efflux of HL60 cells, a highly glycolytic leukemia cell line expressing MCT1 but not MCT4.164 Furthermore, similar to AZD3965, AR-C155858 shows limited activity in tumor cells with MCT4 expression; in tumor cells expressing both MCT1 and MCT4, MCT4 was able to promote lactate efflux when MCT1 was blocked.35
Coumarin Carboxylic Acids
Coumarin is an important structural unit found in many important drug molecules with favorable pharmaceutical properties.165 A variety of derivatives of coumarin have MCT inhibitory effects. Draoui et al reported that the 7-alkyl amino substituent on the 3-carboxycoumarin scaffold was required for significant inhibition of MCT.166 Tateishi et al showed that 7-amino-carboxycoumarin derivatives bind MCT1 in astrocytes, and targeted imaging of MCT1 by labeled 7-amino-carboxycoumarin derivatives may help further study the pathological mechanism of central nervous system diseases.167 Furthermore, 7-aminocarboxycoumarin derivatives caused significant tumor growth retardation by inhibiting lactate influx in cervical cancer SiHa xenografts and HCT-116 cell transplantation models.164
Bay-8002
BAY-8002 with a 5-(phenylsulfonyl benzamide) benzoic acid scaffold is an effective and specific oral hMCT1 inhibitor. BAY-8002 inhibits the proliferation of lymphoma cells and has no inhibitory effect in MCT4-expressing oocytes. BAY-8002 and AZD3965 have the same or overlapping binding sites on MCT1.168
Inhibitors of MCT4
So far, only two MCT4-specific inhibitors, VB124 and Bindarit, have been reported. VB124 selectively inhibits MCT4 and has a very low inhibitory effect on MCT1. Cluntun et al found that VB124 specifically inhibits MCT4 and inhibits lactate efflux.169 VB124 inhibits MCT4 in mouse H9c2 cells to prevent and reverse cardiac hypertrophy. In an in vitro HCC model, VB124 inhibited MCT4, resulting in less lactate output, which enhanced the killing effect of immune cells on HCC cells.170
Bindarit, an anti-inflammatory agent that inhibits the production of inflammatory cytokines, is a highly selective and non-competitive hMCT4 inhibitor that selectively inhibits hMCT4 more than hMCT1 in Xenopus laevis oocytes.171,172
Nonselective Inhibitors of MCTs
α-Cyano-4-Hydroxycinnamate and Analogs
The MCT1 inhibitor α-cyano-4-hydroxycinnamate (CHC), a cyanoacetic acid, has been extensively studied for its inhibitory effect on MCT1 and MCT4. In vitro cell experiments and xenograft models showed that CHC inhibited MCT1 to reduce tumor size, sensitize hypoxic tumor regions to radiotherapy and induce cell death when lactate was the only energy source.47 Colen et al used CHC to inhibit lactate efflux in glioblastoma tumors, affecting tumor invasion and proliferation capacity.173 CHC also inhibits MCT in the mitochondrial membrane, which reduces the flow of pyruvate to mitochondria and inhibits cell proliferation.174,175 Other studies have shown that CHC affects DNA repair by inhibiting MCT to limit lactate flux.176
α-Cyano-4-hydroxy-3-methoxycinnamic acid (ACCA) is a derivative of CHC that inhibits MCTs and has a high affinity for MCT1.11 Hamdan et al demonstrated that ACCA selectively inhibited the growth of breast cancer cells in vitro by inhibiting MCT1.177
Jonnalagadda et al recently reported that N,N-dialkyl cyanocinnamic acids not only showed high potency against MCT1 but also showed excellent MCT4 inhibition.178 In vivo xenograft model studies revealed that N,N-dialkyl cyanocinnamic acids exhibit excellent efficacy and selectivity in MCT1-expressing tumors.165
Isobutyrate Derivatives
Fibrate lipid-lowering agents, such as bezafibrate, fenofibrate anion (the active metabolite of fenofibrate) and clinofibrate, contain butyrate, which has an isobutyrate moiety, and exhibit inhibitory effects on both MCT1 and MCT4.171,179 The inhibitory potency of clinofibrate on hMCT4 was similar to that of bezafibrate and fenofibrate anions; its inhibitory potency on hMCT1 was slightly higher than that of other fibrates.171
A previous study showed that 1-benzyl-1-hindazole-3-carboxylate, a derivative of benzylindazole and dechlorinated form of clonamide, slightly inhibits hMCT1 and hMCT4. Bendazak, a non-steroidal anti-inflammatory drug, showed a slight inhibitory effect on hMCT1 and hMCT4.180
Other Inhibitors
Some early MCT inhibitors, such as phloretin, quercetin and 4.4′-di-iso-thiocyanostilbene-2,2′-disulfonate (DIDS), showed low affinity and poor specificity.7 Nancolas et al found that the antitumor drug ionidamine inhibited the ability of MCTs to transport lactate in Xenopus oocytes, but it showed nonselective inhibitory activity against transporters.181 Other inhibitors include the MCT1 inhibitor indolecyanoacrylate, pteridine dione and trione and the MCT4 inhibitor acriflavine (ACF).42,182,183 ACF disrupted the interaction of MCT4 with CD147 to inhibit MCT4 function in glioblastoma stem cells; ACF also significantly reduced angiogenesis and tumor progression under hypoxic conditions.184
Some inhibitors of MCTs are summarized in Table 2.
 |
Table 2 Summary of Inhibitors |
Conclusion
MCT1 and MCT4 are highly expressed in a variety of tumor cells; they play key roles in transporting monocarboxylate in the intracellular and extracellular environment and connect the lactate shuttle between tumor cells, tumor cells and non-tumor cells. MCT1 and MCT4 affect the glucose metabolism of tumor cells and play a crucial role in tumor cell proliferation, migration and invasion. MCT1 expression is closely related to the prognosis and drug resistance of tumors. Therefore, targeting MCTs has become a possible strategy for the treatment of cancer. In drug-resistant tumor cells, the combination of targeted MCT and chemotherapeutic drugs reversed the drug resistance of tumor cells. These findings suggest a potential new strategy for cancer treatment.
In recent years, increasing studies have been performed on drugs that inhibit MCTs, most of which are non-specific inhibitors. Considering the role of MCTs in tumor progression, research on specific MCT inhibitors is becoming important. Nano-drug delivery carriers have been recently developed and are widely used in pre-clinical trials because of their good biocompatibility and low toxicity. Nano-drug delivery vectors can carry not only genes but also drugs. A nano-drug delivery vector loaded with gene targeting MCTs may specifically and effectively inhibit MCTs, making this approach a potential method to inhibit MCT for cancer treatment.
The role of MCT1 and MCT4 in tumor cells goes far beyond these described above, and more studies are needed to explore other roles of MCTs in tumor cells. Inhibitors for MCTs also need to be continuously developed and explored in clinical trials.
Acknowledgments
We thank Gabrielle White Wolf, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript. This study was partly funded by the Natural Science Foundation of Shandong Province (ZR2021MH354), Medical and health research program of Qingdao (2021-WJZD170).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447(5):619–628. doi:10.1007/s00424-003-1067-2
2. Bisbach CM, Hass DT, Thomas ED, Cherry TJ, Hurley JB. Monocarboxylate Transporter 1 (MCT1) mediates succinate export in the retina. Invest Ophthalmol Vis Sci. 2022;63(4):1. doi:10.1167/iovs.63.4.1
3. Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol. 1993;264(4 Pt 1):C761–782. doi:10.1152/ajpcell.1993.264.4.C761
4. Hosoya K, Kondo T, Tomi M, Takanaga H, Ohtsuki S, Terasaki T. MCT1-mediated transport of L-lactic acid at the inner blood-retinal barrier: a possible route for delivery of monocarboxylic acid drugs to the retina. Pharm Res Dordr. 2001;18(12):1669–1676. doi:10.1023/A:1013310210710
5. Halestrap AP, Poole RC, Cranmer SL. Mechanisms and regulation of lactate, pyruvate and ketone body transport across the plasma membrane of mammalian cells and their metabolic consequences. Biochem Soc Trans. 1990;18(6):1132–1135. doi:10.1042/bst0181132
6. Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343(Pt 2):281–299. doi:10.1042/bj3430281
7. Wang N, Jiang X, Zhang S, et al. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell. 2021;184(2):370–383 e313. doi:10.1016/j.cell.2020.11.043
8. Adeva-Andany M, Lopez-Ojen M, Funcasta-Calderon R, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion. 2014;17:76–100. doi:10.1016/j.mito.2014.05.007
9. Halestrap AP. The SLC16 gene family - structure, role and regulation in health and disease. Mol Aspects Med. 2013;34(2–3):337–349. doi:10.1016/j.mam.2012.05.003
10. Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350(Pt 1):219–227. doi:10.1042/bj3500219
11. Halestrap AP. The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life. 2012;64(1):1–9. doi:10.1002/iub.573
12. Manning Fox JE, Meredith D, Halestrap AP. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol. 2000;529(Pt 2):285–293. doi:10.1111/j.1469-7793.2000.00285.x
13. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med. 2016;94(2):155–171. doi:10.1007/s00109-015-1307-x
14. Philp NJ, Yoon HY, Lombardi L. Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. Am J Physiol Cell Ph. 2001;280(5):C1319–C1326. doi:10.1152/ajpcell.2001.280.5.C1319
15. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi:10.1126/science.1160809
16. Doyen J, Trastour C, Ettore F, et al. Expression of the hypoxia-inducible monocarboxylate transporter MCT4 is increased in triple negative breast cancer and correlates independently with clinical outcome. Biochem Bioph Res Co. 2014;451(1):54–61. doi:10.1016/j.bbrc.2014.07.050
17. Fei F, Guo X, Chen Y, et al. Polymorphisms of monocarboxylate transporter genes are associated with clinical outcomes in patients with colorectal cancer. J Cancer Res Clin Oncol. 2015;141(6):1095–1102. doi:10.1007/s00432-014-1877-y
18. Payen VL, Mina E, Van Hee VF, Porporato PE, Sonveaux P. Monocarboxylate transporters in cancer. Mol Metab. 2020;33:48–66. doi:10.1016/j.molmet.2019.07.006
19. Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121(1):29–40. doi:10.1016/j.pharmthera.2008.09.005
20. Wu PH, Zhou Y, Guo YZ, Zhang SL, Tam KY. Recent developments of human monocarboxylate transporter (hMCT) inhibitors as anticancer agents. Drug Discov Today. 2021;26(3):836–844. doi:10.1016/j.drudis.2021.01.003
21. Galic S, Schneider HP, Broer A, Deitmer JW, Broer S. The loop between helix 4 and helix 5 in the monocarboxylate transporter MCT1 is important for substrate selection and protein stability. Biochem J. 2003;376(2):413–422. doi:10.1042/bj20030799
22. Futagi Y, Sasaki S, Kobayashi M, Narumi K, Furugen A, Iseki K. The flexible cytoplasmic loop 3 contributes to the substrate affinity of human monocarboxylate transporters. Biochim Biophys Acta Biomembr. 2017;1859(10):1790–1795. doi:10.1016/j.bbamem.2017.05.014
23. Price NT, Jackson VN, Halestrap AP. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem J. 1998;329(Pt 2):321–328. doi:10.1042/bj3290321
24. Rahman B, Schneider HP, Broer A, Deitmer JW, Broer S. Helix 8 and helix 10 are involved in substrate recognition in the rat monocarboxylate transporter MCT1. Biochemistry. 1999;38(35):11577–11584. doi:10.1021/bi990973f
25. Manoharan C, Wilson MC, Sessions RB, Halestrap AP. The role of charged residues in the transmembrane helices of monocarboxylate transporter 1 and its ancillary protein basigin in determining plasma membrane expression and catalytic activity. Mol Membr Biol. 2006;23(6):486–498. doi:10.1080/09687860600841967
26. Nancolas B, Sessions RB, Halestrap AP. Identification of key binding site residues of MCT1 for AR-C155858 reveals the molecular basis of its isoform selectivity. Biochem J. 2015;467(1):192. doi:10.1042/bj4670192
27. Sasaki S, Kobayashi M, Futagi Y, Ogura J, Yamaguchi H, Iseki K. Involvement of Histidine Residue His382 in pH regulation of MCT4 activity. PLoS One. 2015;10(4):e0122738. doi:10.1371/journal.pone.0122738
28. Sasaki S, Kobayashi M, Futagi Y, et al. Crucial residue involved in L-lactate recognition by human monocarboxylate transporter 4 (hMCT4). PLoS One. 2013;8(7):e67690. doi:10.1371/journal.pone.0067690
29. Li XF, Yu XZ, Dai D, Song XY, Xu WG. The altered glucose metabolism in tumor and a tumor acidic microenvironment associated with extracellular matrix metalloproteinase inducer and monocarboxylate transporters. Oncotarget. 2016;7(17):23141–23155. doi:10.18632/oncotarget.8153
30. Igakura T, Kadomatsu K, Kaname T, et al. A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol. 1998;194(2):152–165. doi:10.1006/dbio.1997.8819
31. Muramatsu T, Miyauchi T. Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol Histopathol. 2003;18(3):981–987. doi:10.14670/HH-18.981
32. Hanna SM, Kirk P, Holt OJ, Puklavec MJ, Brown MH, Barclay AN. A novel form of the membrane protein CD147 that contains an extra Ig-like domain and interacts homophilically. BMC Biochem. 2003;4(1):17. doi:10.1186/1471-2091-4-17
33. Ochrietor JD, Moroz TP, van Ekeris L, et al. Retina-specific expression of 5A11/Basigin-2, a member of the immunoglobulin gene superfamily. Invest Ophthalmol Vis Sci. 2003;44(9):4086–4096. doi:10.1167/iovs.02-0995
34. Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19(15):3896–3904. doi:10.1093/emboj/19.15.3896
35. Le Floch R, Chiche J, Marchiq I, et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci U S A. 2011;108(40):16663–16668. doi:10.1073/pnas.1106123108
36. Wilson MC, Meredith D, Fox JE, Manoharan C, Davies AJ, Halestrap AP. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). J Biol Chem. 2005;280(29):27213–27221. doi:10.1074/jbc.M411950200
37. Wilson MC, Meredith D, Halestrap AP. Fluorescence resonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. J Biol Chem. 2002;277(5):3666–3672. doi:10.1074/jbc.M109658200
38. Choi JW, Kim Y, Lee JH, Kim YS. Prognostic significance of lactate/proton symporters MCT1, MCT4, and Their Chaperone CD147 expressions in urothelial carcinoma of the bladder. Urology. 2014;84(1):
39. Afonso J, Santos LL, Miranda-Goncalves V, et al. CD147 and MCT1-potential partners in bladder cancer aggressiveness and cisplatin resistance. Mol Carcinog. 2015;54(11):1451–1466. doi:10.1002/mc.22222
40. Als AB, Dyrskjot L, von der Maase H, et al. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13(15):4407–4414. doi:10.1158/1078-0432.CCR-07-0109
41. Xue YJ, Lu Q, Sun ZX. CD147 overexpression is a prognostic factor and a potential therapeutic target in bladder cancer. Med Oncol. 2011;28(4):1363–1372. doi:10.1007/s12032-010-9582-4
42. Puri S, Juvale K. Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: a review with structure-activity relationship insights. Eur J Med Chem. 2020;199:112393. doi:10.1016/j.ejmech.2020.112393
43. Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. doi:10.1038/nrc704
44. Sonveaux P, Copetti T, De Saedeleer CJ, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012;7(3):e33418. doi:10.1371/journal.pone.0033418
45. Pavlides S, Whitaker-Menezes D, Castello-Cros R, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8(23):3984–4001. doi:10.4161/cc.8.23.10238
46. Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10(11):1772–1783. doi:10.4161/cc.10.11.15659
47. Sonveaux P, Vegran F, Schroeder T, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118(12):3930–3942. doi:10.1172/JCI36843
48. Ayala FRR, Rocha RM, Carvalho KC, et al. Glut1 and Glut3 as potential prognostic markers for oral squamous cell carcinoma. Molecules. 2010;15(4):2374–2387. doi:10.3390/molecules15042374
49. Fenske W, Volker HU, Adam P, et al. Glucose transporter GLUT1 expression is an stage-independent predictor of clinical outcome in adrenocortical carcinoma. Endocr Relat Cancer. 2009;16(3):919–928. doi:10.1677/ERC-08-0211
50. Wang X, Liu H, Ni Y, Shen P, Han X. Lactate shuttle: from substance exchange to regulatory mechanism. Hum Cell. 2022;35(1):1–14. doi:10.1007/s13577-021-00622-z
51. Dhup S, Dadhich RK, Porporato PE, Sonveaux P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des. 2012;18(10):1319–1330. doi:10.2174/138161212799504902
52. Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–3692. doi:10.1172/JCI69741
53. Amrutkar M, Berg K, Balto A, et al. Pancreatic stellate cell-induced gemcitabine resistance in pancreatic cancer is associated with LDHA- and MCT4-mediated enhanced glycolysis. Cancer Cell Int. 2023;23(1). doi:10.1186/s12935-023-02852-7
54. Meijer TW, Schuurbiers OC, Kaanders JH, et al. Differences in metabolism between adeno- and squamous cell non-small cell lung carcinomas: spatial distribution and prognostic value of GLUT1 and MCT4. Lung Cancer. 2012;76(3):316–323. doi:10.1016/j.lungcan.2011.11.006
55. Rademakers SE, Lok J, van der Kogel AJ, Bussink J, Kaanders JH. Metabolic markers in relation to hypoxia; staining patterns and colocalization of pimonidazole, HIF-1alpha, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer. 2011;11(1):167. doi:10.1186/1471-2407-11-167
56. Hashimoto T, Masuda S, Taguchi S, Brooks GA. Immunohistochemical analysis of MCT1, MCT2 and MCT4 expression in rat plantaris muscle. J Physiol. 2005;567(1):121–129. doi:10.1113/jphysiol.2005.087411
57. Murray CM, Hutchinson R, Bantick JR, et al. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat Chem Biol. 2005;1(7):371–376. doi:10.1038/nchembio744
58. Mathupala SP, Parajuli P, Sloan AE. Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study. Neurosurgery. 2004;55(6):1410–1419. doi:10.1227/01.NEU.0000143034.62913.59
59. De Saedeleer CJ, Porporato PE, Copetti T, et al. Glucose deprivation increases monocarboxylate transporter 1 (MCT1) expression and MCT1-dependent tumor cell migration. Oncogene. 2014;33(31):4060–4068. doi:10.1038/onc.2013.454
60. Dietl K, Renner K, Dettmer K, et al. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J Immunol. 2010;184(3):1200–1209. doi:10.4049/jimmunol.0902584
61. Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–3819. doi:10.1182/blood-2006-07-035972
62. Certo M, Tsai CH, Pucino V, Ho PC, Mauro C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol. 2021;21(3):151–161. doi:10.1038/s41577-020-0406-2
63. Yuan C, Zhang J, Lou J, et al. Comprehensive Analysis of Monocarboxylate Transporter 4 (MCT4) expression in breast cancer prognosis and immune infiltration via integrated bioinformatics analysis. Bioengineered. 2021;12(1):3850–3863. doi:10.1080/21655979.2021.1951928
64. Long Y, Gao Z, Hu X, et al. Downregulation of MCT4 for lactate exchange promotes the cytotoxicity of NK cells in breast carcinoma. Cancer Med. 2018;7(9):4690–4700. doi:10.1002/cam4.1713
65. Sun X, Wang M, Wang M, et al. Role of proton-coupled monocarboxylate transporters in cancer: from metabolic crosstalk to therapeutic potential. Front Cell Dev Biol. 2020;8:651. doi:10.3389/fcell.2020.00651
66. Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280(51):41928–41939. doi:10.1074/jbc.M508718200
67. Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277(26):23111–23115. doi:10.1074/jbc.M202487200
68. Hunt TK, Aslam RS, Beckert S, et al. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal. 2007;9(8):1115–1124. doi:10.1089/ars.2007.1674
69. Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72(11):1493–1505. doi:10.1016/j.bcp.2006.04.011
70. Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappa B/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71(7):2550–2560. doi:10.1158/0008-5472.CAN-10-2828
71. Zhao Z, Wu MS, Zou C, et al. Downregulation of MCT1 inhibits tumor growth, metastasis and enhances chemotherapeutic efficacy in osteosarcoma through regulation of the NF-kappaB pathway. Cancer Lett. 2014;342(1):150–158. doi:10.1016/j.canlet.2013.08.042
72. Hong CS, Graham NA, Gu W, et al. MCT1 modulates cancer cell pyruvate export and growth of tumors that co-express MCT1 and MCT4. Cell Rep. 2016;14(7):1590–1601. doi:10.1016/j.celrep.2016.01.057
73. Pertega-Gomes N, Vizcaino JR, Miranda-Goncalves V, et al. Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer. Bmc Cancer. 2011;11:11. doi:10.1186/1471-2407-11-11
74. Giannoni E, Bianchini F, Calorini L, Chiarugi P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Sign. 2011;14(12):2361–2371. doi:10.1089/ars.2010.3727
75. Giannoni E, Bianchini F, Masieri L, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70(17):6945–6956. doi:10.1158/0008-5472.CAN-10-0785
76. Fiaschi T, Marini A, Giannoni E, et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72(19):5130–5140. doi:10.1158/0008-5472.CAN-12-1949
77. Shi H, Jiang H, Wang L, et al. Overexpression of monocarboxylate anion transporter 1 and 4 in T24-induced cancer-associated fibroblasts regulates the progression of bladder cancer cells in a 3D microfluidic device. Cell Cycle. 2015;14(19):3058–3065. doi:10.1080/15384101.2015.1053666
78. Zhang G, Zhang Y, Dong D, et al. MCT1 regulates aggressive and metabolic phenotypes in bladder cancer. J Cancer. 2018;9(14):2492–2501. doi:10.7150/jca.25257
79. Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007;67(9):4182–4189. doi:10.1158/0008-5472.CAN-06-3184
80. Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6(3):1670–1690. doi:10.3390/cancers6031670
81. Chen P, Zuo H, Xiong H, et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U S A. 2017;114(3):580–585. doi:10.1073/pnas.1614035114
82. Mu X, Shi W, Xu Y, et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle. 2018;17(4):428–438. doi:10.1080/15384101.2018.1444305
83. Halestrap AP, Wilson MC. The monocarboxylate transporter family--role and regulation. IUBMB Life. 2012;64(2):109–119. doi:10.1002/iub.572
84. Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8(9):705–713. doi:10.1038/nrc2468
85. Draoui N, Feron O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech. 2011;4(6):727–732. doi:10.1242/dmm.007724
86. Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3):177–185. doi:10.1016/j.cmet.2006.02.002
87. Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3(3):187–197. doi:10.1016/j.cmet.2006.01.012
88. Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1 alpha-dependent mechanism. J Biol Chem. 2006;281(14):9030–9037. doi:10.1074/jbc.M511397200
89. Ke X, Fei F, Chen YK, et al. Hypoxia upregulates CD147 through a combined effect of HIF-1 and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors. Carcinogenesis. 2012;33(8):1598–1607. doi:10.1093/carcin/bgs196
90. Jamali S, Klier M, Ames S, et al. Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci Rep. 2015;5(1):13605. doi:10.1038/srep13605
91. Nagpal I, Yuan ZM. The basally expressed p53-mediated homeostatic function. Front Cell Dev Biol. 2021;9:775312. doi:10.3389/fcell.2021.775312
92. Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137(3):413–431. doi:10.1016/j.cell.2009.04.037
93. Vousden KH, Ryan KM. p53 and metabolism. Nat Rev Cancer. 2009;9(10):691–700. doi:10.1038/nrc2715
94. Boidot R, Vegran F, Meulle A, et al. Regulation of Monocarboxylate Transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Res. 2012;72(4):939–948. doi:10.1158/0008-5472.CAN-11-2474
95. Doherty JR, Yang C, Scott KE, et al. Blocking lactate export by inhibiting the Myc target MCT1 Disables glycolysis and glutathione synthesis. Cancer Res. 2014;74(3):908–920. doi:10.1158/0008-5472.CAN-13-2034
96. Gabay M, Li YL, Felsher DW. MYC Activation is a hallmark of cancer initiation and maintenance. Csh Perspect Med. 2014;4(6):a014241. doi:10.1101/cshperspect.a014241
97. Baluapuri A, Wolf E, Eilers M. Target gene-independent functions of MYC oncoproteins. Nat Rev Mol Cell Biol. 2020;21(5):255–267. doi:10.1038/s41580-020-0215-2
98. Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006;16(4):253–264. doi:10.1016/j.semcancer.2006.07.014
99. Ji H, Wu G, Zhan X, et al. Cell-type independent MYC target genes reveal a primordial signature involved in biomass accumulation. PLoS One. 2011;6(10):e26057. doi:10.1371/journal.pone.0026057
100. Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13(6):472–482. doi:10.1016/j.ccr.2008.05.005
101. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi:10.1126/science.123.3191.309
102. Gan L, Xiu R, Ren P, et al. Metabolic targeting of oncogene MYC by selective activation of the proton-coupled monocarboxylate family of transporters. Oncogene. 2016;35(23):3037–3048. doi:10.1038/onc.2015.360
103. Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F. Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr. 2012;44(1):127–139. doi:10.1007/s10863-012-9428-1
104. Curtis NJ, Mooney L, Hopcroft L, et al. Pre-clinical pharmacology of AZD3965, a selective inhibitor of MCT1: DLBCL, NHL and Burkitt’s lymphoma anti-tumor activity. Oncotarget. 2017;8(41):69219–69236. doi:10.18632/oncotarget.18215
105. Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–132. doi:10.1016/j.neuint.2016.06.011
106. Gassull MA. Review article: the intestinal lumen as a therapeutic target in inflammatory bowel disease. Aliment Pharm Ther. 2006;24:90–95. doi:10.1111/j.1365-2036.2006.03067.x
107. Cuff MA, Shirazi-Beechey SP. The importance of butyrate transport to the regulation of gene expression in the colonic epithelium. Biochem Soc Trans. 2004;32(Pt 6):1100–1102. doi:10.1042/BST0321100
108. Cuff MA, Lambert DW, Shirazi-Beechey SP. Substrate-induced regulation of the human colonic monocarboxylate transporter, MCT1. J Physiol. 2002;539(Pt 2):361–371. doi:10.1113/jphysiol.2001.014241
109. Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF-kappa B pathway. J Cell Biochem. 2008;103(5):1452–1463. doi:10.1002/jcb.21532
110. Borthakur A, Priyamvada S, Kumar A, et al. A novel nutrient sensing mechanism underlies substrate-induced regulation of monocarboxylate transporter-1. Am J Physiol Gastrointest Liver Physiol. 2012;303(10):G1126–G1133. doi:10.1152/ajpgi.00308.2012
111. Brown TP, Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther. 2020;206:107451. doi:10.1016/j.pharmthera.2019.107451
112. Ge H, Weiszmann J, Reagan JD, et al. Elucidation of signaling and functional activities of an orphan GPCR, GPR81. J Lipid Res. 2008;49(4):797–803. doi:10.1194/jlr.M700513-JLR200
113. Kuei C, Yu J, Zhu J, et al. Study of GPR81, the lactate receptor, from distant species identifies residues and motifs critical for GPR81 functions. Mol Pharmacol. 2011;80(5):848–858. doi:10.1124/mol.111.074500
114. Roland CL, Arumugam T, Deng DF, et al. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. 2014;74(18):5301–5310. doi:10.1158/0008-5472.CAN-14-0319
115. Lee YJ, Shin KJ, Park SA, et al. G-protein-coupled receptor 81 promotes a malignant phenotype in breast cancer through angiogenic factor secretion. Oncotarget. 2016;7(43):70898–70911. doi:10.18632/oncotarget.12286
116. Liu C, Kuei C, Zhu J, et al. 3,5-Dihydroxybenzoic acid, a specific agonist for hydroxycarboxylic acid 1, inhibits lipolysis in adipocytes. J Pharmacol Exp Ther. 2012;341(3):794–801. doi:10.1124/jpet.112.192799
117. Wu Y, Wang M, Zhang K, et al. Lactate enhanced the effect of parathyroid hormone on osteoblast differentiation via GPR81-PKC-Akt signaling. Biochem Biophys Res Commun. 2018;503(2):737–743. doi:10.1016/j.bbrc.2018.06.069
118. Hanson J, Gille A, Zwykiel S, et al. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J Clin Invest. 2010;120(8):2910–2919. doi:10.1172/JCI42273
119. Tunaru S, Kero J, Schaub A, et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9(3):352–355. doi:10.1038/nm824
120. Alavi MS, Karimi G, Roohbakhsh A. The role of orphan G protein-coupled receptors in the pathophysiology of multiple sclerosis: a review. Life Sci. 2019;224:33–40. doi:10.1016/j.lfs.2019.03.045
121. Bardhan K, Paschall AV, Yang DF, et al. IFN gamma induces DNA methylation-silenced GPR109A expression via pSTAT1/p300 and H3K18 acetylation in colon cancer. Cancer Immunol Res. 2015;3(7):795–805. doi:10.1158/2326-6066.CIR-14-0164
122. Stein U, Walther W, Arlt F, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15(1):59–67. doi:10.1038/nm.1889
123. Radhakrishnan H, Walther W, Zincke F, et al. MACC1-The first decade of a key metastasis molecule from gene discovery to clinical translation. Cancer Metast Rev. 2018;37(4):805–820. doi:10.1007/s10555-018-9771-8
124. Wang C, Wen Z, Xie J, et al. MACC1 mediates chemotherapy sensitivity of 5-FU and cisplatin via regulating MCT1 expression in gastric cancer. Biochem Biophys Res Commun. 2017;485(3):665–671. doi:10.1016/j.bbrc.2017.02.096
125. Alrefai WA, Tyagi S, Gill R, et al. Regulation of butyrate uptake in Caco-2 cells by phorbol 12-myristate 13-acetate. Am J Physiol Gastrointest Liver Physiol. 2004;286(2):G197–G203. doi:10.1152/ajpgi.00144.2003
126. Saksena S, Dwivedi A, Gill RK, et al. PKC-dependent stimulation of the human MCT1 promoter involves transcription factor AP2. Am J Physiol Gastrointest Liver Physiol. 2009;296(2):G275–G283. doi:10.1152/ajpgi.90503.2008
127. Fanjul C, Barrenetxe J, Lostao MP, Ducroc R. Modulation of intestinal L-glutamate transport by luminal leptin. J Physiol Biochem. 2015;71(2):311–317. doi:10.1007/s13105-015-0414-z
128. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi:10.1038/372425a0
129. Buyse M, Sitaraman SV, Liu X, Bado A, Merlin D. Luminal leptin enhances CD147/MCT-1-mediated uptake of butyrate in the human intestinal cell line Caco2-BBE. J Biol Chem. 2002;277(31):28182–28190. doi:10.1074/jbc.M203281200
130. Ranasinghe A, Schwarz MA. Integrating epigenetics and metabolomics to advance treatments for pulmonary arterial hypertension. Biochem Pharmacol. 2022;204:115245. doi:10.1016/j.bcp.2022.115245
131. Felmlee MA, Jones RS, Rodriguez-Cruz V, Follman KE, Morris ME. Monocarboxylate Transporters (SLC16): function, regulation, and role in health and disease. Pharmacol Rev. 2020;72(2):466–485. doi:10.1124/pr.119.018762
132. Fisel P, Kruck S, Winter S, et al. DNA methylation of the SLC16A3 promoter regulates expression of the human lactate transporter MCT4 in renal cancer with consequences for clinical outcome. Clin Cancer Res. 2013;19(18):5170–5181. doi:10.1158/1078-0432.CCR-13-1180
133. Asada K, Miyamoto K, Fukutomi T, et al. Reduced expression of GNA11 and silencing of MCT1 in human breast cancers. Oncology. 2003;64(4):380–388. doi:10.1159/000070297
134. Ng HK, Pang J, Kwok K. miRNA-124 is frequently down-regulated in medulloblastoma and is a negative regulator of SLC16A1. J Neuropath Exp Neur. 2009;68(5):567.
135. Liang D, Zhang Y, Han J, et al. Embryonic stem cell-derived pancreatic endoderm transplant with MCT1-suppressing miR-495 attenuates type II diabetes in mice. Endocr J. 2015;62(10):907–920. doi:10.1507/endocrj.EJ15-0186
136. Romero-Cordoba SL, Rodriguez-Cuevas S, Bautista-Pina V, et al. Loss of function of miR-342-3p results in MCT1 over-expression and contributes to oncogenic metabolic reprogramming in triple negative breast cancer. Sci Rep. 2018;8(1):12252.
137. Xu WF, Zhang ZJ, Zou KJ, et al. MiR-1 suppresses tumor cell proliferation in colorectal cancer by inhibition of Smad3-mediated tumor glycolysis. Cell Death Dis. 2017;8(5):e2761.
138. Zhao Y, Li W, Li M, et al. Targeted inhibition of MCT4 disrupts intracellular pH homeostasis and confers self-regulated apoptosis on hepatocellular carcinoma. Exp Cell Res. 2019;384(1):111591. doi:10.1016/j.yexcr.2019.111591
139. Luo EF, Wang D, Yan GL, et al. The NF-kappa B/miR-425-5p/MCT4 axis: a novel insight into diabetes-induced endothelial dysfunction. Mol Cell Endocrinol. 2020;500:110641.
140. Fan Q, Yang L, Zhang X, et al. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/beta-catenin signaling pathway activation in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2018;37(1):9. doi:10.1186/s13046-018-0673-y
141. Sprowl-Tanio S, Habowski AN, Pate KT, et al. Lactate/pyruvate transporter MCT-1 is a direct Wnt target that confers sensitivity to 3-bromopyruvate in colon cancer. Cancer Metab. 2016;4(1):20. doi:10.1186/s40170-016-0159-3
142. Hadjiagapiou C, Borthakur A, Dahdal RY, et al. Role of USF1 and USF2 as potential repressor proteins for human intestinal monocarboxylate transporter 1 promoter. Am J Physiol Gastr L. 2005;288(6):G1118–G1126.
143. Diehl K, Dinges LA, Helm O, et al. Nuclear factor E2-related factor-2 has a differential impact on MCT1 and MCT4 lactate carrier expression in colonic epithelial cells: a condition favoring metabolic symbiosis between colorectal cancer and stromal cells. Oncogene. 2018;37(1):39–51. doi:10.1038/onc.2017.299
144. Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007;21(10):2602–2612. doi:10.1096/fj.07-8174com
145. Becker HM, Hirnet D, Fecher-Trost C, Sultemeyer D, Deitmer JW. Transport activity of MCT1 expressed in Xenopus oocytes is increased by interaction with carbonic anhydrase. J Biol Chem. 2005;280(48):39882–39889. doi:10.1074/jbc.M503081200
146. Klier M, Andes FT, Deitmer JW, Becker HM. Intracellular and extracellular carbonic anhydrases cooperate non-enzymatically to enhance activity of monocarboxylate transporters. J Biol Chem. 2014;289(5):2765–2775. doi:10.1074/jbc.M113.537043
147. Mboge MY, Chen Z, Khokhar D, et al. A non-catalytic function of carbonic anhydrase IX contributes to the glycolytic phenotype and pH regulation in human breast cancer cells. Biochem J. 2019;476(10):1497–1513. doi:10.1042/BCJ20190177
148. Aspatwar A, Tolvanen MEE, Schneider HP, et al. Catalytically inactive carbonic anhydrase-related proteins enhance transport of lactate by MCT1. FEBS Open Bio. 2019;9(7):1204–1211. doi:10.1002/2211-5463.12647
149. Choi SH, Kim MY, Yoon YS, et al. Hypoxia-induced RelA/p65 derepresses SLC16A3 (MCT4) by downregulating ZBTB7A. Biochim Biophys Acta Gene Regul Mech. 2019;1862(8):771–785. doi:10.1016/j.bbagrm.2019.06.004
150. Furugen A, Kobayashi M, Narumi K, et al. AMP-activated protein kinase regulates the expression of monocarboxylate transporter 4 in skeletal muscle. Life Sci. 2011;88(3–4):163–168. doi:10.1016/j.lfs.2010.11.003
151. Kobayashi M, Narumi K, Furugen A, Iseki K. Transport function, regulation, and biology of human monocarboxylate transporter 1 (hMCT1) and 4 (hMCT4). Pharmacol Ther. 2021;226:107862. doi:10.1016/j.pharmthera.2021.107862
152. Narumi K, Kobayashi M, Otake S, et al. Regulation of human monocarboxylate transporter 4 in skeletal muscle cells: the role of protein kinase C (PKC). Int J Pharm. 2012;428(1–2):25–32. doi:10.1016/j.ijpharm.2012.02.021
153. Wang D, Wang Q, Yan G, et al. High glucose and interleukin 1beta-induced apoptosis in human umbilical vein endothelial cells involves in down-regulation of monocarboxylate transporter 4. Biochem Biophys Res Commun. 2015;466(4):607–614. doi:10.1016/j.bbrc.2015.09.016
154. Tong YH, Hu XP, Xiang XP, Fang L. High expression of monocarboxylate transporter 4 (MCT 4), but not MCT 1, predicts poor prognosis in patients with non-small cell lung cancer. Transl Cancer Res. 2021;10(3):1336–1345. doi:10.21037/tcr-20-3117
155. Eilertsen M, Andersen S, Al-Saad S, et al. Monocarboxylate transporters 1–4 in NSCLC: MCT1 is an independent prognostic marker for survival. PLoS One. 2014;9(9):e105038. doi:10.1371/journal.pone.0105038
156. Guan X, Morris ME. In vitro and in vivo efficacy of AZD3965 and Alpha-Cyano-4-hydroxycinnamic acid in the murine 4T1 breast tumor model. AAPS J. 2020;22(4):84. doi:10.1208/s12248-020-00466-9
157. Izumi H, Takahashi M, Uramoto H, et al. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci. 2011;102(5):1007–1013. doi:10.1111/j.1349-7006.2011.01908.x
158. Noble RA, Bell N, Blair H, et al. Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel therapeutic approach for diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica. 2017;102(7):1247–1257. doi:10.3324/haematol.2016.163030
159. Noble RA, Thomas H, Zhao Y, et al. Simultaneous targeting of glycolysis and oxidative phosphorylation as a therapeutic strategy to treat diffuse large B-cell lymphoma. Brit J Cancer. 2022;127(5):937–947. doi:10.1038/s41416-022-01848-w
160. Zhao B, Aggarwal A, Im SY, Viswanathan K, Landa I, Nehs MA. Effect of lactate export inhibition on anaplastic thyroid cancer growth and metabolism. J Am Coll Surg. 2022;234(6):1044–1050. doi:10.1097/XCS.0000000000000226
161. Plummer R, Halford S, Jones P, et al. A first-in-human first-in-class (FIC) trial of the monocarboxylate transporter 1 (MCT1) inhibitor AZD3965 in patients with advanced solid tumours. Ann Oncol. 2018;29:iii9. doi:10.1093/annonc/mdy048.008
162. Beloueche-Babari M, Wantuch S, Casals Galobart T, et al. MCT1 inhibitor AZD3965 increases mitochondrial metabolism, facilitating combination therapy and noninvasive magnetic resonance spectroscopy. Cancer Res. 2017;77(21):5913–5924. doi:10.1158/0008-5472.CAN-16-2686
163. Ovens MJ, Davies AJ, Wilson MC, Murray CM, Halestrap AP. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7–10. Biochem J. 2010;425(3):523–530. doi:10.1042/BJ20091515
164. Draoui N, Schicke O, Seront E, et al. Antitumor activity of 7-aminocarboxycoumarin derivatives, a new class of potent inhibitors of lactate influx but not efflux. Mol Cancer Ther. 2014;13(6):1410–1418. doi:10.1158/1535-7163.MCT-13-0653
165. Gurrapu S, Jonnalagadda SK, Alam MA, et al. Coumarin carboxylic acids as monocarboxylate transporter 1 inhibitors: in vitro and in vivo studies as potential anticancer agents. Bioorg Med Chem Lett. 2016;26(14):3282–3286. doi:10.1016/j.bmcl.2016.05.054
166. Draoui N, Schicke O, Fernandes A, et al. Synthesis and pharmacological evaluation of carboxycoumarins as a new antitumor treatment targeting lactate transport in cancer cells. Bioorgan Med Chem. 2013;21(22):7107–7117. doi:10.1016/j.bmc.2013.09.010
167. Tateishi H, Tsuji AB, Kato K, et al. Synthesis and evaluation of C-11-labeled coumarin analog as an imaging probe for detecting monocarboxylate transporters expression. Bioorg Med Chem Lett. 2017;27(21):4893–4897. doi:10.1016/j.bmcl.2017.09.033
168. Quanz M, Bender E, Kopitz C, et al. Preclinical efficacy of the novel monocarboxylate transporter 1 inhibitor BAY-8002 and associated markers of resistance. Mol Cancer Ther. 2018;17(11):2285–2296. doi:10.1158/1535-7163.MCT-17-1253
169. Cluntun AA, Badolia R, Lettlova S, et al. The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 2021;33(3):629–648 e610. doi:10.1016/j.cmet.2020.12.003
170. Fang Y, Liu WR, Tang Z, et al. Monocarboxylate transporter 4 inhibition potentiates hepatocellular carcinoma immunotherapy through enhancing T cell infiltration and immune attack. Hepatology. 2022. doi:10.1002/hep.32348
171. Futagi Y, Kobayashi M, Narumi K, Furugen A, Iseki K. Identification of a selective inhibitor of human monocarboxylate transporter 4. Biochem Biophys Res Commun. 2018;495(1):427–432. doi:10.1016/j.bbrc.2017.10.025
172. Mora E, Guglielmotti A, Biondi G, Sassone-Corsi P. Bindarit An anti-inflammatory small molecule that modulates the NF kappa B pathway. Cell Cycle. 2012;11(1):159–169. doi:10.4161/cc.11.1.18559
173. Colen CB, Shen YM, Ghoddoussi F, et al. Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: an in vivo study. Neoplasia. 2011;13(7):620–632. doi:10.1593/neo.11134
174. Diers AR, Broniowska KA, Chang CF, Hogg N. Pyruvate fuels mitochondrial respiration and proliferation of breast cancer cells: effect of monocarboxylate transporter inhibition. Biochem J. 2012;444(3):561–571. doi:10.1042/BJ20120294
175. Hildyard JC, Halestrap AP. Identification of the mitochondrial pyruvate carrier in Saccharomyces cerevisiae. Biochem J. 2003;374(Pt 3):607–611. doi:10.1042/bj20030995
176. Wagner W, Ciszewski WM, Kania KD. L- and D-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Commun Signal. 2015;13(1):36. doi:10.1186/s12964-015-0114-x
177. Hamdan L, Arrar Z, Al Muataz Y, et al. Alpha cyano-4-hydroxy-3-methoxycinnamic acid inhibits proliferation and induces apoptosis in human breast cancer cells. PLoS One. 2013;8(9):e72953. doi:10.1371/journal.pone.0072953
178. Jonnalagadda S, Jonnalagadda SK, Ronayne CT, et al. Novel N,N-dialkyl cyanocinnamic acids as monocarboxylate transporter 1 and 4 inhibitors. Oncotarget. 2019;10(24):2355–2368. doi:10.18632/oncotarget.26760
179. Derosa G, Sahebkar A, Maffioli P. The role of various peroxisome proliferator-activated receptors and their ligands in clinical practice. J Cell Physiol. 2018;233(1):153–161. doi:10.1002/jcp.25804
180. Abdelkader H, Alany RG, Pierscionek B. Age-related cataract and drug therapy: opportunities and challenges for topical antioxidant delivery to the lens. J Pharm Pharmacol. 2015;67(4):537–550. doi:10.1111/jphp.12355
181. Nancolas B, Guo LL, Zhou R, et al. The anti-tumour agent lonidamine is a potent inhibitor of the mitochondrial pyruvate carrier and plasma membrane monocarboxylate transporters. Biochem J. 2016;473(7):929–936. doi:10.1042/BJ20151120
182. Puri S, Stefan K, Khan SL, Pahnke J, Stefan SM, Juvale K. Indole derivatives as new structural class of potent and antiproliferative inhibitors of monocarboxylate transporter 1 (MCT1; SLC16A1). J Med Chem. 2022;66(1):657–676. doi:10.1021/acs.jmedchem.2c01612
183. Wang H, Yang C, Doherty JR, Roush WR, Cleveland JL, Bannister TD. Synthesis and structure-activity relationships of pteridine dione and trione monocarboxylate transporter 1 inhibitors. J Med Chem. 2014;57(17):7317–7324. doi:10.1021/jm500640x
184. Voss DM, Spina R, Carter DL, Lim KS, Jeffery CJ, Bar EE. Disruption of the monocarboxylate transporter-4-basigin interaction inhibits the hypoxic response, proliferation, and tumor progression. Sci Rep. 2017;7(1):4292. doi:10.1038/s41598-017-04612-w
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



