Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Role of Genetic Polymorphisms in IL12Rβ2 in Chronic Obstructive Pulmonary Disease
Received 17 March 2022
Accepted for publication 25 June 2022
Published 27 July 2022 Volume 2022:17 Pages 1671—1683
DOI https://doi.org/10.2147/COPD.S366844
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Yihui Fu, Lirong Liu, Haihong Wu
Department of Respiratory and Critical Care Medicine, Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University), Haikou, 570311, People’s Republic of China
Correspondence: Haihong Wu, Department of Respiratory and Critical Care Medicine, Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University), #19, Xiuhua Road, Xiuying District, Haikou, 570311, People’s Republic of China, Tel/Fax +86 13976906068, Email [email protected]
Background: Chronic obstructive pulmonary disease (COPD) is the most common chronic inflammatory airway disease. Il-12r beta 2 (IL-12Rβ 2) is important for the production of pathogenic Th1 cells. We aimed to explore the association between IL-12Rβ 2 genetic variants and COPD risk among southern Chinese Han population.
Methods: We recruited 996 participants to perform an association analysis through SNPStats online software. We used false-positive report probability analysis to detect whether the positive findings were noteworthy. Haploview 4.2 software and SNPStats were used to conduct the haplotype analysis and linkage disequilibrium. Finally, the interaction of SNP-SNP in COPD risk was evaluated by multi-factor dimensionality reduction.
Results: The study found evidence that genetic loci in IL-12Rβ 2 (rs2201584, rs1874791, rs6679356, and rs3790567) were potentially associated with the COPD susceptibility. In particular, IL-12Rβ 2-rs2201584 and -rs1874791 showed close associations with COPD risk in both overall and several stratified analyses. Overall analysis or several stratified analyses indicated that allele A or homozygous genotype AA of IL-12Rβ 2-rs2201584 were risk factors for COPD (Allele A: OR (95% CI) = 1.23 (1.02– 1.48), p = 0.033; genotype AA: OR (95% CI) = 1.76 (1.15– 2.69), p = 0.009). The allele A or homozygous genotype AA of IL-12Rβ 2- rs1874791 were also risk factors for COPD (Allele A: OR (95% CI) = 1.36 (1.10– 1.68), p = 0.004; genotype AA: OR (95% CI) = 2.17 (1.18– 3.99), p = 0.013).
Conclusion: Intronic variants in IL-12Rβ 2 (rs2201584, rs1874791, rs6679356, and rs3790567) were associated with the COPD susceptibility. In particular, there were sufficient evidences that IL-12Rβ 2-rs2201584 and -rs1874791 were associated with the increasing risk of COPD.
Keywords: chronic obstructive pulmonary disease, IL-12Rβ 2, genetic variants, Chinese Han population
Introduction
Chronic obstructive pulmonary disease (COPD) is a common and frequently-occurring disease of respiratory system. It is a preventable and treatable chronic airway inflammatory disease.1,2 For a long time, exploring the potential mechanism related to the occurrence and development of COPD has been a research hotspot. At present, it is widely accepted that airway remodeling in COPD is closely related to repeated inflammatory stimulation and abnormal inflammatory response induced by harmful particles such as cigarette smoke and gas stimulation. Although smoking is an important factor affecting the development of COPD,3,4 many studies have reported the importance of genetic factors in the development of COPD in recent years.5–7
Airway inflammation is mediated by the expression of inflammatory genes.8–10 Chemokines and cytokines are the most important inflammatory mediators of chronic obstructive pulmonary airway inflammation, such as IL-17,11 IL-4, IL-1212 and IL-5,13 etc. Interleukin-12 receptor (IL-12R), mainly expressed in Th1 cells and natural killer cells (NK cells) producing interferon-γ (IFN-γ), is a heterodimer composed of two different subunits IL-12Rβ1 and IL-12Rβ2.14 IL-12/IL-12Rβ2 pathway is a key mediator in Th1 differentiation.14 More importantly, COPD is related to a series of Th cell-mediated biological processes.15,16 Based on the above evidence, we believe that IL-12β2 is potentially associated with the development of COPD. Up to now, we have found that many researchers have carried out studies on the association between IL-12Rβ2 gene polymorphism and pulmonary tuberculosis susceptibility in a variety of populations.17–19 However, the association between IL-12Rβ2 and the occurrence and development of COPD has not been reported.
Therefore, this study intends to conduct a case-control study in southern Chinese Han population. To investigate the association between IL-12Rβ2 genetic polymorphism and COPD susceptibility. We also conducted a stratified analysis based on some potential risk factors for COPD (age, gender, smoking, drinking, etc.). The association between IL-12Rβ2 genetic polymorphism and these influencing factors was also evaluated. This study laid a foundation for the study of the mechanism of IL-12Rβ2 in the occurrence and development of COPD, and provided new ideas for the individualized prevention and treatment of COPD in southern Chinese Han population.
Materials and Methods
Sample Source
We recruited 498 patients with COPD and 498 healthy individuals for this case-control study. The 996 participants were recruited in the same hospital (Hainan General Hospital). We continuously recruited 498 COPD patients in the outpatient or inpatient department of respiratory medicine. These patients were all newly diagnosed. Patients met the clear and diagnostic criteria of the World Health Organization (WHO) on COPD. Incomplete reversibility of airflow restriction is a prerequisite for COPD diagnosis. The main basis was FEV1/FVC <70% and FEV1 <80% predicted value of lung function after inhaling bronchodilators. A few patients without common cough, expectoration, shortness of breath and other clinical symptoms were diagnosed with COPD only after FEV1/FVC <70% and FEV1 ≥80% were tested for lung function, excluding other diseases. Exclusion criteria: (1) Patients with pneumonia, bronchial asthma, bronchiectasis, pulmonary tuberculosis, lung cancer, silicosis, interstitial pneumonia and other lung diseases; (2) Patients with serious diseases of heart, liver, kidney and endocrine organs. Patients treated with antibiotics, bronchodilators, and glucocorticoids due to recent severe lung infections. During the same period, 498 healthy individuals were recruited in the health examination center as the control group in this study. We obtained the demographic information and basic epidemiological information of the participants by consulting medical records or professional questionnaire survey. After obtaining the informed consent of all participants, we collected their blood samples for further study. This study has been approved by the ethics committee of the Hainan General Hospital before the beginning.
Selection of SNPs
As shown in Figure 1, the selection process of candidate SNPs is as follows: first, we found that the physical position of the IL-12Rβ2 was on the Chromosome 1: 67307364–67398724 through the e!GRCh37 database (http://asia.ensembl.org/Homo_sapiens/Info/Index). Then, we use the online converter window (VCF to PED: http://grch37.ensembl.org/Homo_sapiens/Tools/VcftoPed) to download the related files of IL-12Rβ2 genetic variants. For the population, we chose CHB and CHS. Finally, after setting specific conditions, six candidate IL-12Rβ2 SNPs (rs2201584, rs10489626, rs6659932, rs1874791, rs6679356, and rs3790567) were selected using Haploview software. These special conditions are as follows: Tagger r2 > 0.8, Min Genotype > 75%, MAF > 0.05 and HWE > 0.01.
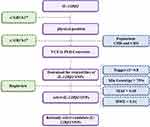 |
Figure 1 Flowchart for screening candidate SNPs. Abbreviations: CHB, Han Chinese population in Beijing, China; CHS, Han Chinese population in Shanghai, China. |
DNA Extraction and Genotyping
In this study, we chose the kit (GoldMag Co. Ltd. Xi’an, China) to complete the extraction and purification of genomic DNA, and the specific experimental steps were carried out according to the instructions. According to the DNA sequence of IL-12Rβ2, we designed specific amplification primers and extension primers for candidate genetic loci by MassARRAY Assay Design software. Genotyping was performed by the MassARRAY® -IPLEX SNP genotyping technology.
At the same time, we randomly selected 5% DNA samples for repeated experiments. The repetition rate of the results should reach >99% to ensure the reliability and repeatability of the experimental results.
Data Analysis
We used online software (HaploReg v4.1: https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php) to predict the potential function of candidate SNPs. In this study, continuous variables such as age, forced vital capacity (FVC), and forced the first second of expiratory volume (FEV1) were expressed as “mean ± standard deviation”. Categorical variables such as sex, smoking or alcohol status were expressed as frequency. The p value of continuous variables was calculated by t-test, and the p value of categorical variables was calculated by Chi-square test. The statistical analysis of this study was mainly completed by SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). In this study, the association between COPD risk and candidate genetic polymorphism under multiple genetic models was completed by SNPStats online software (https://www.snpstats.net/start.htm?q=snpstats/start.htm). We mainly evaluated the impact of candidate SNPs on the risk of COPD through the Odds ratios (OR) and 95% confidence intervals (CI). In order to avoid the influence of confounding factors on the results, all the results were adjusted by the confounding factors, such as age, gender, smoking or drinking. In addition, we also used false-positive report probability (FPRP) analysis to detect whether all positive results are noteworthy at a prior probability level of 0.25 and FPRP threshold of 0.2. Haploview 4.2 software and SNPStats online software were used to perform the haplotype analysis of candidate SNPs and evaluation of linkage disequilibrium (LD). Finally, the interaction of candidate SNPs in COPD risk was evaluated by multi-factor dimensionality reduction (MDR). The p < 0.05 indicated statistically significant.
Results
There were 996 participants in the case group (498) and the control group (498) enrolled in this study. The average ages of the case and control groups were 70.89 ± 10.19 years and 65.86 ± 5.56 years (Table 1). The number of males and females in the case group were 328 (65.9%) and 170 (34.1%), and in the control group were 322 (64.7%) and 176 (35.3%), respectively. The basic information of the participants can be found in Table 1.
 |
Table 1 Characteristics of Patients with COPD and Healthy Individuals |
Genotyping and Information About Candidate SNPs
Six IL-12Rβ2 candidate genetic loci (rs2201584, rs10489626, rs6659932, rs1874791, rs6679356, and rs3790567) have been successfully genotyped. These genetic variants are all intronic variants in IL-12Rβ2. After testing, the distribution of all candidate genetic loci is in accordance with Hardy-Weinberg equilibrium (HWE p> 5%). HaploReg online software was used to predict the potential functions of candidate genetic loci and found that the six SNPs may be regulated by a variety of factors (Table 2).
 |
Table 2 The Basic Information and HWE About the Candidate SNPs of IL-12Rβ2 |
Association Analysis Between IL-12RΒ2 SNPS and COPD Risk (Overall Analysis)
The association analysis between candidate SNPs and the COPD risk showed that IL-12Rβ2-rs2201584 and rs1874791 were significantly associated with the susceptibility of COPD among participants (Table 3). Specifically, compared with the allele ‘G’ or homozygous genotype ‘GG’, ‘A’ and ‘AA’ of IL-12Rβ2-rs2201584 were risk factors for COPD (A vs G: OR (95% CI) = 1.23 (1.02–1.48), p = 0.033; AA vs GG: OR (95% CI) = 1.76 (1.15–2.69), p = 0.009). IL-12Rβ2-rs2201584 can significantly increase the COPD risk under recessive (AA vs GG-GA, OR (95% CI) = 1.72 (1.15–2.57), p = 0.008) and log-additive genetic models (OR (95% CI) = 1.24 (1.02–1.50), p = 0.028).
 |
Table 3 Intronic Variants in IL-12Rβ2 Associated with Susceptibility of COPD |
In addition, compared with the allele ‘G’ or homozygous genotype ‘GG’, ‘A’ and ‘AA’ of IL-12Rβ2-rs1874791 were risk factors for COPD (A vs G: OR (95% CI) = 1.36 (1.10–1.68), p = 0.004; AA vs GG: OR (95% CI) = 2.17 (1.18–3.99), p = 0.013). IL-12Rβ2-rs1874791 can significantly increase the COPD risk under dominant (GA-AA vs GG, OR (95% CI) = 1.36 (1.04–1.77), p = 0.025), recessive (AA vs GG-GA, OR (95% CI) = 1.99 (1.09–3.64), p = 0.022) and log-additive genetic models (OR (95% CI) = 1.35 (1.09–1.69), p = 0.007).
We found that other intronic variants in IL-12Rβ2 were not associated with the COPD risk in overall analysis.
Association Analysis Between IL-12Rβ2 SNPS and COPD Risk (Stratified Analysis)
In order to further avoid the influence of other factors on the reliability of the association analysis results, we divided the participants according to different influencing factors and carried out stratified analyses (age, gender, smoking, drinking).
Age (≤68 Years Old)
The analysis showed (Table 4) that the IL-12Rβ2-rs2201584 has a significant association with COPD risk among participants ≤68 years old under recessive genetic model (AA vs GG-GA, OR (95% CI) = 1.74 (1.06–2.84), p = 0.029). Heterozygous genotype ‘GA’ of -rs1874791 is a risk factor for COPD AA (GA vs GG: OR (95% CI) = 1.48 (1.00–2.18), p = 0.048). And IL-12Rβ2-rs1874791 can significantly increase the COPD risk under dominant (GA-AA vs GG, OR (95% CI) = 1.49 (1.04–2.13), p = 0.029) and log-additive genetic models (OR (95% CI) = 1.38 (1.02–1.85), p = 0.035). We also found evidence that IL-12Rβ2-rs6679356 was associated with COPD risk under multiple genetic models (Allele: OR (95% CI) = 0.57 (0.42–0.79), p = 0.001; dominant: OR (95% CI) = 0.49 (0.23–1.04), p = 0.048; log-additive: OR (95% CI) = 0.49 (0.23–1.02), p = 0.042).
 |
Table 4 Intronic Variants in IL-12Rβ2 Associated with Susceptibility of COPD in the Subgroup Analysis (Age and Gender) |
In addition, there are no intronic variants in IL-12RΒ2 associated with the COPD risk among participants >68 years old.
Gender (Female)
Among female participants, only IL-12Rβ2-rs2201584 was associated with COPD risk. Specifically, compared with the homozygous genotype ‘GG’, ‘AA’ of IL-12Rβ2-rs2201584 were risk factors for COPD (OR (95% CI) = 2.57 (1.25–5.29), p = 0.010). And IL-12Rβ2-rs2201584 was significantly associated with increasing risk of COPD under multiple genetic models (recessive: OR (95% CI) = 2.33 (1.11–4.89), p = 0.021); (log-additive: OR (95% CI) = 1.43 (1.02–2.01), p = 0.038).
Gender (Male)
Among male participants, only IL-12Rβ2-rs1874791 was associated with COPD risk. Specifically, compared with the allele ‘G’ or homozygous genotype ‘GG’, ‘A’ and ‘AA’ of IL-12Rβ2-rs1874791 were risk factors for COPD (A vs G: OR (95% CI) = 1.40 (1.08–1.82), p = 0.012; AA vs GG: OR (95% CI) = 2.56 (1.17–5.6), p = 0.018). IL-12Rβ2-rs1874791 can significantly increase the COPD risk under dominant (GA-AA vs GG, OR (95% CI) = 1.45 (1.03–2.04), p = 0.032), recessive (AA vs GG-GA, OR (95% CI) = 2.20 (1.02–4.75), p = 0.039) and log-additive genetic models (OR (95% CI) = 1.44 (1.08–1.91), p = 0.011).
Smoking (Yes)
Among smoking participants, we only found that IL-12Rβ2-rs1874791 was associated with COPD risk under allele (A vs G: OR (95% CI) = 1.40 (1.08–1.82), p = 0.012) and log-additive genetic models (OR (95% CI) = 1.43 (1.01–2.03), p = 0.041).
In addition, there are no intronic variants in IL-12Rβ2 associated with the COPD risk among non-smoking participants.
Drinking (Yes)
Among drinking participants, homozygous genotype ‘AA’ of IL-12Rβ2-rs2201584 was a risk factor for COPD (AA vs GG: OR (95% CI) = 2.00 (1.09–3.67), p = 0.025). -rs2201584 also associated with increasing risk of COPD under recessive genetic models (AA vs GG-GA, OR (95% CI) = 2.11 (1.18–3.76), p = 0.010). Similarly, homozygous genotype ‘AA’ of IL-12Rβ2-rs1874791 was a risk factor for COPD (AA vs GG: OR (95% CI) = 3.77 (1.45–9.80), p = 0.007). -rs1874791 also associated with increasing risk of COPD under recessive (AA vs GG-GA, OR (95% CI) = 3.66 (1.42–9.43), p = 0.004) and log-additive genetic models (OR (95% CI) = 1.41 (1.03–1.93), p = 0.030). In addition, we have found evidence that IL-12Rβ2-rs3790567 have certain association with COPD risk (homozygous genotype ‘AA’: OR (95% CI) = 2.68 (1.18–6.09), p = 0.019; recessive genetic model: AA Vs GG-GA, OR (95% CI) = 2.92 (1.31–6.53), p = 0.006) (Table 5).
 |
Table 5 Intronic Variants in IL-12Rβ2 Associated with Susceptibility of COPD in the Subgroup Analysis (Drinking and Smoking) |
In addition, there are no intronic variants in IL-12Rβ2 associated with the COPD risk among non-drinking participants.
FPRP Analysis
The results of FPRP analysis showed that a very few positive results may be accidental, and most of them were noteworthy findings at the prior probability level of 0.25 and FPRP threshold of 0.2 (Supplemental Table 1).
IL-12Rβ2-rs6679356 may be associated with the COPD risk among participants ≤68 years (dominant: prior probability (0.25) = 0.284; log-additive: prior probability (0.25) = 0.262). IL-12Rβ2-rs1874791 may be associated with the COPD risk among participants >68 years (genotype AA (0.25): prior probability = 0.204) or male participants (recessive: prior probability (0.25) = 0.249). The above positive results may not be worth noting. The remaining positive results were all noteworthy findings. In particular, it is a noteworthy finding that the allele ‘A’ of IL-12Rβ2- rs2201584 (allele A (overall analysis): prior probability = 0.078, statistical power = 100%) was risk factor for COPD. Similarly, it is also noteworthy that allele ‘A’ is a risk factor for COPD (allele A (overall analysis): prior probability = 0.013, statistical power = 100%).
Haplotype Analysis
The result of linkage disequilibrium showed that (Figure 2) the two candidate IL-12Rβ2 SNPs (rs2201584, rs1874791) composed one LD block (D’ = 0.968, R2 = 0.108). Haplotype analysis showed that the haplotype ‘Ars2201584Grs1874791’ (OR (95% CI) = 1.46 (1.19–1.80), p = 0.0004) and ‘Ars2201584Ars1874791’ (OR (95% CI) = 1.61 (1.26–2.05), p = 0.0001) were associated with increasing risk of COPD (Table 6).
 |
Table 6 Haplotype Analysis About IL-12Rβ2 Genetic Polymorphisms with COPD Risk |
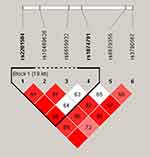 |
Figure 2 One linkage disequilibrium block composed by the IL-12Rβ2 genetic loci (rs2201584 and rs1874791). The numbers inside the diamonds indicate The D’ for pairwise analyses. |
MDR Analysis
As is shown in Figure 3, the dendrogram has described the interaction between the six candidate SNPs. The color of the lines in the dendrogram represents the level of redundancy or synergy. The closer to the red, the stronger the synergy, and the closer to the blue, the stronger the redundancy. The MDR results showed that the three loci model (rs2201584, rs1874791, rs3790567) has the highest test accuracy (Table 7). Therefore, the three loci model composed of rs2201584, rs1874791, and rs3790567 was chosen as the best model for predicting COPD risk (p< 0.0001), with a test accuracy of 0.551 and a perfect CVC = 10/10.
 |
Table 7 IL-12Rβ2 SNP–SNP Interaction Models Analyzed by the MDR Method |
Discussion
Smoking has long been considered a major environmental risk factor for COPD, but only a small number of smokers develop airway obstruction.20 It has been reported that it may be due to the genetic factors that play an indispensable role in the occurrence and development of COPD.21 With the development of molecular biology, many genetic polymorphisms associated with lung function have been reported, such as HLA-DQB1-rs9273410,22 TNF-A gene-308 G/A polymorphism,23 ADRB2-rs1265477824 and SREK1-rs74794265.25 However, limited by race and sample size, only a small proportion of the population’s susceptibility to COPD can be explained.
The association between IL-12Rβ2 genetic polymorphism and COPD susceptibility was studied in a case-control study involving 998 participants. Combined with association analysis and FPRP results, four genetic loci in IL-12Rβ2 (rs2201584, rs1874791, rs6679356, and rs3790567) were found to be associated with COPD risk in southern Chinese Han population. IL-12Rβ2-rs2201584 and -rs1874791 are not only significantly associated with COPD risk in the overall analysis, but also in multiple stratified analysis. To our knowledge, this study is the first to report that IL-12Rβ2 genetic polymorphisms are associated with COPD risk in southern Chinese Han population.
Overall analysis showed that the presence of allele ‘A’ or genotype ‘AA’ of IL-12Rβ2-rs2201584 or -rs1874791 was significantly associated with increased risk of COPD. In addition, in order to eliminate the influence of confounding factors on the reliability of this study, we have conducted stratified analyses according to potential risk factors of COPD. IL-12Rβ2-rs2201584 was significantly associated with an increased risk of COPD among participants younger than 68 years of age, women or drinkers. IL-12Rβ2-rs1874791 was significantly associated with increased COPD risk among participants younger than 68 years of age, men, drinking or smoking participants. It is worth noting that no candidate genetic polymorphism in this study was associated with the COPD risk of participants older than 68 years old. We analyzed that IL-12Rβ2-rs2201584 and -rs1874791 were associated with an increased risk of COPD, which may not be affected by aging, although age growth has been reported as a potential risk factor for COPD.26 COPD is considered to be a disease of older men. However, more and more studies have shown that women are increasingly burdened with COPD.27,28 According to the gender-specific prevalence rate of COPD, it is necessary to prevent and treat COPD for different genders. Combined with the results of this study, IL-12Rβ-rs2201584 and -rs1874791 may be expected to become new targets for the prevention and treatment of COPD in female and male Han populations in southern China, respectively. Similarly, IL-12Rβ2-rs2201584 (associated with increased risk of COPD in drinking participants) and -rs1874791 (associated with increased risk of COPD in smoking participants) are also expected to become new targets for individualized prevention and treatment of COPD in southern Chinese Han population. Combined with the FPRP analysis, the above new findings are worth noting.
The up-regulation of IL-12Rβ2 can promote the differentiation of cells into Th1 cells. Th1 cells are closely related to the occurrence and development of COPD: Th1 cells cause inflammatory infiltration and pathological changes by secreting pro-inflammatory factors (IL-12, TNF-α and IFN-γ).29 Combined with the results of this study, we speculated that IL-12Rβ2-rs2201584 and-rs1874791 may cause the up-regulation of IL-12Rβ2 expression to promote cell differentiation into Th1 cells, thereby secreting proinflammatory cytokines, and ultimately increasing the risk of COPD. The above is only speculation, and further molecular mechanism studies are necessary to explore how IL-12Rβ2-rs2201584 and -rs1874791 affect COPD susceptibility in southern Chinese Han population by affecting IL-12Rβ2 expression.
In addition, we also found evidence that IL-12Rβ2-rs6679356 was associated with COPD susceptibility. IL-12Rβ2-rs6679356 has been reported to be associated with a variety of inflammatory diseases, such as primary biliary cholangitis30 and allergic rhinitis.31 However, IL-12Rβ2-rs6679356 was only associated with COPD risk in participants younger than 68 years old in our study. IL-12Rβ2-rs3790567 has also been reported to be associated with the occurrence and development of systemic lupus erythematosus,32 allergic rhinitis31 and other diseases. But we only found IL-12Rβ2-rs3790567 was associated with COPD risk in female participants. Therefore, the evidence that IL-12Rβ2-rs6679356 and -rs3790567 are identified as susceptibility loci of COPD in Han population from southern China is insufficient, and further validation experiments are necessary.
In any case, this study has laid a reliable theoretical foundation for the study on mechanism of IL-12Rβ2 in the development of COPD. At the same time, it provides a new idea for the risk assessment and clinical individualized prevention and treatment of COPD in southern Chinese Han population. There are still some limitations in this study: on the one hand, in order to ensure the reliability and repeatability of the results, a large sample size verification study is necessary; on the other hand, necessary functional tests should be added in subsequent studies to further understand the molecular mechanism of IL-12Rβ2 in the occurrence and development of COPD. In any case, this study is the first to explore the association between the genetic polymorphisms of IL-12Rβ2 and the susceptibility to COPD in Han population from southern China and found noteworthy positive results.
Conclusion
Genetic polymorphisms in IL-12Rβ2 (rs2201584, rs1874791, rs6679356, and rs3790567) were associated with the COPD susceptibility. In particular, there were sufficient evidences that IL-12Rβ2-rs2201584 and -rs1874791 were associated with the increasing risk of COPD. This study has laid a theoretical foundation for clinical COPD risk assessment and individualized treatment.
Data Sharing Statement
The datasets used and analyzed in the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted under the standard approved by the ethics committee of Hainan General Hospital. And conformed to the ethical principles for medical research involving humans of the World Medical Association Declaration of Helsinki. All participants signed informed consent forms before participating in this study.
Consent to Publication
All authors agreed to publish the manuscript.
Acknowledgments
We thank all authors for their contributions and support.
Funding
This study was supported by the National Natural Science Foundation of China (81860015 and 81660013).
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi:10.1016/S0140-6736(12)61728-0
2. Halpin DMG, Criner GJ, Papi A, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD Science Committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):24–36. doi:10.1164/rccm.202009-3533SO
3. Shastri MD, Shukla SD, Chong WC, et al. Smoking and COVID-19: what we know so far. Respir Med. 2021;176:106237. doi:10.1016/j.rmed.2020.106237
4. Shih YM, Chang YJ, Cooke MS, et al. Alkylating and oxidative stresses in smoking and non-smoking patients with COPD: implications for lung carcinogenesis. Free Radic Biol Med. 2021;164:99–106. doi:10.1016/j.freeradbiomed.2020.12.442
5. Silverman EK. Genetics of COPD. Annu Rev Physiol. 2020;82:413–431. doi:10.1146/annurev-physiol-021317-121224
6. Yuan C, Chang D, Lu G, Deng X. Genetic polymorphism and chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:1385–1393. doi:10.2147/COPD.S134161
7. Leap J, Arshad O, Cheema T, Balaan M. Pathophysiology of COPD. Crit Care Nurs Q. 2021;44(1):2–8. doi:10.1097/CNQ.0000000000000334
8. Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J. 2005;25(3):552–563. doi:10.1183/09031936.05.00117504
9. Barnes PJ. Role of HDAC2 in the pathophysiology of COPD. Annu Rev Physiol. 2009;71:451–464. doi:10.1146/annurev.physiol.010908.163257
10. Caramori G, Casolari P, Barczyk A, Durham AL, Di Stefano A, Adcock I. COPD immunopathology. Semin Immunopathol. 2016;38(4):497–515. doi:10.1007/s00281-016-0561-5
11. Tan HL, Rosenthal M. IL-17 in lung disease: friend or foe? Thorax. 2013;68(8):788–790. doi:10.1136/thoraxjnl-2013-203307
12. Bal SM, Bernink JH, Nagasawa M, et al. IL-1β, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016;17(6):636–645. doi:10.1038/ni.3444
13. Narendra DK, Hanania NA. Targeting IL-5 in COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:1045–1051. doi:10.2147/COPD.S155306
14. Shevach EM, Chang JT, Segal BM. The critical role of IL-12 and the IL-12R beta 2 subunit in the generation of pathogenic autoreactive Th1 cells. Springer Semin Immunopathol. 1999;21(3):249–262. doi:10.1007/BF00812256
15. Jogdand P, Siddhuraj P, Mori M, et al. Eosinophils, basophils and type 2 immune microenvironments in COPD-affected lung tissue. Eur Respir J. 2020;55(5):1900110. doi:10.1183/13993003.00110-2019
16. Gao W, Gao L, Yang F, Li Z. Circulating JNK pathway-associated phosphatase: a novel biomarker correlates with Th17 cells, acute exacerbation risk, and severity in chronic obstructive pulmonary disease patients. J Clin Lab Anal. 2022;36(1):e24153. doi:10.1002/jcla.24153
17. Boisson-Dupuis S, El Baghdadi J, Parvaneh N, et al. IL-12Rβ1 deficiency in two of fifty children with severe tuberculosis from Iran, Morocco, and Turkey. PLoS One. 2011;6(4):e18524. doi:10.1371/journal.pone.0018524
18. Akar HH, Kose M, Ceylan O, et al. Congenital IL-12R1β receptor deficiency and thrombophilia in a girl homozygous for an IL12RB1 mutation and compound heterozygous for MTFHR mutations: a case report and literature review. Eur J Microbiol Immunol (Bp). 2014;4(1):83–87. doi:10.1556/EuJMI.4.2014.1.8
19. Sahiratmadja E, Baak-Pablo R, de Visser AW, et al. Association of polymorphisms in IL-12/IFN-gamma pathway genes with susceptibility to pulmonary tuberculosis in Indonesia. Tuberculosis. 2007;87(4):303–311. doi:10.1016/j.tube.2007.02.001
20. Weiss ST. Lung function and airway diseases. Nat Genet. 2010;42(1):14–16. doi:10.1038/ng0110-14
21. Zhou Y, Wang C, Yao W, et al. [A cross-sectional survey of familial aggregation of chronic obstructive pulmonary disease: in seven provinces/cities in China]. Zhonghua nei ke za zhi. 2014;53(5):354–358. Chinese.
22. Zhou Y, Zhang Y, Zhao R, et al. Integrating RNA-Seq with GWAS reveals a novel SNP in immune-related HLA-DQB1 gene associated with occupational pulmonary fibrosis risk: a multi-stage study. Front Immunol. 2021;12:796932. doi:10.3389/fimmu.2021.796932
23. Yang LT, Liu X, Wu GH, Chen LF. Association between tumor necrosis factor-α −308 Gauss/A polymorphism and risk of silicosis and coal workers pneumoconiosis in Chinese population. Inhal Toxicol. 2018;30(6):213–217. doi:10.1080/08958378.2018.1494766
24. Li JX, Fu WP, Zhang J, et al. A functional SNP upstream of the ADRB2 gene is associated with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:917–925. doi:10.2147/COPD.S151153
25. Abudureheman Z, Li L, Zhong X, et al. The rs74794265 SNP of the SREK1 gene is associated with COPD in Kashi, China. Int J Chron Obstruct Pulmon Dis. 2021;16:2631–2636. doi:10.2147/COPD.S321150
26. Soriano JB, Polverino F, Cosio BG. What is early COPD and why is it important? Eur Respir J. 2018;52(6).
27. Ntritsos G, Franek J, Belbasis L, et al. Gender-specific estimates of COPD prevalence: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:1507–1514. doi:10.2147/COPD.S146390
28. Sørheim IC, Johannessen A, Gulsvik A, Bakke PS, Silverman EK, DeMeo DL. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax. 2010;65(6):480–485. doi:10.1136/thx.2009.122002
29. Sun J, Liu T, Yan Y, et al. The role of Th1/Th2 cytokines played in regulation of specific CD4 (+) Th1 cell conversion and activation during inflammatory reaction of chronic obstructive pulmonary disease. Scand J Immunol. 2018;88(1):e12674. doi:10.1111/sji.12674
30. Wasik U, Wunsch E, Norman GL. Polymorphisms of IL12RB2 may affect the natural history of primary biliary cholangitis: a single centre study. J Immunol Res. 2017;2017:2185083. doi:10.1155/2017/2185083
31. Wei P, Kou W, Sun R, et al. Erratum to: association study between interleukin-12 receptor β1/β2 genes and allergic rhinitis in the Chinese Han population. Eur Archiv Oto-Rhino Laryngol. 2015;272(4):895–896. doi:10.1007/s00405-014-3204-2
32. You Y, Zhai ZF, Chen FR, Chen W, Hao F. Autoimmune risk loci of IL12RB2, IKZF1, XKR6, TMEM39A and CSK in Chinese patients with systemic lupus erythematosus. Tissue Antigens. 2015;85(3):200–203. doi:10.1111/tan.12522
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.