Back to Journals » Cancer Management and Research » Volume 14
Role of Emergency Surgery for Fatal Complications of Gestational Trophoblastic Neoplasia: A Single-Center Experience
Authors Wang Z , Han P, Zhu X, Ying J, Qian J
Received 9 November 2021
Accepted for publication 11 February 2022
Published 27 February 2022 Volume 2022:14 Pages 851—861
DOI https://doi.org/10.2147/CMAR.S346421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Zhe Wang, Peilin Han, Xiaoxu Zhu, Jun Ying, Jianhua Qian
Department of Gynecology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
Correspondence: Jianhua Qian, Tel +86 13858028056, Email [email protected]
Purpose: High-risk gestational trophoblastic neoplasia (GTN) can lead to fatal complications; however, few reports have assessed emergency surgery as a treatment option for such complications. Thus, this study aimed to analyze the clinical features and prognosis of patients with GTN who underwent emergency surgery.
Patients and Methods: Thirteen patients with high-risk or ultra-high-risk GTN who underwent emergency surgery for fatal complications in the First Affiliated Hospital of Zhejiang University, School of Medicine from 2013 to 2020 were analyzed, and their medical records were reviewed. The patients’ characteristics and treatment were evaluated with respect to outcomes.
Results: Thirteen patients with GTN who underwent 15 emergency surgical procedures were identified in our center. The mean International Federation of Gynecology and Obstetrics score of these patients was 14.8 (range, 11– 19). Of the 13 patients, six underwent brain surgeries, such as tumor resection (n = 5) and conservative surgery (n = 1). All the patients received multi-agent chemotherapy after emergency surgery, and the mean time from emergency surgery to subsequent chemotherapy was 12.7 days. Of the 13 patients, 10 (77%) were cured and disease-free, with a follow-up period ranging from 3 months to 8 years. All the patients (n = 6) who underwent emergency brain surgery survived and achieved complete remission.
Conclusion: For patients with high-risk GTN with fatal complications, especially brain lesions, emergency surgery combined with subsequent chemotherapy may provide a favorable prognosis.
Keywords: ultra high-risk, multidrug chemotherapy, multidisciplinary, brain metastasis, prognosis
Introduction
Gestational trophoblastic neoplasia (GTN) is a group of gynecological malignancies related to pregnancy, including choriocarcinoma, placental site trophoblastic tumor, and epithelioid trophoblastic tumor. GTN is a rare condition, with an estimated incidence of 1 case per 40,000 pregnancies.1–3 The main treatment for GTN is chemotherapy, which should be administered based on the disease risk, classified according to the International Federation of Gynecology and Obstetrics (FIGO) staging system and the World Health Organization prognostic scoring system.4
Because of GTN’s remarkable sensitivity to chemotherapy, its cure rate can reach 100% and >90% in patients with low-risk (FIGO score <7) and high-risk (7 ≤ FIGO score ≤12) GTN, respectively.5 However, the prognosis of patients with ultra-high-risk (FIGO score >12) GTN and refractory GTN remains poor,6–8 and their death is mostly linked to chemoresistance and severe complications, including hemorrhagic metastases, infection, multisystem organ failure, and tumor lysis syndrome.9,10 Patients with high-risk GTN can experience life-threatening events, including severe tumorous hemorrhage, digestive tract perforation, and brain herniation, any time before or during treatment. These patients die easily as it is difficult for them to receive timely treatment. Emergency surgery is the only treatment option that is potentially effective. However, performing an emergency surgery in these patients with complications is quite challenging, given their clinical features: on the one hand, they are in a critical condition (hemorrhagic shock and cerebral hemorrhage that inhibit breathing), and on the other, surgical sites often occur in where gynecologist are not often involved. The surgical procedures used for these patients include pulmonary wedge resection, craniotomy, nephrectomy, partial liver resection, and bowel resection. Moreover, as patients with GTN who have metastases (brain and digestive tract metastases) are rare, studies on emergency surgeries, postoperative treatment, and prognosis of these patients are scarce.
Hence, this study aimed to retrospectively analyze the data of patients with high-risk GTN who underwent emergency surgeries in our center to evaluate the effectiveness of emergency surgeries performed for such patients.
Materials and Methods
Patients
In this retrospective study, the data of 13 patients with ultra-high risk GTN who underwent 15 emergency surgeries before diagnosis or during treatment at the First Affiliated Hospital of Zhejiang University, School of Medicine from 2013 to 2020 were analyzed. Our hospital is a comprehensive regional medical center, and several patients with high-risk GTN and concurrent multiorgan metastasis, particularly GTN diagnosed in the context of emergency surgeries for different organs, are referred to our hospital.
Of the 13 patients, four underwent emergency surgeries in other hospitals and were transferred to our hospital for further management; one underwent emergency surgery, received initial and salvage chemotherapy in other hospitals, and was transferred to our hospital after drug resistance occurred; and the remaining patients received treatment at our hospital.
This study was approved by the Institutional Review Board of the First Affiliated Hospital, Zhejiang University School of Medicine (2020IIT339) and complies with the Declaration of Helsinki. The patients provided their written informed consent to participate in this study. Written informed consent was obtained for the publication of any potentially identifiable images or data included in the present study.
Pretreatment Evaluation
Medical history investigation, physical examination, serum beta-human chorionic gonadotropin (β-hCG) level measurements; lung radiography; chest, abdominal, and brain computed tomography (CT); and/or brain magnetic resonance imaging (MRI) with positron emission tomography/computed tomography when indicated were performed for all the patients. The FIGO staging and risk score was used to identify the disease stage and score.
Treatment
All the GTN patients suffering fatal complications in our hospital, which needed emergency surgery, were managed by a dedicated team led by a senior gynecological oncologist and a multidisciplinary team (MDT), comprising gynecological oncologists, general surgeons, urologists, neurosurgeons, radiotherapists, radiologists, pathologists, and intensive care unit (ICU) doctors. When needed, doctors of different specialties are involved in the disease management. MDT exerted its functions on diagnosis, indications for emergency surgery, surgical procedures, postoperative monitoring, and timing of postoperative chemotherapy for every patient involved.
All patients with ultra-high risk GTN received multi-agent chemotherapy. In our center, the first-line chemotherapy was EMA-CO regimen (etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine), except for one drug-resistant patient who was referred from another hospital had accepted EMA-EP regimen (etoposide, methotrexate, actinomycin D, and cisplatin) as initial treatment. For those who did not have a satisfactory serum β-HCG level decline or developed drug resistance to initial treatment, the salvage treatment was employed, we generally preferred FAVE regimen (floxuridine, actinomycin D, vincristine, and etoposide). If FAVE regimen failed, other regimens would be took into consideration, which comprised (1) paclitaxel/cisplatin, alternated with paclitaxel/etoposide (TP/TE); (2) bleomycin, etoposide, and cisplatin (BEP); (3) paclitaxel, etoposide, and cisplatin (TEP); and/or (4) methotrexate, bleomycin, etoposide (MBE). Programmed cell death protein 1 (PD-1) inhibitors were administered to some patients who were insensitive to salvage treatment. After hCG levels normalized, 3–5 cycles of consolidation chemotherapy were administered.
Assessments After Treatment
During the treatment period, measurement of serum β-hCG levels, complete blood counts, and liver and kidney function tests were performed weekly to assess the treatment effect and monitor toxicity. Complete remission (CR) was defined as serum β-hCG levels within the normal range, revealed in measurements that were performed once weekly for three consecutive weeks. Recurrence was defined as an increase in serum β-hCG levels 1 month after CR. Disease progression (PD) was defined as an increase or stabilization of serum β-hCG levels or the appearance of new metastases after at least two consecutive treatment courses. The serum β-hCG concentration of each patient was measured weekly until 1 month after the end of treatment, then monthly for the first year of remission and then at 3-month intervals during the second year of follow-up.
Results
Patient Demographics
Thirteen patients were included in this study. The average age of the patients was 32 (range 21–50) years, and 11 (85%) patients were less than 40 years old (Table 1). Regarding the commonest entities in the patients’ obstetrical histories, eight (62%), three (23%), and two (15%) patients had term deliveries, molar pregnancies, and non-molar abortion. The interval from the last pregnancy to GTN diagnosis ranged from <1 month to 15 years. The serum β-hCG levels of 11 (84%) patients at diagnosis were >100,000 mIU/mL before treatment in our hospital. Regarding the lesion site, the primary lesion was observed in the uterus in eight (62%) patients, and the lesion was not detected in five (38%) patients. The metastases distribution was as follows: lung, spleen, kidney, gastrointestinal tract, liver, and brain metastases in 12 (92%), two (15%), four (31%), six (46%), eight (62%), and seven (54%) patients, respectively. Furthermore, one (8%) patient had ilium metastasis, and one patient had no distant metastases; the tumor in the latter was classified as FIGO stage I. The remaining 12 patients had FIGO stage IV tumors. Besides lung metastases, two patients (17%) had solitary brain metastases, and the remaining 11 (83%) had three or more distant metastases. The mean FIGO score of the patients was 14.8 (range 11–19).
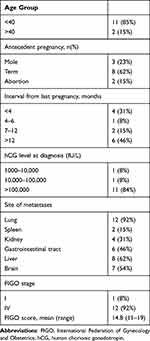 |
Table 1 Clinical Characteristics of Gestational Trophoblastic Neoplasia Patients Suffering Emergency Surgery |
Treatments
All the patients received multi-agent chemotherapy (Table 2). Regarding the initial treatment, 12 (92%) and one (8%) patient received the EMA/CO and EP/EMA regimens, respectively. Of the patients who received EMA/CO, treatment was discontinued in six of them because of no response (five patients) and death (one patient), and these five patients with no response to EMA/CO accepted salvage treatment with the FAVE chemotherapy regimen. Four of these patients were no response to FAVE and accepted the TP/TE regimen; of those, one died during treatment with the TP/TE regimen, and one failed to respond to TP/TE and died after receiving subsequent TOG, TEP, BEP, MBE, and PD-1 inhibitors.
 |
Table 2 Clinical Characteristics of Emergency Surgery, Postoperative Treatment Plan, and Outcome of Patients with High-Risk Gestational Trophoblastic Neoplasia |
The 13 patients underwent 15 emergency surgical procedures. Six of them underwent brain surgeries, including tumor resection (n = 5) and conservative surgery (n = 1) due to cerebral hemorrhage (n = 4) or brain herniation (n = 2). Three patients underwent partial liver resection due to hemorrhage; resection of the affected bowel was performed simultaneously in one of them. Three patients underwent bowel operations for hemorrhage (n = 2) or perforation (n = 1); hysterectomy was performed in one of them simultaneously. One patient underwent nephrectomy for hemorrhage. Two patients underwent uterine surgeries (hysterectomy [n = 1] and cornual resection [n = 1]). Each of the two patients underwent two emergency surgeries.
In nine patients, surgeries were performed before chemotherapy initiation; the underlying disease was known in only one patient since it occurred after the emergency brain surgery, permitting pathological diagnosis. Five surgeries were performed during the first course of chemotherapy, and one was performed after the first course of chemotherapy cycle, given that multi-line chemotherapy drug resistance had occurred. The mean time from emergency surgery to subsequent chemotherapy was 12.7 (range 1–31) days.
Moreover, one, three, and one patient underwent elective hysterectomy during chemotherapy, interventional therapy for a liver lesion, and whole-brain radiotherapy, respectively.
Outcomes
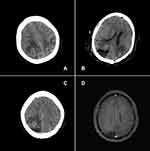
Of the 13 patients, 10 (77%) were cured and disease-free, with a follow-up duration ranging from 3 months to 8 years. All the patients (n = 6) who underwent emergency brain surgery survived and achieved CR (Table 3); among them, only two patients had neurological sequelae, which were consistent with their previous symptoms: one had impaired vision of the right eye (anisocoria) and one had impaired left limb function. Figure 1 shows the imaging findings of one patient during the treatment; the patient had cerebral hemorrhage and brain herniation during the first cycle of chemotherapy. After the patient underwent life-saving emergency surgery, she received chemotherapy again and was finally healed. Three patients died, among whom two developed drug resistance and died from respiratory failure (Table 4). One patient died owing to uncontrolled postoperative bleeding during the first chemotherapy cycle after undergoing emergency surgery.
 |
Table 3 Clinical Characteristics of Six Patients with Gestational Trophoblastic Neoplasia and Brain Metastases |
 |
Table 4 Clinical Characteristics of Patients with Gestational Trophoblastic Neoplasia Who Died After Emergency Surgery or Subsequent Treatment |
Discussion
In this study, most emergency surgeries for GTN were performed for fatal bleeding, which mainly occurred when the disease was not clearly diagnosed or during the first chemotherapy cycle. These patients were all high-risk (FIGO scores >11), and they had multiple metastatic lesion sites. With the help of the MDT, the patients received multi-drug chemotherapy after undergoing emergency surgery and finally achieved a good prognosis. Particularly, patients who underwent emergency brain surgery were cured and had no obvious residual neurological sequelae, thereby achieving a better quality of life.
Since GTN is highly sensitive to chemotherapy, the cure rate of low-risk GTN can be 100%. The treatment difficulties are mainly observed in patients with high-risk GTN, particularly those with ultra-high-risk GTN. Besides chemotherapy, surgery is an important supplementary treatment option for GTN. Emergency surgery often saves patients with life-threatening conditions and provides opportunities for follow-up treatments. According to reports, the mortality rate of patients with high-risk GTN is 5–10%,11–13 while that of patients with ultra-high-risk GTN is 40%.6,14 In this study, except for one patient with a FIGO score of 11, the patients had FIGO scores above 13. These patients all had life-threatening emergencies; however, they had a satisfactory survival after they underwent emergency surgery and received subsequent multi-agent chemotherapy. Based on our experience, this good result was dependent on multiple factors that are related to the platform of a general hospital with competent departments (internal medicine, surgery, radiology intervention, intensive care unit, and infection).
First, diagnosis of the disease and its complications is difficult; this poses great challenges to later treatments, given that approximately 80% of GTN cases have remote metastatic lesions with different clinical manifestations.15 Many studies report that women with ruptured GTN lesions in the liver, spleen, or pelvis can present with abdominal pain and a massive hemoperitoneum.16,17 Infringement of the nervous system can manifest as hemiparesis, paresthesia, or seizures.16,18 Acute intracranial hemorrhage can lead to unconsciousness and sudden collapse. Digestive tract metastasis, including gastric, colon, rectal, and small bowel metastasis, may present as upper gastrointestinal bleeding, melena, perforation, and intestinal obstruction.19–22 In our center, early recognition and processing relies on effective in-hospital education regarding GTN and a high degree of professionalism among doctors in each clinical department. Second, in the MDT, each team member performs their functions smoothly, efficiently, and integrally to save lives. The gynecological doctors lead the MDT in formulating patients’ treatment plans, which mainly include the timing of emergency surgery, ICU stay, timing of chemotherapy after emergency surgery, and management of postoperative and chemotherapy complications. Third, patients with ultra-high-risk GTN present with emergency situations requiring emergency surgery, which is an opportunity for subsequent chemotherapy after a successful surgery. This treatment choice may prevent death in many patients with ultra-high-risk GTN who could die from emergency events, and therefore increase survival rates. In our center, surgery for GTN, especially in emergency and critical conditions, is performed to save patients’ lives and to restore stable vital functions; it is not performed for radical resection. Hence, we often select conservative surgery, including local resection of the lesion, instead of resection of the whole organ, to avoid large-scale resection of organs, to preserve functions, and to achieve fast recovery so as to administer chemotherapy. Our treatment protocol for GTN is similar to that in Europe; this includes referring patients with GTN to large centers for centralized treatment management. Standardized treatment protocols for diseases are beneficial to GTN-related disciplines as they help to improve patient prognosis.
In this study, besides patients being admitted to the emergency department before diagnosis, most patients experienced emergency events during the first EMA-CO course. This result was consistent with that of Charing Cross Center study, wherein patients with GTN having a FIGO score >12 were prone to premature death, especially during the chemotherapy induction period.14 We deem that this may be related to patients’ high tumor burden and tumor characteristics. Intraoperative findings and dissection of the surgical specimen revealed uterus, brain, liver, and kidney lesions close to the affected organ’s serosal surface, which are more likely to rupture and cause hemorrhage and perforation. Because GTN is highly responsive to chemotherapy drugs, after chemotherapy, the tumor undergoes lysis and necrosis, leading to bleeding, perforation, and intracranial hypertension; these conditions predispose patients to early death; one patient in our study died from uncontrollable multiorgan bleeding after chemotherapy. To this end, the Charing Cross Center proposed a low-dose EP induction chemotherapy regimen (etoposide 100 mg/m2 and cisplatin 20 mg/m2 on days 1 and 2, repeated weekly for 1–2 cycles before administration of the EMA-CO chemotherapy regimen) to reduce early mortality of patients. This chemotherapy regimen resulted in a decrease in the early mortality rate from 7.2% to 0.7% in their study.23 However, these data came from high-risk patients with a large disease burden. For patients with a FIGO score >12, the effect of the low-dose EP regimen is not ideal. Among 6 ultra high-risk patients who received induction low-dose EP, 3 patients died; 1 was an early death. At present, there is no good solution for preventing early death or emergency events, and we can only pay more attention to the patient’s situation and deal with it in a timely manner.
GTN rarely results in brain metastases, and the prognosis of brain metastases is not good.7 However, in the current study, the prognosis and recovery of five patients were very good after surgery, similar to the results in Xiao et al’s study, in which patients who underwent surgery had a better prognosis and recovery rate than those who did not undergo emergency surgery.24 For such result, it may be due to the blood-brain barrier that makes difficult for the entrance of chemotherapeutic drugs into the brain. Besides chemotherapy, surgical resection might be an irreplaceable treatment option for brain metastases. Moreover, experts have proposed a multi-modal treatment plan, ie, chemotherapy plus radiotherapy; however, both curative effects and potential problems of radiotherapy are unclear. Because GTN is rare and patients with central nervous system involvement account for only a small proportion of cases and usually require emergency treatments, well-designed clinical research and clinical trials for such patients with the aim of optimizing therapy are unlikely to be conducted. Based on our findings, in patients in whom whole-brain irradiation is unable to proceed due to lack of conditions or patients with similar presentations like our study patients, with the improvement of surgical techniques and postoperative monitoring techniques, surgery is safe and reliable, especially for GTN with lesions located near the serosal surface of the affected organ.
This study had few limitations. First, this was a single-center retrospective study. Since the region does not yet have an established medical center dedicated for treating GTN, most patients select a hospital with no clear direction. Compared to obstetrics and gynecology hospitals, a general hospital (like ours) is more likely to accept patients with both GTN and other systemic clinical manifestations; this resulted in selection bias. Second, because GTN is rare and the number of patients undergoing emergency surgery for ultra-high-risk GTN is even rarer, it is difficult to conduct prospective, controlled clinical studies to evaluate the effect of emergency surgery and further assess the beneficiary population. Hence, it is very important that research organizations worldwide unite to conduct GTN research.
Conclusion
We demonstrated that emergency surgery with multi-agent chemotherapy saved the lives of patients with high-risk GTN who had fatal complications, resulting in favorable prognoses. In particular, emergency surgery combined with chemotherapy resulted in great benefits and few sequelae in patients with brain complications. Most of the patients had emergency events during the administration of the first chemotherapy, and this situation requires great attention and emphasis. Based on our study findings, for better prognosis, it is best that patients be admitted to a general hospital with competence for diagnosis and treatment and an MDT.
Nevertheless, our experience is limited and the findings may not be generalized to the total population. We call for larger scale and higher quality research which focus on the following areas: role of emergency surgery in improving prognosis of high-risk GTN, and prediction and prevention of fatal complications in patients with GTN.
Acknowledgments
We thank Dr. Jie Zhou for his valuable comments on the manuscript, Dr. Jingfeng Xu for his interpretation of the CT/MRI images, and Ms. Li Zhou for editing the images in the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ngan S, Seckl MJ. Gestational trophoblastic neoplasia management: an update. Curr Opin Oncol. 2007;19(5):486–491. doi:10.1097/CCO.0b013e3282dc94e5
2. Brinton LA, Bracken MB, Connelly RR. Choriocarcinoma incidence in the United States. Am J Epidemiol. 1986;123(6):1094–1100. doi:10.1093/oxfordjournals.aje.a114337
3. Berkowitz RS, Goldstein DP. Current management of gestational trophoblastic diseases. Gynecol Oncol. 2009;112(3):654–662. doi:10.1016/j.ygyno.2008.09.005
4. Tsakiridis I, Giouleka S, Kalogiannidis I, Mamopoulos A, Athanasiadis A, Dagklis T. Diagnosis and management of gestational trophoblastic disease: a comparative review of national and international guidelines. Obstet Gynecol Surv. 2020;75(12):747–756. doi:10.1097/OGX.0000000000000848
5. Freitas F, Braga A, Viggiano M, et al. Gestational trophoblastic neoplasia lethality among Brazilian women: a retrospective national cohort study. Gynecol Oncol. 2020;158(2):452–459. doi:10.1016/j.ygyno.2020.04.704
6. Kong Y, Yang J, Jiang F, et al. Clinical characteristics and prognosis of ultra high-risk gestational trophoblastic neoplasia patients: a retrospective cohort study. Gynecol Oncol. 2017;146(1):81–86. doi:10.1016/j.ygyno.2017.04.010
7. Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376(9742):717–729. doi:10.1016/S0140-6736(10)60280-2
8. Agarwal R, Alifrangis C, Everard J, et al. Management and survival of patients with FIGO high-risk gestational trophoblastic neoplasia: the U.K. experience, 1995–2010. J Reprod Med. 2014;59(1–2):7–12.
9. Lybol C, Centen DW, Thomas CM, et al. Fatal cases of gestational trophoblastic neoplasia over four decades in the Netherlands: a retrospective cohort study. BJOG. 2012;119(12):1465–1472. doi:10.1111/j.1471-0528.2012.03480.x
10. Schuman S, Pearson JM, Lucci JA
11. Neubauer NL, Strohl AE, Schink JC, Lurain JR. Fatal gestational trophoblastic neoplasia: an analysis of treatment failures at the Brewer Trophoblastic Disease Center from 1979–2012 compared to 1962–1978. Gynecol Oncol. 2015;138(2):339–342. doi:10.1016/j.ygyno.2015.05.041
12. Al-Husaini H, Soudy H, Darwish A, et al. Gestational trophoblastic neoplasia: treatment outcomes from a single institutional experience. Clin Transl Oncol. 2015;17(5):409–415. doi:10.1007/s12094-014-1251-1
13. Jiang F, Wan XR, Xu T, et al. Evaluation and suggestions for improving the FIGO 2000 staging criteria for gestational trophoblastic neoplasia: a ten-year review of 1420 patients. Gynecol Oncol. 2018;149(3):539–544. doi:10.1016/j.ygyno.2018.04.001
14. Bolze PA, Riedl C, Massardier J, et al. Mortality rate of gestational trophoblastic neoplasia with a FIGO score of ≥13. Am J Obstet Gynecol. 2016;214(3):
15. Berkowitz RS, Goldstein DP. Chorionic tumors. N Engl J Med. 1996;335(23):1740–1748. doi:10.1056/NEJM199612053352306
16. Kim SJ. Difficult cases of trophoblastic disease and its classification and management. Semin Oncol. 1982;9(2):234–238.
17. Erb RE, Gibler WB. Massive hemoperitoneum following rupture of hepatic metastases from unsuspected choriocarcinoma. Am J Emerg Med. 1989;7(2):196–198. doi:10.1016/0735-6757(89)90138-1
18. Ghaemmaghami F, Behtash N, Ayatollahi H, Hanjani P. Successful treatment of two patients with gestational trophoblastic neoplasm presenting with emergent neurologic symptoms. Int J Gynecol Cancer. 2006;16(2):937–940. doi:10.1111/j.1525-1438.2006.00213.x
19. Balagopal P, Pandey M, Chandramohan K, Somanathan T, Kumar A. Unusual presentation of choriocarcinoma. World J Surg Oncol. 2003;1(1):4. doi:10.1186/1477-7819-1-4
20. Galloway SW, Yeung EC, Lau JY, Chung SC. Laparoscopic gastric resection for bleeding metastatic choriocarcinoma. Surg Endosc. 2001;15(1):100. doi:10.1007/s004640010052
21. Kristoffersson A, Emdin S, Järhult J. Acute intestinal obstruction and splenic hemorrhage due to metastatic choriocarcinoma. A case report. Acta Chir Scand. 1985;151(4):381–384.
22. Deligdisch L, Waxman J. Metastatic gestational trophoblastic neoplasm. A study of two cases in unusual clinical settings and review of the literature. Gynecol Oncol. 1984;19(3):323–328. doi:10.1016/0090-8258(84)90199-9
23. Alifrangis C, Agarwal R, Short D, et al. EMA/CO for high-risk gestational trophoblastic neoplasia: good outcomes with induction low-dose etoposide-cisplatin and genetic analysis. J Clin Oncol. 2013;31(2):280–286. doi:10.1200/JCO.2012.43.1817
24. Xiao C, Yang J, Zhao J, et al. Management and prognosis of patients with brain metastasis from gestational trophoblastic neoplasia: a 24-year experience in Peking union medical college hospital. BMC Cancer. 2015;15:318. doi:10.1186/s12885-015-1325-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.