Back to Journals » International Journal of General Medicine » Volume 15
Role of Ca2+, Calnexin and Calreticulin in Platelet from Adult Patients with Chronic Immune Thrombocytopenic Purpura
Authors Xu DM, Zhang ZW, Yi JX, Xie L, Yu WJ, Qiu JF, Xu CW, He CL, Xu XR, Yin J
Received 2 November 2021
Accepted for publication 25 January 2022
Published 24 February 2022 Volume 2022:15 Pages 2119—2125
DOI https://doi.org/10.2147/IJGM.S347301
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Da-Ming Xu,1,* Ze-Wen Zhang,2,* Jing-Xing Yi,3 Long Xie,3 Wen-Jun Yu,2 Jin-Feng Qiu,4 Cheng-Wei Xu,5 Chun-Ling He,6 Xian-Ru Xu,7 Jun Yin3
1Division of Urological Surgery, The Second Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong Province, People’s Republic of China; 2Division of Hematology, The Second Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong Province, People’s Republic of China; 3Department of Clinical Laboratory Medicine, The Second Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong Province, People’s Republic of China; 4Division of Respirology, The Second Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong Province, People’s Republic of China; 5Department of Blood Purification, The Second Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong Province, People’s Republic of China; 6Department of Pathology, The Second Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong Province, People’s Republic of China; 7Division of Inventional Ultrasonic Therapeutics, The Second Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Yin, Department of Clinical Laboratory Medicine, The Second Affiliated Hospital of Shantou University Medical College, Dongxia Road North, Shantou, Guangdong Province, 515041, People’s Republic of China, Tel +86 754 8891 5950, Email [email protected]
Background: Adult chronic immune thrombocytopenia (chronic ITP) is a common autoimmune hemorrhagic disease characterized by decreased platelet production and increased platelet destruction, leading to thrombocytopenia. In this study, Ca2+, calnexin (CNX) and calreticulin (CRT) within platelets from adult patients with chronic ITP were investigated.
Methods: Platelets were isolated from blood specimen collected from 20 adult patients with chronic ITP and 20 healthy volunteers. Ca2+, CNX and CRT were determined by flow cytometry, and the results were analyzed with EXPO32 ADC software.
Results: Flow cytometry showed the expressions of Ca2+ (74.19± 19.40% vs 22.79± 10.47%) was elevated (P< 0.05). However, CNX (15.10± 7.32% vs 41.79± 14.45%) and CRT (25.11± 12.66% vs 38.58± 12.02%) were decreased markedly in platelets from adult patients with chronic ITP (P< 0.05 compared with healthy volunteers).
Conclusion: Based on enhanced expression of Ca2+ and attenuated expression of CNX and CRT in patients with chronic ITP, Ca2+ concentration and its associated down-regulated proteins may be important regulatory signals in the pathogenesis of chronic ITP.
Keywords: platelets, chronic immune thrombocytopenia, Ca2+, calnexin, calreticulin
Introduction
Immune thrombocytopenia (ITP) is a common hemorrhagic disease with thrombocytopenia and skin or mucosal bleeding as the main clinical features. The two main causes of ITP are the reduction of megakaryocytes in the producing platelet or the enhancement of platelet destruction. ITP can generally be divided into two types: acute and chronic. Acute ITP tends to occur in children and adolescents, but the disorder onset is often self-limiting, while chronic ITP usually occurs in adults, especially young women, and is a chronic hemorrhagic disease.1 There are many different theories and viewpoints on the pathogenesis of ITP in recent studies. Immune disorders and abnormal proliferation of platelets are the common intersection of many complex pathogenic mechanisms.2 Of concern, in our clinical work, we have found that the uses of anti-immunotherapy and thrombogenesis therapy are generally significantly effective in patients with acute ITP, whereas in patients with chronic ITP, only a transient increase in platelet count is shown, and the bleeding symptoms recur once treatment is reduced or stopped. Therefore, the underlying pathogenic events leading to chronic ITP remain elusive and the mechanisms by which platelets are destroyed by immune abnormalities in chronic ITP are unknown.
In a previous study,3,4 in adults with chronic ITP, platelet death was characterized by loss of mitochondrial membrane potential, phosphatidylserine exposure, caspase-3 activation, enhanced expression of Bax and Bak, and attenuated expression of Bcl-xL, which revealed a possible mechanism pathway of thrombocytopenia in ITP. We found that platelet mitochondrial function is impaired in patients with chronic ITP, and Ca2+ has been shown to be the initial messenger causing endoplasmic reticulum stress (ERS)-related apoptosis. However, there are currently no studies on platelet Ca2+ levels in patients with chronic ITP. In order to identify whether platelets, in chronic ITP patients, exhibit a link between endoplasmic reticulum and mitochondria and determine the pathogenic mechanisms involved in chronic ITP, the expression of Ca2+, CNX and CRT of platelets from patients with chronic ITP were investigated in this study.
Materials and Methods
Patient Enrollment and Blood Specimen Collection
Patients were diagnosed with chronic ITP according to the guidelines and criteria from the International Working Group. Twenty adult patients with 5 males and 15 females (age >18 years) diagnosed as having chronic primary ITP, as well as 20 adult healthy volunteers (control, 5 males and 15 females, normal platelet count and no concurrent illnesses or medications at the time of blood draw) were enrolled into the study which received Institutional Review Board–approved consent in accordance with the Declaration of Helsinki. Patient inclusion criteria were as follows: A. a peripheral blood platelet count ≤3×109/l. B. the course of ITP lasted more than 1 year. For each patient or healthy control, 15 mL whole blood was collected into a tube containing ethylene-diamine-tetraacetic-acid dipotassium salt (EDTA-K2). The tubes were kept at ambient temperature and transported within 1 hr from the clinical ward to the analytical laboratory where it was immediately processed. The platelet count in peripheral blood of patients with chronic ITP was (5–30) ×109/l, and that in healthy controls was (152–299) ×109/l.
Platelet Isolation
Once collected blood samples reached the laboratory, platelet-rich plasma (PRP) was obtained from whole blood by centrifugation at 200×g for 10 min at 25°C.
PRP was centrifuged at 1000×g for another 10 min. The supernatant was discarded, and 1 mL phosphate-buffered saline (PBS) was added for washing once. Platelets were resuspended in PBS and the platelet concentration was adjusted to 1×105/mL.
Detection of Ca2+ in Platelets
Eighty μL of PBS-platelet suspension was incubated with 10 μL of phycoerythrin (PE)-labeled mouse monoclonal antibody against human CD61 and 10 μL of Fluo-3AM (final concentration: 5 mmol/l) were added to detect Ca2+ in platelets. After incubation at 25°C for 30 min, platelets were washed twice with 1 mL PBS, then resuspended in 1 mL PBS. In addition, 80 μL PBS-platelet suspension was taken and 10 μL PE-labeled mouse antibody against human CD61 and 10 μL mouse IgG1 antibody labeled with fluorescein isothiocyanate (FITC) were added. Also, after incubation at 25°C in the dark for 30 min, platelets were washed twice with 1 mL PBS, then resuspended with 1 mL PBS. Ca2+ in platelets was detected by flow cytometry, and the results were analyzed with EXPO32 ADC software.
Detection of CNX in Platelets
Eighty μL PBS-platelet suspension was taken and 10 μL PE-labeled mouse anti-human CD61 monoclonal antibody was added to label the platelets. After incubation in the dark at 25°C for 30 min, platelets were washed once with 1 mL PBS. After adding 100 μL 4% paraformaldehyde solution at 25°C for 10 min in dark, the solution was washed once with 1 mL PBS. The solution was added with 100 μL 0.1% saponin and incubated in dark for 10 min at 25°C to perforate the platelet membrane. Then, 10 μL FITC-labeled mouse anti-human CNX monoclonal antibody was added. For the matching control group, 10 μL FITC-labeled mouse IgG1 antibody was added, incubated at 25°C in dark for 30 min, washed twice with 1 mL PBS, and resuspended with 1 mL PBS. CNX in platelets was detected by flow cytometry, and the results were analyzed with EXPO32 ADC software.
Detection of CRT in Platelets
Ten μL FITC-labeled mouse monoclonal antibody against human CD61 was added to 80μL PBS-platelet suspension and incubated at 25°C for 30 min. After washing once with 1 mL PBS, 100 μL 4% paraformaldehyde solution at 25°C was added and platelets were fixed in dark for 10 min and washed again. Then, 100 μL 0.1% saponin was added and platelets were incubated in the dark at 25°C for 10 min to perforate the platelet membrane, followed by addition of 10 μL PE-labeled with mouse monoclonal antibody against human CRT and 10 μL PE-labeled mouse IgG1 antibody was added to the homozygous control group. After incubated in dark at 25°C for 30 min, 1 mL PBS was added and washed twice and platelets were resuspended in 1 mL PBS. CRT in platelets was detected by flow cytometry, and the results were analyzed with EXPO32 ADC software.
Statistical Analysis
All measured data were normally distributed, as judged by the K–S test, and the results were expressed as mean ± standard deviation. SPSS 20.0 statistical software was used to conduct t-tests for the comparison of the two sample mean data. P<0.05 was considered statistically significant.
Results
Ca2+, CNX and CRT Detection in Platelets
Flow cytometry showed that the percentage of platelets expressing Ca2+ in patients with chronic ITP was (74.19±19.40) %. The percentage of platelets expressing Ca2+ in healthy controls was (22.79±10.47) %. The percentage of platelets expressing Ca2+ in patients with chronic ITP was significantly higher than that in healthy controls (P<0.05).
Flow cytometry showed that the percentage of platelets expressing CNX and CRT in patients with chronic ITP was (15.10±7.32) % and (25.11±12.02) %. The percentage of platelets expressing CNX and CRT in healthy controls was (41.79±14.45) % and (38.58±12.66) %, whereas the percentage of platelets expressing CNX and CRT in patients with chronic ITP was significantly lower than that in healthy controls (P<0.05) (Figures 1 and 2).
 |
Figure 1 Flow cytometry results for the expression of Ca2+, CNX and CRT in platelets. |
 |
Figure 2 Comparison between MFI of Ca2+, CNX and CRT in platelets. (*Comparison with the group of healthy controls, P < 0.05.). |
Discussion
Role of Ca2+ in Platelet Apoptosis
The Ca2+ pools in platelets are mainly divided into two categories: a dense tubular system (DTS) and lysosome-like acidic organelles (LLAO). The former is relieved of Ca2+ by the inositol 1, 4, 5-triphosphate (IP3) signaling pathway, while the latter increases the intracellular Ca2+ concentration by the role of the second messenger, nicotinamide adenine dinucleotide phosphoric acid. Then, store-energized calcium channels (SOCC) are responsible for the free Ca2+ influx in most of the platelet matrix. Under the joint regulation of stromal interaction molecule 1 (STIM1) and the calcium release-activated calcium modulator 1 (Orai1), the effect of Ca2+ release from the calcium pool and increase the concentration of Ca2+ in the cytoplasm can be amplified. When the cytoplasmic Ca2+ concentration is increased, Ca2+ pools can re-uptake Ca2+ by the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), or be pumped into the extracellular environment by the plasma membrane Ca2+-ATPase (PMCA) on the platelet membrane, both of which are required to maintain the low intracellular Ca2+ concentration in the resting state.5
In our study, we have found that the platelet Ca2+ level of patients with chronic ITP is significantly higher than that of healthy controls, indicating the presence of calcium overload in platelets. This may be due to the increased concentration of Ca2+ in the cytoplasm of platelets, which leads to the uptake of Ca2+ by mitochondria from the cytoplasm, further leading to an increased Ca2+ load in mitochondria.6,7 We hypothesize that Ca2+ may be important in the initiation of the platelet mitochondrial apoptosis pathway. Blocking related Ca2+ channel proteins and inhibiting the occurrence of platelet calcium overload may reduce abnormal platelet apoptosis in patients with chronic ITP.
Role of CNX in Platelets Apoptosis
CNX is a Ca2+-regulated transmembrane protein present on the endoplasmic reticulum. CNX contains an endoplasmic reticulum functional domain, diaphragm segment and cytoplasmic tail. The RKPRRE motif at the C terminus may be related to the localization of CNX on the endoplasmic reticulum. The cytoplasmic region of CNX contains sites that can be phosphorylated by casein kinase II, and the luminal region contains Ca2+ binding sites that may be involved in regulating the binding of CNX to proteins that are not fully folded. CNX can bind to the oligosaccharide chains of newly synthesized proteins that have not been fully folded to prevent protein aggregation and ubiquitination and prevent incomplete proteins from leaving the endoplasmic reticulum.8 It also facilitates the binding of other chaperones to these proteins, allowing complete protein folding. The separation of CNX from its bound protein marks the completion of the folding or assembly of the new protein. CNX is part of a CNX/CRT cycle in the endoplasmic reticulum, which can specifically recognize N-junction glycoproteins and is an important mechanism for eukaryotic cells to promote correct protein folding. CNX, under stress, responds to the unfolded protein response, and improves the load-carrying capacity of the endoplasmic reticulum collectively through up-regulation of its own expression.9
The results of this study show that the expression level of CNX in platelets of patients with chronic ITP is significantly lower than that of healthy controls. CNX can maintain Ca2+ homeostasis and signal transduction by regulating the storage and release of Ca2+ from the endoplasmic reticulum.10 In our analysis, there may be endoplasmic reticulum disruption in patient platelets, leading to the down-regulation of the expression of two important Ca2+-related proteins (CRT and CNX) in the endoplasmic reticulum resulting in disruption of platelet intracellular Ca2+ homeostasis and Ca2+ overload.
Role of CRT in Platelet Apoptosis
CRT is one of the major calcium-binding proteins in the endoplasmic reticulum. There are three functional regions in CRT, namely, N-terminal region, P-terminal region and C-terminal region. The C terminus region is similar to calsequestrin, the calcium storage protein of the sarcoplasmic reticulum. This region is highly acidic and has a high Ca2+ binding capacity. The C-terminal contains a tetrapeptide (K-D-E-L) that acts as an endoplasmic reticulum retention signal and is generally thought to be localized in the endoplasmic reticulum.11 When CRT was first discovered, it was considered as a molecular chaperone, coordinating the folding and transport of membrane surface proteins, exocrine proteins, or endoplasmic reticulum-reticulum residing proteins. CRT is found on the surface of the platelet membrane and on the granular membrane in the cytoplasm, interacting with integrin α2β1 and glycoprotein IV, and anti-CRT antibodies can cause activation and aggregation of platelets.
CRT has been shown to be more highly expressed on the surfaces of tumor cells, apoptotic cells and cells that have been treated with drugs such as anthracycline. CRT can bind to CD91/lRP1, a receptor on the surface of innate immune cells, such as macrophages, dendritic cells and other immune cells, to enhance the immune response.12 In addition, studies have shown that high concentrations of CRT and its antibodies can be detected in the serum of rheumatoid or SLE patients, suggesting that CRT is related to autoimmune diseases.13 We show the expression of CRT in platelets of patients with chronic ITP is significantly lower than that of healthy controls. CRT has a high affinity for Ca2+, which buffers the intracellular Ca2+ concentration by binding to Ca2+, thereby regulating intracellular Ca2+ levels and reducing intracellular Ca2+ overload. At present, most studies on the relationship between CRT and apoptosis have focused on eukaryotic cells, while there are few reports about platelets.14 We hypothesize that platelets in patients with chronic ITP are deficient in CRT, resulting in the disruption of Ca2+ homeostasis and Ca2+ overload, then leading to the activation and apoptosis of platelets in the hyperactive state of chronic ITP.
Association Between Ca2+ Overload and Bcl-2 Family Proteins
In a previous study, we found that Bcl-xL levels in platelets of patients with chronic ITP are significantly lower than those of healthy controls, while the expression levels of Bax and Bak are markedly increased. Under the stimulation of immune factors, platelets in patients with chronic ITP experience depolarization of mitochondrial membrane, decreased mitochondrial transmembrane potential and abnormal opening of mitochondrial permeability transition pore (MPTP), which eventually leads to the imbalance of expression of anti-apoptotic and pro-apoptotic proteins in platelets, thereby causing platelet apoptosis.15,16 Studies have shown that Bcl-2 family proteins can interact with the endoplasmic reticulum, control the release of Ca2+, and regulate signal transmission between the endoplasmic reticulum and mitochondria.17 Another study has found that Bak and Bax, pro-apoptotic members of the Bcl-2 family, can bind Ca2+ in the endoplasmic reticulum and sensitize mitochondria to Ca2+ influx, thus regulating the release of cytochrome C and causing platelet apoptosis.18 Platelets contain both Ca2+ and Bcl-2 family proteins, so Ca2+ could be an important regulator of Bcl-2 family proteins. The endoplasmic reticulum is the primary storage site for Ca2+. Ca2+ exists in free form or in the form of binding with intracavitary proteins such as CRT and CNX. When CRT and CNX fail to function normally, the concentration of Ca2+ in platelets is increased, leading to an increase in the uptake of Ca2+ by mitochondria from the cytoplasm. The increase of Ca2+ concentration caused abnormal opening of MPTPs. The continuous opening of MPTP further leads to disappearance of the mitochondrial transmembrane potential, which leads to the rupture of mitochondrial respiratory chain and the release of apoptotic factors such as cytochrome C and caspase, thus initiating platelet apoptosis. Ca2+ plays an important role in accelerating cell death and triggering cell apoptosis in the mitochondria-endoplasmic reticulum positive feedback pathway (Figure 3).
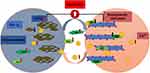 |
Figure 3 Association between Ca2+ overload and Bcl-2 family proteins. |
Conclusion
Based on enhanced expression of Ca2+ and attenuated expression of CNX and CRT in patients with chronic ITP, Ca2+ overload is an important regulatory signal to initiate the mitochondria-mediated apoptosis in platelets. Ca2+ concentration and its associated down-regulated proteins may be potential targets for treatment of chronic ITP.
Statement of Ethics
All participants gave the informed consent before their inclusion in the study. The study protocols were conducted according to the principles of the Declaration of Helsinki and were approved by the Scientific and Medical Ethical Committee of the Second Affiliated Hospital of Shantou University Medical College.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Da-Ming Xu and Ze-Wen Zhang are co-first authors for this study. The authors do not have any conflicts of interest to report in this work.
References
1. Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129(21):
2. Cooper N, Ghanima W, Solomon CG. Immune thrombocytopenia. N Engl J Med. 2019;381(10):
3. Xie L, Xu DM, Cai XJ, et al. Apoptosis in platelets from adult patients with chronic idiopathic thrombocytopenic purpura. Blood Coagul Fibrinolysis. 2021;32(7):434–442. doi:10.1097/MBC.0000000000001054
4. Yung KC, Zhang ZW, Yu WJ, et al. Study on the role of calreticulin within platelet from adult patients with chronic immune thrombocytopenic purpura. Indian J Hematol Blood Transfus. 2018;34(4):711–718. doi:10.1007/s12288-018-0955-8
5. Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7(7):1057–1066. doi:10.1111/j.1538-7836.2009.03455.x
6. Marchi S, Patergnani S, Missiroli S, et al. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018;69:62–72. doi:10.1016/j.ceca.2017.05.003
7. Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27(50):6407–6418. doi:10.1038/onc.2008.308
8. Coe H, Bedard K, Groenendyk J, Jung J, Michalak M. Endoplasmic reticulum stress in the absence of calnexin. Cell Stress Chaperones. 2008;13(4):497–507. doi:10.1007/s12192-008-0049-x
9. Chevet E, Smirle J, Cameron PH, Thomas DY, Bergeron JJ. Calnexin phosphorylation: linking cytoplasmic signalling to endoplasmic reticulum lumenal functions. Semin Cell Dev Biol. 2010;21(5):486–490. doi:10.1016/j.semcdb.2009.12.005
10. Zhang S, Zheng H, Chen Q, et al. The lectin chaperone calnexin is involved in the endoplasmic reticulum stress response by regulating Ca2+ homeostasis in Aspergillus nidulans. Appl Environ Microbiol. 2017;83(15):e00673–17. doi:10.1128/AEM.00673-17
11. Gelebart P, Opas M, Michalak M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol. 2005;37(2):260–266. doi:10.1016/j.biocel.2004.02.030
12. Raghavan M, Wijeyesakere SJ, Peters LR, Del Cid N. Calreticulin in the immune system: ins and outs. Trends Immunol. 2013;34(1):13–21. doi:10.1016/j.it.2012.08.002
13. Wang Y, Xie J, Liu Z, et al. Association of calreticulin expression with disease activity and organ damage in systemic lupus erythematosus patients. Exp Ther Med. 2017;13(5):2577–2583. doi:10.3892/etm.2017.4235
14. Jo SH, Choi JA, Lim YJ, et al. Calreticulin modulates the intracellular survival of mycobacteria by regulating ER-stress-mediated apoptosis. Oncotarget. 2017;8(35):58686–58698. doi:10.18632/oncotarget.17419
15. Melchinger H, Jain K, Tyagi T, Hwa J. Role of platelet mitochondria: life in a nucleus-free zone. Front Cardiovasc Med. 2019;6:153. doi:10.3389/fcvm.2019.00153
16. Gyulkhandanyan AV, Mutlu A, Freedman J, Leytin V. Mitochondrial permeability transition pore (MPTP)-dependent and -independent pathways of mitochondrial membrane depolarization, cell shrinkage and microparticle formation during platelet apoptosis. Br J Haematol. 2015;169(1):142–145. doi:10.1111/bjh.13180
17. Weston RT, Puthalakath H. Endoplasmic reticulum stress and BCL-2 family members. Adv Exp Med Biol. 2010;687:65–77. doi:10.1007/978-1-4419-6706-0_4
18. Scorrano L, Oakes SA, Opferman JT, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300(5616):135–139. doi:10.1126/science.1081208
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
