Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Role of Atogepant in the Treatment of Episodic Migraines: Clinical Perspectives and Considerations
Received 21 February 2022
Accepted for publication 19 April 2022
Published 22 April 2022 Volume 2022:18 Pages 447—456
DOI https://doi.org/10.2147/TCRM.S348724
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Fred Cohen, Hsiangkuo Yuan
Jefferson Headache Center, Department of Neurology, Thomas Jefferson University, Philadelphia, PA, 19107, USA
Correspondence: Hsiangkuo Yuan, Jefferson Headache Center, Department of Neurology, Thomas Jefferson University, 900 Walnut St. Suite 200, Philadelphia, PA, 19107, USA, Tel +1 215-955-2243, Fax +1 215-955-2060, Email [email protected]
Abstract: Advances in molecular biology and neuroscience have led to the discovery of calcitonin gene-related peptide (CGRP), a 37 amino-acid neuropeptide that plays a critical role in the pathogenesis of migraine. CGRP receptor antagonist, also known as gepant, is an oral medication that inhibits the CGRP-related nociceptive signaling pathway. To date, three gepants are approved by the FDA for migraine treatment. Atogepant is a 2nd-generation gepant that non-competitively antagonizes CGRP receptors inhibiting neurogenic inflammation and pain sensitization. With its long half-life and minimal cardiovascular or liver toxicity, it is the first in its class approved primarily for migraine prevention. This article will discuss the evidence, safety, and rationale of atogepant for use in clinical practice.
Keywords: treatment, migraine, CGRP, gepant
Introduction
Migraine is one of the most common neurological disorders seen in medical practice, affecting nearly 16% of the US population.1 It can be a severely debilitating condition, for over 43% of patients with migraine reported moderate-to-severe disability.2 The Global Burden of Disease Survey 2019 has ranked migraine as the 2nd highest cause of years lived with disability, 1st among women between 15 and 49 years of age.3 The annual direct health care costs for patients with migraine in the United States is estimated at $22,364 per person, and a greater total indirect cost estimate of over 19 billion dollars.4 This comprehensive socioeconomic impact warrants expanded efforts to broaden the understanding of migraine pathophysiology and bolster the development of new treatments.
Migraine is a primary headache disorder typically characterized by a pulsating or throbbing head pain lasting between 4 and 72 hours. Pain during a migraine attack is commonly unilateral in location, aggravated by physical activity, and associated with photophobia, phonophobia, nausea, and/or vomiting. Migraine can also be accompanied by muscle tenderness and abnormal skin sensitivity (allodynia).5 In addition to pain, 30% of patients with migraine report having an aura, a complex presentation of reversible sensory, visual, and/or other central nervous system (CNS) symptoms.6 Visual auras are the most common type, reported to occur in 98% of cases of migraine with aura, although speech/motor and sensory symptoms can occur.7 Auras generally occur prior to the onset of headache pain but may persist afterward.6 One of the oldest accounts of migraine was described by Hippocrates, who wrote
Most of the time he saw something shining before him like a light, usually in part of the right eye; at the end of a moment, a violent pain supervened in the right temple.8
Additionally, migraine attacks can have prodromal symptoms, with the most common being fatigue, irritability, yawning/sighing, neck stiffness, and mood change.9 Common migraine triggers include stress, sleep disturbances, weather changes, menstrual cycle changes, and alcohol in other foods.10 Migraine can be classified as episodic migraine (EM) or chronic migraine (CM), with the latter being defined as having 15 or more headache days per month (8 days of which meet criteria for migraine attacks).
Migraine was initially believed to be a vascular condition, with headache pain associated with vasodilation.11 Although the pathophysiology of this disorder is not yet completely understood, progression in the understanding of migraine attacks has suggested vasodilation is not the primary cause of pain, but rather the result of neurogenic inflammation and other mechanisms.12 Neuropeptides have been demonstrated to play a major role in pain signaling and modulation in migraine.13 Calcitonin-gene-related peptide (CGRP), a 37 amino-acid neuropeptide, has been suggested to be implicated in the pathogenesis of migraine.
CGRP and Migraine Pathophysiology
CGRP provides many functions throughout our body, including vascular, digestive, sensory, vestibular, hematopoietic, immunomodulatory, and nociceptive processes.14 CGRP exists as two isomers: α-CGRP and β-CGRP.15 α-CGRP is largely expressed within primary sensory neurons within the trigeminal system and dorsal root ganglia, and is an isoform primarily involved in the pathogenesis of migraine. While not permeable across the blood–brain barrier, it acts on nearby receptive cells and is able to diffuse farther from the release site via volume transmission. The CGRP receptor consists of a G-protein-coupled receptor (GPCR), calcitonin receptor-like receptor (CLR), and an accessory protein known as receptor activity modifying protein 1 (RAMP1).16 When activated, adenylyl cyclase catalyzes the synthesis of cAMP, in addition to other subsequent intracellular messengers, generating a range of functions depending on the associated cell type.17,18 CGRP receptors are found in large concentrations within the thalamus, pineal gland, colliculi, cerebral cortex, cerebellum, striatum, trigeminal ganglion, and trigeminal nucleus caudalis.19 While CGRP receptors are also found within the smooth muscle layer of cerebral blood vessels, there is no evidence of venous CGRP receptors. The neuropeptide can also be found in ganglionic satellite glial cells, astrocytes, immune cells, perivascular mast cells, and hepatocytes, but not central glial cells. CGRP does not have a known reuptake system and is degraded by mast cell tryptase, neutral endopeptidase, and matrix metalloproteinase II.19
Since CGRP is a potent cerebral vasodilator, CGRP is thought to have a role in migraine pathophysiology. It was later discovered that the CGRP serum level increased in the jugular venous outflow following acute migraine attacks,20 where the level reached maximum in the first hour.21 CGRP levels were found to be normalized following treatment of migraine with triptans, topiramate, and onabotulinumtoxin A.22–24 Furthermore, infusion of CGRP triggered migraine-like attacks in the majority of patients with migraine, but not in healthy control participants.25,26 It is worth noting that CGRP is more than just a vasodilator. CGRP is thought to have several mechanisms contributing to the pathophysiology of migraine, and prominent processes include modulating nociceptive transmission and facilitating neurogenic inflammation.27,28 Trigeminovascular neuronal activation triggers a sterile inflammatory response, which results in the release of substance P, neurokinin A, and CGRP. CGRP has been found to be the most abundant neuropeptide within the trigeminal system. It is found primarily in the C and Aδ sensory fibers, and is thought to cause cerebral vasodilation and dysfunctional activation of the trigeminovascular nociceptive system. Neuroinflammation is also generated by CGRP-induced mast cell granulation and IL-1 release from satellite glial cells.29,30 Through these various functions, CGRP contributes to the development and maintenance of a sensitized hyperresponsive state.31
Disrupting this sensitization process by inhibiting CGRP has become a clinically relevant target in the management of migraine. To date, there are two treatment classes of drugs that directly inhibit the function of CGRP: CGRP-targeted monoclonal antibodies (mAbs) and small-molecule CGRP antagonists (gepants). Four CGRP-targeted mAbs have obtained approval from the Food and Drug Administration (FDA) for the preventive treatment of migraine (Table 1), namely erenumab (Aimovig®, Amgen, Thousand Oaks, CA), galcanezumab (Emgality®, Eli Lilly, Indianapolis, IN), fremanezumab (Ajovy®, Teva, Petah Tikva, Israel), and eptinezumab (Vyepti®, Lundbeck, Deerfield, IL). In addition, there are 3 gepants (Table 1) approved by the FDA for abortive or preventive treatment of migraine, namely, ubrogepant (UbrelvyTM, Allergan, Dublin, Ireland), rimegepant (Nurtec®, Biohaven, New Haven, CT), and atogepant (QuliptaTM, Abbvie, North Chicago, IL), with atogepant being the latest released. This article will discuss the efficacy, tolerability, and clinical rationale for atogepant for the preventive treatment of migraine.
 |
Table 1 CGRP-targeted mAbs and Gepants |
Gepants
Gepants were initially developed in the 1990s for the treatment of migraine. Their mechanism of action consists of binding to CGRP receptors and reversing CGRP-induced neurogenic inflammation and vasodilation (Figure 1).18 Gepants bind to a hydrophobic gap between CLR and RAMP1.32 Gepants also target cAMP production and cAMP response element-binding protein (CREB) phosphorylation, and inhibit trigeminovascular nociceptive activation.33 Triptans were initially thought to relieve migraine pain through vasoconstriction via 5-HT1B and 5-HT1D receptors. A sizeable number of CGRP neurons found within the trigeminal ganglion express 5-HT1B, 5-HT1D, and 5-HT1B and 5-HT1F receptors. Therefore, 5-HT agonists (triptans and lasmiditan) can possibly share a similar mechanism of action with gepants, by inhibition of the release of CGRP (as well as other neurotransmitters) from primary trigeminal neurons. Importantly, gepant inhibits CGRP-induced vasodilation without causing vasoconstriction, rendering gepant a unique alternative for patients contraindicated to triptan. Antagonism of CGRP-receptors on mast cells, satellite glial cells, and astrocytes prevent CGRP-induced neuroinflammation.34 Gepants also bind to Amylin receptors; their significance in migraine treatment remains unknown.35
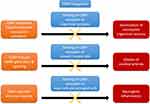 |
Figure 1 Proposed mechanism of actions of CGRP antagonism. |
The first generation of gepants were olcegepant (Boehringer Ingelheim GmbH, Germany), telcagepant (Merck, Kenilworth, NJ), and MK-3207 (Merck, Kenilworth, NJ). These treatments were reported to be more effective than placebo and were comparable in efficacy to triptans.36 However, further investigation of these gepants was discontinued due to concerns for hepatotoxicity when dosed regularly.36
The second generation of gepant includes ubrogepant, rimegepant, and atogepant. Ubrogepant was the first gepant to receive FDA approval for the abortive treatment of migraine. The ACHIEVE (I & II) Phase III studies assessed ubrogepant 50 mg and 100 mg against placebo. Ubrogepant was found to be more efficacious than placebo in providing freedom of pain after 2 hours and resolution of most bothersome symptom (MBS; photophobia, phonophobia, or nausea).37 Patients receiving ubrogepant also reported improved patient satisfaction and functional disability compared to placebo. The most common adverse effects (AEs) reported were nausea and somnolence. Pooled analysis revealed no clinical presentations of hepatoxicity.38
Rimegepant, an oral disintegrating tablet, was also investigated for the acute treatment of migraine. Two phase III randomized controlled trials (RCTs) assessed rimegepant 75 mg against placebo, concluding the drug to be more effective in achieving freedom of pain after 2 hours and resolution of MBS.39,40 A phase II/III RCT assessed the efficacy of rimegepant for migraine prophylaxis. Rimegepant 75 mg taken every other day, was found to be more effective in reducing migraine days than placebo (4.3 vs 3.5 days, 95% CI −1.46 to −0.20; p = 0.0099).41 Common AEs of rimegepant include nausea, dizziness, and urinary tract infections (UTIs). Rimegepant, when dosed every other day, was established to have no statistically significant difference in liver function test abnormalities compared to placebo. On May 27th, 2021, rimegepant became the first gepant to obtain FDA approval for the preventive treatment of migraine. A Phase IV RCT comparing galcanezumab and rimegepant (NCT05127486) is currently in progress.
Atogepant
Atogepant is a 2nd generation oral CGRP receptor antagonist with a time to peak concentration of ~2 hours and a half-life of ~11 hours.42 It is a potent CGRP antagonist that non-competitively binds to the CGRP receptor.43 Atogepant is a substrate of CYP3A4 and P-gp but does not significantly induce or inhibit many CYPs. Unlike other gepants, atogepant was developed specifically as a preventive treatment due to its long half-life. In a pharmacokinetic study, overall systemic exposures to atogepant were 15% to 38% higher in participants with hepatic impairment compared with those with normal hepatic function.42 Over 28 days of treatment (170 mg supratherapeutic dose), no participant receiving atogepant had an ALT elevation above 1.5 × upper limit of normal (ULN).44 A Phase I clinical study reported atogepant can be tolerated in single doses as high as 300 mg.45 No cardiovascular AEs, such as prolonged QT/QTc, were observed. It remains uncertain whether chronic CGRP antagonism leads to receptor upregulation or tolerance.
Goadsby et al conducted a phase IIb/III RCT assessing atogepant 10 mg once daily, 30 mg once daily, 60 mg once daily, 30 mg twice daily, and 60 mg twice daily against placebo (Table 2). A total of 834 patients with EM underwent a 12-week double-blind period, followed by a 4-week safety follow-up period. The primary outcome measure was the change in monthly migraine days (MMDs) from baseline. Secondary outcome measures were change in monthly headache days (MHDs) from baseline, change in mean acute medication use days from baseline, and the number of proportions of participants who had at least a 50% reduction in MMDs.
 |
Table 2 Summary of Atogepant RCTs |
Atogepant was reported to decrease MMDs by −4.0 (p = 0.024) for 10 mg daily, −3.8 (p = 0.039) for 30 mg once daily, −3.6 (p = 0.039) for 60 mg once daily, −4.2 (p = 0.0034) for 30 mg twice daily, and −4·1 (p = 0.0031) for 60 mg twice daily, compared to −2.9 in the placebo group.46 As for secondary outcomes, atogepant was reported to decrease MHDs by −4.3 (p = 0.024) for 10 mg daily, −4.2 (p = 0.039) for 30 mg once daily, −3.9 (p = 0.039) for 60 mg once daily, −4.2 (p = 0.013) for 30 mg twice daily, and −4·3 (p = 0.0083)7 for 60 mg twice daily, compared to −2.9 in the placebo group. 50% responder rates were 53% (p = 0.11) for 10 mg daily, 53% (p = 0.11) for 30 mg once daily, 52% (p = 0.15) for 60 mg once daily, 58% (p=0.034) for 30 mg twice daily, and 62% (p = 0.0097) for 60 mg twice daily, compared to 40% in the placebo group. Acute medication use days were also reduced: −3.7 (p = 0.11) for 10 mg daily, −3.9 (p = 0.11) for 30 mg once daily, −3.5 (p = 0.15) for 60 mg once daily, −3.8 (p = 0.034) for 30 mg twice daily, and −3.6 (p = 0.0097) for 60 mg twice daily, compared to −2.4 in the placebo group. Of the total patients receiving atogepant, the proportion of patients reporting ≥75% reduction of MMD was 18%, compared to 3% among patients receiving placebo.
Ailani et al. conducted a phase III RCT assessing atogepant 10 mg daily, 30 mg daily, and 60 mg daily compared to placebo. The primary outcome measures were changed from baseline in MMDs over 12 weeks. Secondary outcome measures included the proportion of participants reporting 50% responder rate, change in monthly headache days (MHDs) from baseline, change in mean acute medication use days from baseline, and improvement in quality of life. In total, 910 patients with EM were recruited, with 873 included in the final analysis.
The mean difference from baseline in MMDs after the treatment period was −3.7 for 10-mg atogepant, −3.9 for 30-mg atogepant, −4.2 for 60-mg atogepant, and −2.5 with placebo (p < 0.001 for all dosages compared to placebo).47 The mean difference from baseline in MHDs after the treatment period was −3.9 for 10-mg atogepant, −4.0 for 30-mg atogepant, −3.9 for 60-mg atogepant, and −2.4 with placebo (p < 0.001 for all dosages compared to placebo). Of the total patients receiving atogepant, the proportion of patients reporting ≥50% reduction of MMD was 55.6% in the 10-mg atogepant group, 58.7% in the 30-mg atogepant group, 60.8% in the 60-mg atogepant group, and 29.0% of tin the placebo group (p < 0.001 for all dosages compared to placebo). In an exploratory efficacy analysis, treatment benefit can be observed during the first week with the mean difference (1–4 weeks) being −3.1 for 10-mg atogepant, −3.4 for 30-mg atogepant, −3.9 for 60-mg atogepant, and −1.6 with placebo (p < 0.001 for all dosages compared to placebo). On the first day of treatment, 25.2% of placebo-treated participants reported a migraine in comparison with 10.8–14.1% of participants treated with various doses of atogepant for all atogepant groups (p ≤ 0.0071).48 Improvement in quality of life was assessed by participants’ responses to Activity Impairment in Migraine-Diaries (AIM-D) and Migraine-Specific Quality-of-Life Questionnaires (MSQ). Statistically significant improvement was reported among all treatment groups compared to placebo, expect atogepant 10 mg, which did not show a significant difference in sections of the AIM-D (p = 0.09).
Goadsby et al. reported AE rates ranging from 58% to 66% in the treatment groups, with atogepant 10 mg daily having the highest rate. Ailani et al reported AE rates ranging 52.9%–53.7%. The most common AEs were similar among both studies, reporting constipation, nausea, and upper respiratory tract infections (URTIs) as the most common events. There were several cases of participants having LFTs >3 times the ULN, in both studies; however, no cases met the criteria for Hy’s law. An open-label trial (NCT03700320) assessed the safety and tolerability of atogepant 60 mg daily over the span 52 weeks. Of the participants receiving atogepant, 67% reported AEs, of which the investigators determined 18% were related to atogepant.49 The most commonly reported AEs were URTIs (10.3%), constipation (7.2%), nausea (6.3%), and urinary tract infection (5.2%). Thirteen participants (2.4%) were found to have LFTs >3 times the ULN, however none of these cases met criteria for Hy’s law.
Clinical Rationale
As discussed above, atogepant, in addition to other CGRP inhibiting treatments, has demonstrated clinical efficacy in the prevention of EM. The American Academy of Neurology (AAN) has not yet issued guidelines on when to implement gepants. The American Headache Society (AHS), in a recent consensus statement, recommend trialing a gepant for abortive therapy for patients with an inadequate response to 2 or more triptans.50 However, there is no recommendation on implementing a gepant for migraine prophylaxis in treatment naïve or refractory subjects. The AHS and European Headache Federation (EHF) recommend trialing a CGRP-targeted mAb when patients have failed at least two standard-of-care preventive treatments.51,52 While there are no recommendations on prophylactic gepant use, most payers in the US require treatment failure in at least two classes of migraine prevention before authorizing atogepant.
Currently, atogepant has only been assessed in patients meeting criteria for EM. Participants with CM or medication overuse headaches (MOH) were excluded. Since CGRP mAbs are effective for MOH and CM, this prompts the question of whether an atogepant can also achieve success in these subgroups. It is worth noting that frequent gepant use does not lead to MOH.53,54 Increased expression of CGRP in the trigeminocervical complex was observed in animal models with triptan/lasmiditan-induced MOH.54 This change was not observed in mice treated with olcegepant. Another animal model assessed MOH by observing rats with repeated treatments of sumatriptan and ubrogepant. Rats receiving repeated sumatriptan developed cutaneous allodynia and latent sensitization, while rats' repeated treatments of ubrogepant did not.53 A phase III PROGRESS trial (Table 2) assessed atogepant 30 mg and 60 mg for the preventive treatment of CM. Early results showed that in the United States-focused 755 patients with evaluable headache diary (modified intention-to-treat population), there were 5.05, 6.88 and 7.46 reductions in MMD on placebo, 30 mg twice daily, 60 mg daily, respectively (all p < 0.01). Similarly, in the European Union-focused population, there were 5.09, 6.75, and 7.33 reductions on MMD (all p < 0.01).55 In addition, with galcanezumab being an effective treatment for episodic cluster headache, gepant might also be a potential option.56
CGRP is implicated in having protective effects in several cardiovascular diseases; CGRP is released in the event of ischemia and produces a protective effect against reperfusion injury.57,58 When given in an animal model with induced cerebral ischemic events, gepants caused larger infarcts and more severe neurological deficits.59 Animal models also demonstrated that CGRP reduces blood pressure in pathologic states and is released in a compensatory fashion in response to heart failure.60–64 Phase II and III RCTs of the CGRP-targeted mAbs did not report any cardiovascular safety concerns.65 However, post-marketing surveillance of erenumab revealed an association with hypertension.66 Ubrogepant has not demonstrated a difference in AEs or cardiac AE in patients with moderate-high cardiovascular risk factors.67 There is concern for inhibiting CGRP in patients with small vessel diseases, such as Raynaud’s phenomenon (RP) cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL).68 Further clinical studies and gathering of real-world data are warranted.
Gepants are not assessed in pregnant women, or if their metabolites are found in breastmilk. CGRP has reported to play a role in vascular adaption during pregnancy and in utero-placental functions.69 Both CGRP-targeting mAbs and gepants are not recommended for patients who are pregnant or who are planning to become pregnant,70 With its shorter half-life, gepant can be washed out quicker than CGRP mAbs in case of urgent need.
Utilizing gepants in combination with other migraine treatments is a growing and expanding debate. Atogepant is metabolized in the liver by P-gp and CYP3A4.71 Coadministration of atogepant with potent CYP3A4 inducers and inhibitors can result in significant increase and decrease in atogepant concentration, respectively.72 Dosage adjustments of atogepant are recommended when concurrently taken with potent CYP3A4 inhibitors (eg, itraconazole, ketoconazole, clarithromycin) or potent or moderate CYP3A4 inducers (eg, carbamazepine, phenytoin, rifampin, St. John’s wort).73 Atogepant was found to have no drug–drug interactions with NSAIDS, acetaminophen, triptans, and oral contraceptives.74,75 A phase I study assessing ubrogepant with erenumab or galcanezumab reported no safety concerns or changes in the pharmacokinetics of ubrogepant.76
An open-label study (NCT03266588) assessing the acute treatment of rimegepant reported 13 patients who were using a CGRP-targeted mAb concurrently (erenumab n = 7, galcanezumab n = 2, and fremanezumab n = 4). Of this subgroup, 3 patients reported AEs that were considered potentially treatment-related; however, there were no reported serious AEs or treatment discontinuation.77 Another open-label, longitudinal treatment study also reported no increase in AEs when gepants were combined with CGRP mAbs.78 The mechanism of synergism between gepant and CGRP-targeted mAb is not certain. It is speculated that gepants, due to their smaller physical size, higher inherent membrane permeability, and differential receptor kinetics than mAbs, may produce a complementary functional inhibition of CGRP signaling.77 Moreover, the interaction between atogepant and ubrogepant is unknown; the clinical benefit of dual gepant therapy (for both acute and preventive treatment) is to be determined.
Concurrent use of CGRP-targeted mAbs and onabotulinumtoxinA has been studied and shown efficacy in reducing MHDs without an increase in AEs.79 While there have yet to be any published findings of concurrent gepant and onabotulinumtoxinA efficacy, a study assessing atogepant and onabotulinumtoxinA has been conducted on rats. Rats were given scalp injections of onabotulinumtoxinA, followed by an infusion of atogepant 7 days later. Activity of central trigeminovascular neurons in the dorsal horn were monitored, for response to peripheral stimulation and CSD. A decrease in cortical spreading of depression-induced activation was reported.80 OnabotulinumtoxinA/atogepant pretreatment prevented CSD-induced activation and sensitization in both high-threshold (80% to 10%, 80% to 0%, respectively) and wide-dynamic range neurons (70% to 0%, 60% to 5%).80 More clinical study is still needed.
Conclusion
CGRP has been established to play a significant role in the pathophysiology of migraine. CGRP-targeted mAbs and gepants are two drug classes developed to inhibit the function of CGRP. Atogepant is a second-generation gepant that recently obtained FDA approval to prevent EM. Phase II and III RCTs have demonstrated atogepant as an effective preventive migraine agent. Although studies have shown long-term tolerability, real-world data is warranted. CGRP-targeted mAbs have demonstrated success in migraine prevention in sub-populations, such as CM and MOH, and improved efficacy when used concurrently with onabotulinumtoxinA. Animal models have suggested that atogepants will be successful in the subgroups. However, more clinical studies are warranted.
Disclosure
F.C. has no conflicts of interest or financial disclosures. Over the past 24 months, H.Y. has served as a consultant for Trillen Medical Inc., and currently received research grants from NIH (R44NS115460). He also reports royalties from Cambridge University Press, outside the submitted work. In addition, he has a patent “Nanostars and nanoconstructs for detection, imaging, and therapy” (9,987,358) issued to Tuan Vo-Dinh, Hsiangkuo Yuan, Andrew Fales, Christopher Khoury; a patent “Plasmonics-active metal nanostar compositions and methods of use” (9,789,154) issued to Tuan Vo-Dinh, Hsiangkuo Yuan, Andrew Fales. The authors report no other conflicts of interest in this work.
References
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: updated age, sex, and socioeconomic-specific estimates from government health surveys. Headache. 2021;61(1):60–68. doi:10.1111/head.14024
2. Lipton RB, Munjal S, Alam A, et al. Migraine in America Symptoms and Treatment (MAST) Study: baseline Study Methods, Treatment Patterns, and Gender Differences. Headache. 2018;58(9):1408–1426. doi:10.1111/head.13407
3. Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z. Lifting The Burden: the Global Campaign against H. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137. doi:10.1186/s10194-020-01208-0
4. Yucel A, Thach A, Kumar S, Loden C, Bensink M, Goldfarb N. Estimating the economic burden of migraine on US employers. Am J Manag Care. 2020;26(12):e403–e408.
5. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35(17):6619–6629. doi:10.1523/JNEUROSCI.0373-15.2015
6. Viana M, Tronvik EA, Do TP, Zecca C, Hougaard A. Clinical features of visual migraine aura: a systematic review. J Headache Pain. 2019;20(1):64. doi:10.1186/s10194-019-1008-x
7. Kim KM, Kim BK, Lee W, Hwang H, Heo K, Chu MK. Prevalence and impact of visual aura in migraine and probable migraine: a population study. Sci Rep. 2022;12(1):426. doi:10.1038/s41598-021-04250-3
8. Pearce JM. Historical aspects of migraine. J Neurol Neurosurg Psychiatry. 1986;49(10):1097–1103. doi:10.1136/jnnp.49.10.1097
9. Cuvellier JC. Pediatric vs. Adult Prodrome and Postdrome: a Window on Migraine Pathophysiology? Front Neurol. 2019;10:199. doi:10.3389/fneur.2019.00199
10. Marmura MJ. Triggers, Protectors, and Predictors in Episodic Migraine. Curr Pain Headache Rep. 2018;22(12):81. doi:10.1007/s11916-018-0734-0
11. Wolff H. Headache: And Other Head Pain. Oxford University Press; 1963.
12. Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol. 1984;16(2):157–168. doi:10.1002/ana.410160202
13. Russo AF. Overview of Neuropeptides: awakening the Senses? Headache. 2017;57(Suppl 2):37–46. doi:10.1111/head.13084
14. Messlinger K. The big CGRP flood - sources, sinks and signalling sites in the trigeminovascular system. J Headache Pain. 2018;19(1):22. doi:10.1186/s10194-018-0848-0
15. Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–1142. doi:10.1152/physrev.00034.2013
16. Barwell J, Wheatley M, Conner AC, et al. The activation of the CGRP receptor. Biochem Soc Trans. 2013;41(1):180–184. doi:10.1042/BST20120251
17. McLatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–339. doi:10.1038/30666
18. Yuan H, Spare NM, Silberstein SD. Targeting CGRP for the Prevention of Migraine and Cluster Headache: a Narrative Review. Headache. 2019;59(Suppl 2):20–32. doi:10.1111/head.13583
19. Yuan H, Lauritsen CG, Kaiser EA, Silberstein SD. CGRP Monoclonal Antibodies for Migraine: rationale and Progress. BioDrugs. 2017;31(6):487–501. doi:10.1007/s40259-017-0250-5
20. Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28(2):183–187. doi:10.1002/ana.410280213
21. Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia. 2000;20(10):907–918. doi:10.1046/j.1468-2982.2000.00146.x
22. Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25(3):179–183. doi:10.1111/j.1468-2982.2005.00836.x
23. Akerman S, Goadsby PJ. Topiramate inhibits trigeminovascular activation: an intravital microscopy study. Br J Pharmacol. 2005;146(1):7–14. doi:10.1038/sj.bjp.0706290
24. Cernuda-Morollon E, Ramon C, Martinez-Camblor P, Serrano-Pertierra E, Larrosa D, Pascual J. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain. 2015;156(5):820–824. doi:10.1097/j.pain.0000000000000119
25. Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61. doi:10.1046/j.1468-2982.2002.00310.x
26. Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30(10):1179–1186. doi:10.1177/0333102410368444
27. Wattiez AS, Sowers LP, Russo AF. Calcitonin gene-related peptide (CGRP): role in migraine pathophysiology and therapeutic targeting. Expert Opin Ther Targets. 2020;24(2):91–100. doi:10.1080/14728222.2020.1724285
28. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55(1):533–552. doi:10.1146/annurev-pharmtox-010814-124701
29. Manning BM, Gruba SM, Meyer AF, Haynes CL. Neuropeptide-Induced Mast Cell Degranulation and Characterization of Signaling Modulation in Response to IgE Conditioning. ACS Chem Biol. 2016;11(11):3077–3083. doi:10.1021/acschembio.6b00616
30. Afroz S, Arakaki R, Iwasa T, et al. CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception. Int J Mol Sci. 2019;20:3. doi:10.3390/ijms20030711
31. Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158(4):543–559. doi:10.1097/j.pain.0000000000000831
32. Sheykhzade M, Amandi N, Pla MV, et al. Binding and functional pharmacological characteristics of gepant-type antagonists in rat brain and mesenteric arteries. Vascul Pharmacol. 2017;90:36–43. doi:10.1016/j.vph.2017.02.001
33. Yuan H, Silberstein S. CGRP and Immune Modulation: evidence-Based Therapy. In: Shah S, editor. Migraine. Irvine, CA, USA: Springer; 2021:75–94.
34. Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med. 2011;13:e36. doi:10.1017/S1462399411002067
35. Garelja ML, Walker CS, Hay DL. CGRP receptor antagonists for migraine. Are they also AMY1 receptor antagonists? Br J Pharmacol. 2022;179(3):454–459. doi:10.1111/bph.15585
36. Ho TW, Connor KM, Zhang Y, et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology. 2014;83(11):958–966. doi:10.1212/WNL.0000000000000771
37. Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant, an Acute Treatment for Migraine, Improved Patient-Reported Functional Disability and Satisfaction in 2 Single-Attack Phase 3 Randomized Trials, ACHIEVE I and II. Headache. 2020;60(4):686–700. doi:10.1111/head.13766
38. Hutchinson S, Dodick DW, Treppendahl C, et al. Ubrogepant for the Acute Treatment of Migraine: pooled Efficacy, Safety, and Tolerability From the ACHIEVE I and ACHIEVE II Phase 3 Randomized Trials. Neurol Ther. 2021;10(1):235–249. doi:10.1007/s40120-021-00234-7
39. Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394(10200):737–745. doi:10.1016/S0140-6736(19)31606-X
40. Lipton RB, Croop R, Stock EG, et al. Rimegepant, an Oral Calcitonin Gene-Related Peptide Receptor Antagonist, for Migraine. N Engl J Med. 2019;381(2):142–149. doi:10.1056/NEJMoa1811090
41. Croop R, Lipton RB, Kudrow D, et al. Oral rimegepant for preventive treatment of migraine: a Phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397(10268):51–60. doi:10.1016/S0140-6736(20)32544-7
42. Boinpally R, Jakate A, Butler M, Borbridge L, Periclou A. Single-Dose Pharmacokinetics and Safety of Atogepant in Adults With Hepatic Impairment: results From an Open-Label, Phase 1 Trial. Clin Pharmacol Drug Dev. 2021;10(7):726–733. doi:10.1002/cpdd.916
43. Rubio-Beltran E, Chan KY, Danser AJ, MaassenVanDenBrink A, Edvinsson L. Characterisation of the calcitonin gene-related peptide receptor antagonists ubrogepant and atogepant in human isolated coronary, cerebral and middle meningeal arteries. Cephalalgia. 2020;40(4):357–366. doi:10.1177/0333102419884943
44. Min KC, Kraft WK, Bondiskey P, et al. Atogepant Is Not Associated With Clinically Meaningful Alanine Aminotransferase Elevations in Healthy Adults. Clin Transl Sci. 2021;14(2):599–605. doi:10.1111/cts.12917
45. Boinpally R, McNamee B, Yao L, et al. A Single Supratherapeutic Dose of Atogepant Does Not Affect Cardiac Repolarization in Healthy Adults: results From a Randomized, Single-Dose, Phase 1 Crossover Trial. Clin Pharmacol Drug Dev. 2021;10(9):1099–1107. doi:10.1002/cpdd.940
46. Goadsby PJ, Dodick DW, Ailani J, et al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol. 2020;19(9):727–737. doi:10.1016/S1474-4422(20)30234-9
47. Ailani J, Lipton RB, Goadsby PJ, et al. Atogepant for the Preventive Treatment of Migraine. N Engl J Med. 2021;385(8):695–706. doi:10.1056/NEJMoa2035908
48. Schwedt TJ, Lipton RB, Ailani J, et al. Time course of efficacy of atogepant for the preventive treatment of migraine: results from the randomized, double-blind ADVANCE trial. Cephalalgia. 2022;42(1):3–11. doi:10.1177/03331024211042385
49. Ashina M, Tepper S, Reuter U, et al. Long-term Safety and Tolerability of Atogepant 60 mg Following Once Daily Dosing Over 1 Year for the Preventive Treatment of Migraine (2664). Neurology. 2021;96(15 Supplement):2664.
50. Ailani J, Burch RC, Robbins MS. Board of Directors of the American Headache S. The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021–1039. doi:10.1111/head.14153
51. American Headache S. The American Headache Society Position Statement On Integrating New Migraine Treatments Into Clinical Practice. Headache. 2019;59(1):1–18.
52. Sacco S, Bendtsen L, Ashina M, et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20(1):6. doi:10.1186/s10194-018-0955-y
53. Navratilova E, Behravesh S, Oyarzo J, Dodick DW, Banerjee P, Porreca F. Ubrogepant does not induce latent sensitization in a preclinical model of medication overuse headache. Cephalalgia. 2020;40(9):892–902. doi:10.1177/0333102420938652
54. Saengjaroentham C, Strother LC, Dripps I, et al. Differential medication overuse risk of novel anti-migraine therapeutics. Brain. 2020;143(9):2681–2688. doi:10.1093/brain/awaa211
55. AbbVie Announces Positive Phase 3 Atogepant (QULIPTA™) Data for the Preventive Treatment of Chronic Migraine; 2020. https://news.abbvie.com/news/press-releases/abbvie-announces-positive-phase-3-atogepant-qulipta-data-for-preventive-treatment-chronic-migraine.htm.
56. Villar-Martínez MD, Puledda F, Goadsby PJ. Recent Advances in the Management of Cluster Headache. Curr Treat Options Neurol. 2020;22(12):46. doi:10.1007/s11940-020-00655-z
57. Kee Z, Kodji X, Brain SD. The Role of Calcitonin Gene Related Peptide (CGRP) in Neurogenic Vasodilation and Its Cardioprotective Effects. Front Physiol. 2018;9:1249. doi:10.3389/fphys.2018.01249
58. Song SW, Guo KJ, Shi R, Cheng Y, Liu YF. Pretreatment with calcitonin gene-related peptide attenuates hepatic ischemia/reperfusion injury in rats. Transplant Proc. 2009;41(5):1493–1498. doi:10.1016/j.transproceed.2009.03.056
59. Mulder IA, Li M, de Vries T, et al. Anti-migraine Calcitonin Gene-Related Peptide Receptor Antagonists Worsen Cerebral Ischemic Outcome in Mice. Ann Neurol. 2020;88(4):771–784. doi:10.1002/ana.25831
60. Katori T, Hoover DB, Ardell JL, et al. Calcitonin gene-related peptide in vivo positive inotropy is attributable to regional sympatho-stimulation and is blunted in congestive heart failure. Circ Res. 2005;96(2):234–243. doi:10.1161/01.RES.0000152969.42117.ca
61. Bergdahl A, Valdemarsson S, Nilsson T, Sun XY, Hedner T, Edvinsson L. Dilatory responses to acetylcholine, calcitonin gene-related peptide and substance P in the congestive heart failure rat. Acta Physiol Scand. 1999;165(1):15–23. doi:10.1046/j.1365-201x.1999.00456.x
62. Smillie SJ, King R, Kodji X, et al. An ongoing role of alpha-calcitonin gene-related peptide as part of a protective network against hypertension, vascular hypertrophy, and oxidative stress. Hypertension. 2014;63(5):1056–1062. doi:10.1161/HYPERTENSIONAHA.113.02517
63. Li J, Wang DH. Development of angiotensin II-induced hypertension: role of CGRP and its receptor. J Hypertens. 2005;23(1):113–118. doi:10.1097/00004872-200501000-00020
64. Li J, Zhao H, Supowit SC, DiPette DJ, Wang DH. Activation of the renin-angiotensin system in alpha-calcitonin gene-related peptide/calcitonin gene knockout mice. J Hypertens. 2004;22(7):1345–1349. doi:10.1097/01.hjh.0000125409.50839.f1
65. Favoni V, Giani L, Al-Hassany L, et al. CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? J Headache Pain. 2019;20(1):27. doi:10.1186/s10194-019-0979-y
66. Dodick DW, Tepper SJ, Ailani J, et al. Risk of hypertension in erenumab-treated patients with migraine: analyses of clinical trial and postmarketing data. Headache. 2021;61(9):1411–1420. doi:10.1111/head.14208
67. Hutchinson S, Silberstein SD, Blumenfeld AM, et al. Safety and efficacy of ubrogepant in participants with major cardiovascular risk factors in two single-attack phase 3 randomized trials: ACHIEVE I and II. Cephalalgia. 2021;41(9):979–990. doi:10.1177/03331024211000311
68. de Boer I, MaassenVanDenBrink A, Terwindt GM. The potential danger of blocking CGRP for treating migraine in CADASIL patients. Cephalalgia. 2020;40(14):1676–1678. doi:10.1177/0333102420941814
69. Szperka CL, VanderPluym J, Orr SL, et al. Recommendations on the Use of Anti-CGRP Monoclonal Antibodies in Children and Adolescents. Headache. 2018;58(10):1658–1669. doi:10.1111/head.13414
70. Tepper D. Gepants. Headache. 2020;60(5):1037–1039. doi:10.1111/head.13791
71. Boinpally R, Spaventa J, Chen K, Butler M. Evaluation of the Pharmacokinetic Interaction and Safety of Atogepant Co-Administered with Acetaminophen or Naproxen in Healthy Participants: a Randomized Trial. Clin Drug Investig. 2021;41(6):557–567. doi:10.1007/s40261-021-01034-5
72. Deeks ED. Atogepant: first Approval. Drugs. 2022;82(1):65–70. doi:10.1007/s40265-021-01644-5
73. AbbVie. QULIPTA (atogepant) tablets, for oral use: US prescribing information; 2021.
74. Jakate A, Boinpally R, Butler M, Lu K, McGeeney D, Periclou A. Evaluation of the Pharmacokinetic Interaction of Ubrogepant Coadministered With Sumatriptan and of the Safety of Ubrogepant With Triptans. Headache. 2020;60(7):1340–1350. doi:10.1111/head.13862
75. Ankrom W, Xu J, Vallee MH, et al. Atogepant Has No Clinically Relevant Effects on the Pharmacokinetics of an Ethinyl Estradiol/Levonorgestrel Oral Contraceptive in Healthy Female Participants. J Clin Pharmacol. 2020;60(9):1157–1165. doi:10.1002/jcph.1610
76. Jakate A, Blumenfeld AM, Boinpally R, et al. Pharmacokinetics and safety of ubrogepant when coadministered with calcitonin gene-related peptide-targeted monoclonal antibody migraine preventives in participants with migraine: a randomized phase 1b drug-drug interaction study. Headache. 2021;61(4):642–652. doi:10.1111/head.14095
77. Berman G, Croop R, Kudrow D, et al. Safety of Rimegepant, an Oral CGRP Receptor Antagonist, Plus CGRP Monoclonal Antibodies for Migraine. Headache. 2020;60(8):1734–1742. doi:10.1111/head.13930
78. Freitag FG, Tolebeyan A, Sivakumar D CGRP monoclonal antibodies along with CGRP receptor antagonists are safe and effective together and compared to standard of care.
79. Cohen F, Armand C, Lipton RB, Vollbracht S. Efficacy and Tolerability of Calcitonin Gene-Related Peptide-Targeted Monoclonal Antibody Medications as Add-on Therapy to OnabotulinumtoxinA in Patients with Chronic Migraine. Pain Med. 2021;22(8):1857–1863. doi:10.1093/pm/pnab093
80. Melo-Carrillo A, Strassman AM, Schain AJ, Adams AM, Brin MF, Burstein R. Combined onabotulinumtoxinA/atogepant treatment blocks activation/sensitization of high-threshold and wide-dynamic range neurons. Cephalalgia. 2021;41(1):17–32. doi:10.1177/0333102420970507
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Efficacy and Safety of Rimegepant 75 mg Oral Tablet, a CGRP Receptor Antagonist, for the Acute Treatment of Migraine: A Randomized, Double-Blind, Placebo-Controlled Trial
Lipton RB, Thiry A, Morris BA, Croop R
Journal of Pain Research 2024, 17:2431-2441
Published Date: 22 July 2024
