Back to Archived Journals » Robotic Surgery: Research and Reviews » Volume 4
Robotics in reproduction, fertility preservation, and ovarian transplantation
Received 2 October 2016
Accepted for publication 17 January 2017
Published 27 February 2017 Volume 2017:4 Pages 19—24
DOI https://doi.org/10.2147/RSRR.S123703
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Masoud Azodi
Enes Taylan,1,2 Kutluk H Oktay1,2
1Department of Obstetrics and Gynecology, Laboratory of Molecular Reproduction and Fertility Preservation New York Medical College, Valhalla, NY, USA; 2Innovation Institute for Fertility Preservation and In Vitro Fertilization, NY, USA
Abstract: Robotic technology is one of the most promising and rapidly developing advancements of the twenty-first century with a potential to make significant contributions to reproductive surgery and preservation of fertility. Along with the major advances in cancer therapy, the number of female cancer survivors of reproductive age has dramatically increased. As a consequence, fertility preservation has gained more emphasis in reproductive science in the last few decades. A broad range of surgical procedures such as tubal reanastomosis, ovarian transposition, radical trachelectomy, and ovarian transplantation has been introduced to restore or preserve fertility in selected patients. These procedures can be accomplished through various surgical routes, including open surgery and minimally invasive approaches. In this review, we aim to present the current applications, advantages, and disadvantages of robotic technology in the field of reproductive surgery with a special interest in ovarian transplantation for fertility preservation.
Keywords: robotic surgery, ovarian transplantation, fertility preservation
Introduction
In the last few decades, the significantly increasing rate of major technological advances in minimally invasive surgical techniques revolutionized the view of modern surgical practice. Already, a wide range of diagnostic and therapeutic tools has become available for surgeons in various fields around the world. Recently, minimally invasive approaches have been more commonly practiced in a diverse spectrum of gynecological procedures, from benign conditions such as uterine fibroids and endometriosis to malignancies such as endometrial and cervical cancers.1
In this review, we aim to present the current applications of robotic technology in the field of reproductive surgery with a special interest in ovarian transplantation for fertility preservation.
Robotics and reproductive surgery
Reproductive surgery can be defined as gynecologic procedures that are performed to preserve or restore fertility. In this perspective, minimally invasive technologies transformed the field of reproductive medicine, providing a wide variety of surgical applications ranging from tubal reanastomosis to ovarian transplantation. Along this way, robot-assisted approaches steadily increased their areas of utilization.
Laparoscopic surgery prevailed over laparotomy with its well-proven advantages of better cosmetic results with smaller incisions, reduced tissue trauma, less bleeding, reduced postoperative pain, and faster recovery and return to normal life.2 However, conventional laparoscopy brought some limitations such as loss of in-depth perception with two-dimensional image, diminished tactile feedback, limited range of motion, amplified tremor, unstable optic camera, and low ergonomics that make complex surgical procedures challenging.3
Since the first robotic tubal reanastomosis operation, which was performed in 1998 using ZEUS (Computer Motion Inc., Sunnywale, CA, USA), the first robotic system for surgery, there have been dramatic technological developments in robotic surgery. Robotic surgery was originally conceived as a military project supported by the National Aeronautics and Space Administration and Defense Advanced Research Project Administration to provide remotely controlled surgical interventions (telesurgery) for astronauts in space conditions and soldiers in battlefields.4 In the field of reproduction, robotic surgery holds the promise to overcome the limitations of conventional laparoscopic approach and enables complex surgical procedures such as ovarian transplantation for fertility preservation. Currently, the only robotic surgical system in use is the da Vinci system (Intuitive Surgical Inc., Sunnywale, CA, USA), and ZEUS is no longer commercially available.5
The da Vinci surgical system is a compact, electro-mechanic interface between the patient and the surgeon driven by advanced computer software. It consists of an ergonomically designed surgeon console, patient-side cart with four interactive robotic arms, and tenfold-magnified high-definition 3D vision system. One robotic arm is connected to the camera and is directly controlled by the surgeon, which ensures a stable and precise image. The other robotic arms have the ability to mimic the dexterity of the human hand, providing seven degrees of motion and eliminating tremor. These crucial features of the robotic system enable the surgeon to perform highly complex surgeries and microsurgical procedures that need to handle very fine suture materials compared to conventional laparoscopy.
However, robotic surgery also has its limitations which include 1) high operation costs; 2) lack of tactile feedback, leading to excessive use of force that causes suture breakage or tissue trauma during traction and dissection; 3) bulky size of the machine, limiting its setup in only large operating rooms; and 4) locked position of patient-side cart after docking of the robotic arms, restricting the surgeon from changing the patient’s position.6 Despite these limitations, numbers of the robotic procedures are rapidly growing worldwide, and this proves that robotic assistance is practical for gynecologic applications.
Reversal of tubal ligation
Tubal reanastomosis was introduced as a surgical treatment option to restore the reproductive function of women who regret surgical sterilization via tubal ligation and have no other cause of infertility.7 The laparoscopic approach for tubal reanastomosis introduced in the late 1980s, however, required precise microsurgical suturing using very fine 6-0 or 8-0 sutures that were highly difficult to handle with conventional laparoscopic instruments.8 To overcome this difficulty, Falcone et al9 conducted the first operation of robot-assisted tubal reanastomosis using the ZEUS robotic system in 1999. In their pilot study, 10 women who underwent robotic tubal reanastomosis were compared to 15 women who had undergone conventional laparoscopic surgery. Reported pregnancy rates and recovery time were similar in both groups; however, operating time and estimated blood loss were significantly higher in the conventional laparoscopic group.10
The first experience with the da Vinci surgical system in tubal reanastomosis was reported by Degueldre et al11 in 2000, and since that time several centers have been demonstrating the effectiveness and safety of the robotic platform12,13 for this application. In a recent study of robotic tubal reanastomosis with one-stitch technique in 18 women, a relatively shorter mean operative time of 141 minutes was reported in addition to 94.1% tubal patency and 58.8% pregnancy rates.14
Robotic myomectomy
Although the contribution of non-cavity-disturbing leiomyomas to infertility is not uniformly accepted, several studies demonstrated that even non-cavity-distorting intramural myomas may have a negative impact on fertility outcomes via altered endometrial receptivity, gamete migration, uterine blood perfusion, and contractility.15–18 Therefore, myomectomy is a widely performed surgery in women of reproductive age to restore and preserve fertility. Numerous randomized controlled studies and meta-analysis established the superiority of laparoscopic myomectomy over laparotomy in terms of decreased blood loss, less operative complications, less adhesion formation, shorter hospital stay, and improved fertility.19–21
In a large study of 575 myomectomies from Cleveland Clinic, researchers compared robot-assisted myomectomy, conventional laparoscopic myomectomy, and abdominal myomectomy. They reported decreased blood loss and length of hospital stay but longer operative time with the robotic approach.22 Robotic assistance can provide delicate uterine incision and myoma dissection to avoid breach of endometrial integrity. With improved suturing and tying abilities, a secured closure of the uterine walls can be accomplished. However, currently there is insufficient evidence to confirm the superiority of robotic-assisted myomectomy over conventional laparoscopy myomectomy.23
Endometriosis surgery
Endometriosis is one of the most common and severe gynecological diseases that can cause infertility without any currently available cure.24 It affects 6%–10% of women of reproductive age, and its management is still one of the most controversial issues in reproductive medicine.25 In patients with minimal-to-mild endometriosis, laparoscopic ablation of endometriotic implants and adhesiolysis may improve fertility outcomes via restoration of the anatomy and function.26,27 However, for cases with severe endometriosis, the reproductive benefits of surgical treatment are less clear and aggressive surgery bears the potential risks to harm future fertility.28 In an international multicenter retrospective study, Collinet et al29 observed no significant increase in operating time, blood loss, or surgical complications in 164 cases of robot-assisted deep infiltrating endometriosis operation.29 Although several cases of severe endometriosis involving the bowel and bladder reported successful application of robotic assistance to laparoscopy, the only study comparing robotic assistance to conventional laparoscopy was published by Nezhat et al.30 They found that both approaches have similar results in terms of blood loss, period of hospitalization, and intra- or postoperative complications; however, significantly longer operative time was recorded in the robotic group. Despite the lack of solid evidence of the superiority of robotic assistance, the authors highlighted the advantages of improved visualization, dexterity, and ergonomics that rank robotic assistance as an alternative surgical option for endometriosis surgery.
Fertility-sparing surgery
Fertility preservation is an emerging and highly promising field in modern reproductive medicine. With the increasing numbers of female cancer survivors of reproductive age, fertility preservation has become more an issue. Efforts to prevent ovaries from the harmful effects of chemotherapy and radiotherapy and to preserve the uterus for future pregnancies resulted in the development of various surgical methods and options. Radical trachelectomy (RT) with pelvic lymphadenectomy for early stage cervical cancer using da Vinci robotic system was first reported in 2008 and was followed by many other successful cases.31,32 In a very recent review, Api et al33 reported that both robot-assisted RT and laparoscopic RT have similar pregnancy, preterm, and term birth rates, suggesting robotic approach as a feasible option in patients who wish to preserve their uterus.
Ovarian transposition before undergoing pelvic radiation is also another fertility-sparing procedure that can be performed via either laparotomy or minimally invasive techniques.34 Suspending ovaries out of the pelvis far from the radiation field may prevent early ovarian failure, and thus robot-assisted laparoscopic surgery is demonstrated as a very efficient and appropriate option, especially as a concurrent procedure during oncological surgery in suitable cases.35
Ovarian transplantation
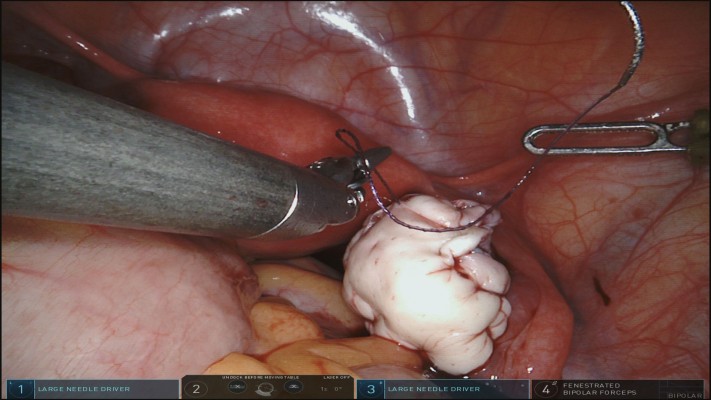
Ovarian transplantation is one of the most remarkable developments in reproductive surgery and fertility preservation. Ovarian tissue cryopreservation followed by transplantation procedure requires multistep efforts. This advanced procedure is currently the only available fertility preservation option for female cancer patients of prepubertal age. It is also a viable option for those who cannot delay chemotherapy treatment.36 Although it is still considered as an experimental procedure, more than sixty babies have already been born.37,38 Oktay and Karlikaya39 presented the first report of successful orthotopic transplantation of cryopreserved ovarian tissue in 2000. Oktay et al40 also reported the first embryo development after heterotopic transplantation of frozen-thawed ovarian cortical pieces beneath the skin of the abdominal wall. Subsequently, numerous live births ensued. Regarding the surgical route, although reimplantation of the ovarian tissue to the original site in the pelvic cavity (orthotopic ovarian transplantation) can be accomplished by laparoscopic surgery, most of the reported cases have been done via laparotomy.41,42 Oktay et al43 described a new method of robot-assisted ovarian transplantation using human extracellular matrix scaffolds. The transplantation of the ovarian tissue is performed without vascular reanastomosis, and the revascularization of the grafted tissue takes up to 10 days.44 This avascular period after transplantation leads to ischemic injury, which has been demonstrated to be the major cause for the loss of more than half of all primordial follicles in several experimental studies.45,46 To reduce ischemic injury by enhancing the revascularization process, Oktay et al43 used Alloderm (LifeCell Corp., Branchburg, NJ, USA), which is a decellularized human extracellular tissue matrix commonly used in cosmetic and reconstructive surgeries.47 In this innovative technique, the preparation of both the tissue graft and the recipient ovary was performed in a synchronized manner. Frozen-thawed ovarian cortical pieces were sutured on the extracellular tissue matrix (ECTM) with 5-0 Monocryl (11 mm 3/8 needle) (Ethicon Inc., Somerville, NJ, USA) in such a position that the stromal side of the cortical pieces was exposed. Then the ECTM was trimmed all around the tissue, leaving a tissue-free zone of ~5 mm. Thereafter, the recipient menopausal ovary was bivalved to expose the medulla and provide a large vascular bed for the reconstructed tissue graft using curved scissors with robotic assistance. Consequently, the graft was juxtaposed on the recipient ovary, exposing the stromal side of the cortical pieces to the stroma of the bivalve ovary, and the edges of the graft were sutured to the ovary using interrupted 4-0 Vicryl (Ethicon Inc.) (Figure 1A–D). The authors also concluded that robotic assistance with improved visualization and increased dexterity of finely controlled instruments catalyzed the surgery and minimized the duration of transplantation of the graft.
Future prospects
A new robot-assisted surgical platform TELELAP ALF-X (TransEnterix, Morrisville, NC, USA) has successfully been applied in hysterectomy.48 The new system provides two novel features of remotely controlled 3D vision through sensors that track the surgeon’s eye movements and also an incorporated haptic feedback system that troubleshoots one of the major disadvantages of robotic surgery. Another robotic platform AVRA Surgical Robotic System (AVRA Medical Robotics Inc., New York, NY, USA) presents a wireless connection between the surgeon’s console and the patient-side cart and also provides a modular arm configuration, which allows the system to be more cost effective for procedures that do not require multiple arms.49 Moreover, robotic single-site surgical platforms such as da Vinci sp™ Surgical System and SPORT (Single-Port Orifice Robotic Technology, Titan Medical, Toronto, ON, Canada) have been recently introduced as highly advanced robotic systems with a futuristic concept of architecturally snake-like flexible instruments to enable surgeons to perform complex procedures through only a single hole.50 Although these systems are currently not available in the US, they are expected to come into use by 2017.
Another potential area of application of robotic assistance is in uterine transplantation. Though all 12 reported cases of uterine transplantation were via the open technique, robotic assistance might find future applications in this area as well.51,52
Conclusion
Robotic technology has the potential of making significant contributions to reproductive surgery, and further studies investigating the feasibility of robotic assistance compared to the conventional laparoscopic approach are needed. Major advances in robotic surgery platforms have irreversibly transformed the vision of modern surgical approach. Surgeons and engineers around the globe are collaborating to find new ways to improve the precision, dexterity, and ergonomics of current robotic systems.
In conclusion, we believe that all these technological advancements and possible future developments in robotics will unequivocally find areas of application in reproductive surgery and will broaden our surgical capabilities in ovarian transplantation.
Acknowledgments
KO was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (RO1HD053112 and R21HD061259) and National Cancer Institute.
Disclosure
The authors report no conflicts of interest in this work.
References
Nezhat C, Nezhat F, Nezhat C. Nezhat’s Operataive Laparoscopy and Hysteroscopy. 3rd ed. New York, NY: Cambridge University Press; 2008. | ||
Velanovich V. Laparoscopic vs open surgery: a preliminary comparison of quality-of-life outcomes. Surg Endosc. 2000;14(1):16–21. | ||
Tulandi T, Marzal A. Redefining reproductive surgery. J Minim Invasive Gynecol. 2012;19(3):296–306. | ||
Diana M, Marescaux J. Robotic surgery. Br J Surg. 2015;102(2):e15–e28. | ||
Bouquet de Joliniere J, Librino A, Dubuisson JB, et al. Robotic surgery in Gynecology. Front Surg. 2016;3:26. | ||
Catenacci M, Flyckt RL, Falcone T. Robotics in reproductive surgery: strengths and limitations. Placenta. 2011;32(Suppl 3):S232–S237. | ||
Zarei A, Al-Ghafri W, Tulandi T. Tubal surgery. Clin Obstet Gynecol. 2009;52(3):344–350. | ||
Cha SH, Lee MH, Kim JH, Lee CN, Yoon TK, Cha KY. Fertility outcome after tubal anastomosis by laparoscopy and laparotomy. J Am Assoc Gynecol Laparosc. 2001;8(3):348–352. | ||
Falcone T, Goldberg J, Garcia-Ruiz A, Margossian H, Stevens L. Full robotic assistance for laparoscopic tubal anastomosis: a case report. J Laparoendosc Adv Surg Tech A. 1999;9(1):107–113. | ||
Goldberg JM, Falcone T. Laparoscopic microsurgical tubal anastomosis with and without robotic assistance. Hum Reprod. 2003;18(1):145–147. | ||
Degueldre M, Vandromme J, Huong PT, Cadiere GB. Robotically assisted laparoscopic microsurgical tubal reanastomosis: a feasibility study. Fertil Steril. 2000;74(5):1020–1023. | ||
Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal reanastomosis: a retrospective study. Fertil Steril. 2010;94(5):1844–1847. | ||
Dharia Patel SP, Steinkampf MP, Whitten SJ, Malizia BA. Robotic tubal anastomosis: surgical technique and cost effectiveness. Fertil Steril. 2008;90(4):1175–1179. | ||
Kavoussi SK, Kavoussi KM, Lebovic DI. Robotic-assisted tubal reanastomosis with one-stitch technique. J Robot Surg. 2014;8(2):133–136. | ||
Arslan AA, Gold LI, Mittal K, et al. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Hum Reprod. 2005;20(4):852–863. | ||
Ng EH, Chan CC, Tang OS, Yeung WS, Ho PC. Endometrial and subendometrial blood flow measured by three-dimensional power Doppler ultrasound in patients with small intramural uterine fibroids during IVF treatment. Hum Reprod. 2005;20(2):501–506. | ||
Nishino M, Togashi K, Nakai A, et al. Uterine contractions evaluated on cine MR imaging in patients with uterine leiomyomas. Eur J Radiol. 2005;53(1):142–146. | ||
Sunkara SK, Khairy M, El-Toukhy T, Khalaf Y, Coomarasamy A. The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and meta-analysis. Hum Reprod. 2010;25(2):418–429. | ||
Alessandri F, Lijoi D, Mistrangelo E, Ferrero S, Ragni N. Randomized study of laparoscopic versus minilaparotomic myomectomy for uterine myomas. J Minim Invasive Gynecol. 2006;13(2):92–97. | ||
Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy: a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21. | ||
Takeuchi H, Kinoshita K. Evaluation of adhesion formation after laparoscopic myomectomy by systematic second-look microlaparoscopy. J Am Assoc Gynecol Laparosc. 2002;9(4):442–446. | ||
Barakat EE, Bedaiwy MA, Zimberg S, Nutter B, Nosseir M, Falcone T. Robotic-assisted, laparoscopic, and abdominal myomectomy: a comparison of surgical outcomes. Obstet Gynecol. 2011;117(2):256–265. | ||
Nezhat C, Lavie O, Hsu S, Watson J, Barnett O, Lemyre M. Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy – a retrospective matched control study. Fertil Steril. 2009;91(2):556–559. | ||
Young VJ, Brown JK, Saunders PT, Horne AW. The role of the peritoneum in the pathogenesis of endometriosis. Hum Reprod Update. 2013;19(5):558–569. | ||
Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction. 2016;152(3):63–78. | ||
Marcoux S, Maheux R, Berube S. Canadian Collaborative Group on Endometriosis. Laparoscopic surgery in infertile women with minimal or mild endometriosis. N Engl J Med. 1997;337(4):217–222. | ||
Jacobson TZ, Duffy JM, Barlow D, Farquhar C, Koninckx PR, Olive D. Laparoscopic surgery for subfertility associated with endometriosis. Cochrane Database Syst Rev. 2010;(1):CD001398. | ||
Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591–598. | ||
Collinet P, Leguevaque P, Neme RM, et al. Robot-assisted laparoscopy for deep infiltrating endometriosis: international multicentric retrospective study. Surg Endosc. 2014;28(8):2474–2479. | ||
Nezhat C, Lewis M, Kotiketa S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94(7):2758–2760. | ||
Geisler JP, Orr CJ, Manahan KJ. Robotically assisted total laparoscopic radical trachelectomy for fertility sparing in stage 1B1 adenosarcoma of the cervix. J Laparoendosc Adv Surg Tech A. 2008;18(5):727–819. | ||
Chuang LT, Lerner DL, Liu CS, Nezhat FR. Fertiltiy-sparing robotic assisted radical trachelectomy and bilateral pelvic lymphadenectomy in early-stage cervical cancer. J Minim Invasive Gynecol. 2008;15(6):767–770. | ||
Api M, Boza A, Ceyhan M. Robotic versus laparoscopic radical trachelectomy for early-stage cervical cancer: case report and review of literature. J Minim Invasive Gynecol. 2016;23(5):677–683. | ||
Morice P, Thiam-Ba R, Castaigne D, et al. Fertility results after ovarian transposition for pelvic malignancies treated by external irradiation or brachytherapy. Hum Reprod. 1998;13(3):660–663. | ||
Molpus KL, Wedergren JS, Carlson MA. Robotically assisted endoscopic ovarian transposition. JSLS. 2003(1);7:59–62. | ||
Sonmezer M, Oktay K. Orthotopic and heterotopic ovarian tissue transplantation. Best Pract Res Clin Obstet Gynaecol. 2010;24(1):113–126. | ||
Donnez J, Dolmans MM, Diaz C, Pellicer A. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104(5):1097–1098. | ||
Dittrich R, Hackl J, Lotz L, Hoffmann I, Beckmann MW. Pregnancies and live births after 20 transplantations of cryopreserved ovarian tissue in a single center. Fertil Steril. 2015;103(2):462–468. | ||
Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342(25):1919. | ||
Oktay K, Buyuk E, Veeck L, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363(9412):837–840. | ||
Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15(6):649–665. | ||
Oktay K, Aydin BA, Karlikaya G. A technique for laparoscopic transplantation of frozen-banked ovarian tissue. Fertil Steril. 2001;75(6):1212–1216. | ||
Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214(1):94.e1–e9. | ||
Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2011;6(4):e19475. | ||
Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at -196 C. Endocrinology. 1999;140(1):462–471. | ||
Lee J, Kong HS, Kim EJ, et al. Ovarian injury during cryopreservation and transplantation in mice: a comparative study between cryoinjury and ischemic injury. Hum Reprod. 2016;31(8):1827–1837. | ||
Jansen LA, De Caigny P, Guay NA, Lineaweaver WC, Shokrollahi K. The evidence base for the acellular dermal matrix AlloDerm: a systematic review. Ann Plast Surg. 2013;70(5):587–594. | ||
Fanfani F, Restaino S, Rossitto C, et al. Total Laparoscopic (S-LPS) versus TELELAP ALF-X robotic-assisted hysterectomy: a case-control study. J Minim Invasive Gynecol. 2016;23(6):933–938. | ||
Laskaris J, Regan K. Soft Tissue Robotics: The Next Generation. Dallas, TX: MD Buyline; 2014. | ||
Titan Medical Inc. (Toronto, ON, Canada). SPORT surgical system. Available from: http://www.titanmedicalinc.com/. Accessed October 10, 2016. | ||
Ozkan O, Dogan NU, Ozkan O, et al. Uterus transplantation: from animal models through the first heart beating pregnancy to the first human live birth. Womens Health (Lond). 2016;12(4):442–449. | ||
Iavazzo C, Gkegkes ID. Possible role of DaVinci Robot in uterine transplantation. J Turk Ger Gynecol Assoc. 2015;16(3):179–180. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


