Back to Journals » Clinical Epidemiology » Volume 13
Risk Stratification in Patients with Ischemic Stroke and Residual Cardiovascular Risk with Current Secondary Prevention
Authors Gynnild MN , Hageman SHJ, Dorresteijn JAN, Spigset O, Lydersen S, Wethal T, Saltvedt I, Visseren FLJ, Ellekjær H
Received 3 June 2021
Accepted for publication 20 August 2021
Published 17 September 2021 Volume 2021:13 Pages 813—823
DOI https://doi.org/10.2147/CLEP.S322779
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eyal Cohen
Mari Nordbø Gynnild,1,2 Steven HJ Hageman,3 Jannick AN Dorresteijn,3 Olav Spigset,4,5 Stian Lydersen,6 Torgeir Wethal,1,2 Ingvild Saltvedt,1,7 Frank LJ Visseren,3 Hanne Ellekjær1,2
1Department of Neuromedicine and Movement Science, Faculty of Medicine and Health Science, NTNU – Norwegian University of Science and Technology, Trondheim, Norway; 2Department of Stroke, Clinic of Medicine, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway; 3Department of Vascular Medicine, University Medical Center Utrecht, Utrecht, The Netherlands; 4Department of Clinical Pharmacology, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway; 5Department of Clinical and Molecular Medicine, NTNU – Norwegian University of Science and Technology, Trondheim, Norway; 6Department of Mental Health, Faculty of Medicine and Health Sciences, NTNU – Norwegian University of Science and Technology, Trondheim, Norway; 7Department of Geriatrics, Clinic of Medicine, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
Correspondence: Mari Nordbø Gynnild
Department of Neuromedicine and Movement Science, Faculty of Medicine and Health Sciences, NTNU – Norwegian University of Science and Technology, Trondheim, Norway
Email [email protected]
Purpose: Suboptimal secondary prevention in patients with stroke causes a remaining cardiovascular risk desirable to reduce. We have validated a prognostic model for secondary preventive settings and estimated future cardiovascular risk and theoretical benefit of reaching guideline recommended risk factor targets.
Patients and Methods: The SMART-REACH (Secondary Manifestations of Arterial Disease-Reduction of Atherothrombosis for Continued Health) model for 10-year and lifetime risk of cardiovascular events was applied to 465 patients in the Norwegian Cognitive Impairment After Stroke (Nor-COAST) study, a multicenter observational study with two-year follow-up by linkage to national registries for cardiovascular disease and mortality. The residual risk when reaching recommended targets for blood pressure, low-density lipoprotein cholesterol, smoking cessation and antithrombotics was estimated.
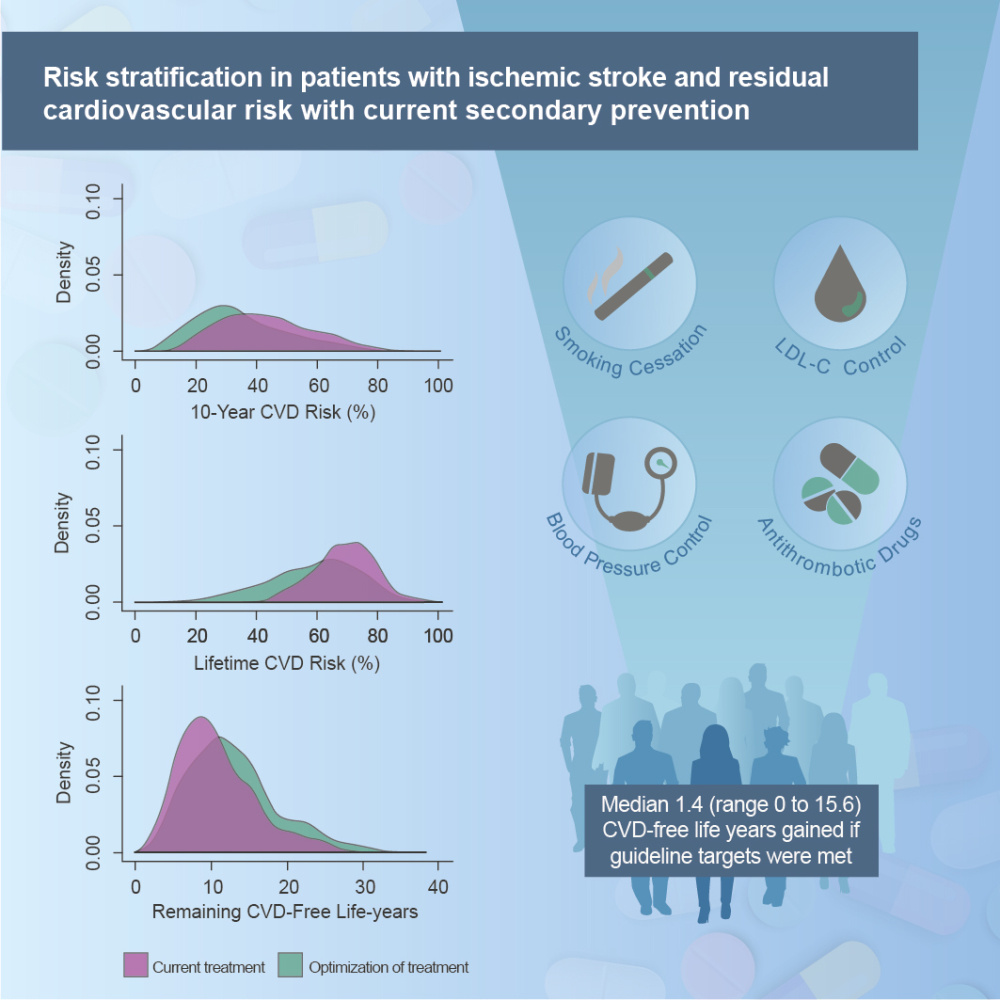
Results: In total, 11.2% had a new event. Calibration plots showed adequate agreement between estimated and observed 2-year prognosis (C-statistics 0.63, 95% confidence interval 0.55– 0.71). Median estimated 10-year risk of recurrent cardiovascular events was 42% (Interquartile range (IQR) 32– 54%) and could be reduced to 32% by optimal guideline-based therapy. The corresponding numbers for lifetime risk were 70% (IQR 63– 76%) and 61%. We estimated an overall median gain of 1.4 (IQR 0.2– 3.4) event-free life years if guideline targets were met.
Conclusion: Secondary prevention was suboptimal and residual risk remains elevated even after optimization according to current guidelines. Considerable interindividual variation in risk exists, with a corresponding variation in benefit from intensification of treatment. The SMART-REACH model can be used to identify patients with the largest benefit from more intensive treatment and follow-up.
Keywords: secondary prevention, ischemic stroke, risk factors, risk assessment, risks and benefits, cardiovascular diseases
Graphical Abstract:
Introduction
Patients with ischemic stroke have an increased risk of recurrent cardiovascular events.1 Secondary prevention aims to reduce the risk of recurrence, but implementation of guideline recommendations in clinical practice is suboptimal with poor risk factor control and low adherence to medications.2–5 Consequently, the residual cardiovascular risk remains elevated. However, there is a substantial interindividual variation in the risk of recurrent events among patients with established cardiovascular disease (CVD).6–8 This variation results from a composite of several prognostic features like age, genetics, cardiovascular risk factors, effectiveness of preventive therapy, competing risks and remaining life-expectancy.6,9,10 Appropriate identification of patients at high risk is important because they most likely gain greatest clinical benefit from intensive treatment of cardiovascular risk factors, novel therapies on top of standard treatment9,11,12 and a more intensive and multidisciplinary follow-up.
Patients with stroke are heterogeneous and systemic atherosclerotic disease and overlapping stroke etiologies are common.13–15 Existing risk stratification tools for stroke patients often focus on short-time risk of recurrent stroke,16–18 while recent long-term follow-up studies have shown that risk of a fatal recurrent stroke and a fatal cardiac event is similar.1 The SMART-REACH (Secondary Manifestations of Arterial Disease-Reduction of Atherothrombosis for Continued Health) model19 is a previously derived, externally validated model estimating individual residual 10-year risk and lifetime risk for recurrent stroke, myocardial infarction and vascular death. The model is intended for use in all patients with clinically manifest atherosclerotic vascular disease and may be useful in routine clinical stroke care. However, it is unknown if this model gives reliable prognostic risk information in a stroke population. Our aim is to estimate future cardiovascular risk using the SMART-REACH model for secondary preventive settings after first validating the model in a stroke cohort. Furthermore, we aim to estimate the theoretical benefit of reaching guideline-recommended risk factor targets.
Materials and Methods
Study Population
We included 729 home-dwelling patients admitted with acute ischemic stroke in the Nor-COAST (Norwegian Cognitive Impairment After Stroke) Study, a multicenter, prospective cohort study consecutively including patients at five Norwegian stroke units from May 2015 to March 2017. Details have been reported previously.2,20
Follow-up for the current substudy started at 3 months poststroke and patients who died before the scheduled 3-month visit (n = 28) were excluded. Since patients expected to have difficulties returning for follow-up visits and patients not independent in daily activities were excluded in the original SMART-REACH derivation and validation cohorts19 and the model is intended for patients with stable vascular disease in which additional preventive therapy is considered, we excluded patients living in nursing homes (n = 36). As the SMART-REACH model was derived in patients aged 45 to 80 years, patients outside this age range were excluded, leaving 465 patients eligible for analysis (Figure 1). Patients were assessed with self-report questionnaires, clinical assessments and blood sampling 3 months poststroke at the outpatient clinic. Patients unable to attend were assessed by telephone or by proxy information. The Regional Committee for Medical and Health Research Ethics in North Norway (REC numbers 2015/171 and 2017/1462) approved the study. All participants gave their written informed consent before inclusion or by proxy if unable. This study was conducted in accordance with the Declaration of Helsinki.
 |
Figure 1 Flowchart of inclusion and exclusion of patients. |
Outcomes
We defined recurrent cardiovascular events as stroke, myocardial infarction (MI) or cardiovascular death, whichever occurred first. All hospitalized events from 3 months poststroke (stable phase) to 31 December 2018 were identified by linkage to the Norwegian Stroke Registry and the Norwegian Cardiovascular Disease Registry. The Norwegian Causes of Death Registry provided follow-up information on the primary cause of death.
We defined recurrent stroke as either registration in the Norwegian Stroke Registry or the Norwegian Cardiovascular Disease Registry (main diagnosis)21 according to the International Classification of Diseases, 10th revision (ICD-10); I61, I63 and I64. Admission with main or secondary diagnosis of MI (ICD-10; I21, I22 and I24) according to the Norwegian Cardiovascular Disease Registry was defined as subsequent MI.22 Cardiovascular death was defined as ICD-code I00-I99 registered as the primary cause of death or death within 28 days after a recurrent stroke or MI. The quality of the information in the registries has been described previously21,22 (Supplementary Methods).
Residual Cardiovascular Risk
The SMART-REACH model19 was used to predict residual cardiovascular risk after initial treatment. The model is a Fine and Gray competing risk model for 10-year and lifetime predictions of cardiovascular events (non-fatal stroke, non-fatal MI and CVD mortality) and non-cardiovascular mortality, where age is used as the underlying time function.9,19 The model uses the following predictors: age, sex, current smoking, diabetes mellitus, history of heart failure, history of atrial fibrillation, systolic blood pressure (BP), serum creatinine concentration, number of locations of CVD (cerebrovascular, coronary and peripheral artery disease) and total and low-density lipoprotein cholesterol (LDL-C). Risks were estimated based on clinical measurements at the 3-month visit since the model is intended for patients with stable CVD in which additional therapy is considered. This timepoint also roughly corresponds to the guideline recommendations to examine risk factors and initiate or modify treatment at 1–3 months after an acute event.23 Table S1 shows detailed definitions of all variables included in the SMART-REACH model and more information about the SMART-REACH model can be found in Supplementary Methods.
External Validation
The external validity of the SMART-REACH model was assessed for risks at 2 years of follow-up. We expressed discrimination (the extent to which patients who develop an event also had higher estimated risk than those who were event-free) with Harrell’s C-statistic.24 We showed the agreement between predicted and observed 2-year risk (calibration) in a flexible calibration curve based on local polynomial regression fitting (loess function in R).25 First, the cohort was divided into 100 quantiles of predicted risk. Then, a local regression was used to smoothly explain the observed cumulative incidence per group by the mean predicted risk per group. The smooth calibration plot and confidence bounds were subsequently predicted from this model over the whole range of relevant predicted risks (cohort predicted risk quantile 0.025 up to 0.975). As event rates vary between geographic locations8,26 and may be influenced by the selection of study participants, recalibration to the population of interest is often necessary.6,19,25 The intercept of the SMART-REACH model for both CVD events and non-CVD mortality was recalibrated (“calibration-in-the-large”) to Nor-COAST by subtracting the expected–observed ratio from the linear predictor (Supplementary Methods).25,27
Impact of Optimization of Risk Factors
Reaching the recommended targets according to Norwegian guidelines23 for systolic BP (≤140 mmHg), LDL-C (≤1.8 mmol/L), smoking cessation and use of antithrombotic agents were defined as optimization of risk factor control and possible benefits if each risk factor was controlled was quantified by the SMART-REACH model.
The relative effect of treating risk factors to recommended targets was retrieved from meta-analyses28–30 (details described in Table S2) and combined with the competing risk-adjusted Cox proportional hazard function from the SMART-REACH model according to previously described methods.9,10,19 A hazard ratio (HR) of 0.80 was assumed per 10 mmHg reduction in systolic BP29 and an HR of 0.78 was assumed per 1.0 mmol/L reduction in LDL-C28 regardless of whether this was achieved by lifestyle changes or medication. Smoking cessation was assumed to reduce the risk of both CVD events (HR 0.60)31 and non-CVD mortality (HR 0.73).32 We assumed that no use of antithrombotic therapy was associated with the inverse effect of starting (at least) aspirin (HR 1/0.81 = 1.23).30 Patients who had already achieved an individual target at 3 months were modeled with an HR of 1.00 for that target.
To estimate the benefit of reaching the guideline-recommended risk factor targets, the cardiovascular risk was estimated twice with the SMART-REACH model for each individual. First, we estimated the risk with the 3-month risk factor levels and treatment, and next we estimated the risk with the assumption that all risk factors met the guideline-recommended targets. The difference between estimated risk with 3-month risk factor levels and estimated risk when risk factors are at target corresponds to an individual’s estimated absolute risk reduction (ARR). We obtained the following estimates from the model: 1) 10-year risk of CVD events, 2) lifetime risk of CVD events, defined as the risk of having an event before the 90th life-year, and 3) the life-expectancy free of CVD events. We calculated the following treatment effects: 1) absolute CVD risk reduction in the next 10 years, 2) absolute lifetime CVD risk reduction and 3) gain in CVD-free life expectancy. The therapy benefits from achieving treatment targets for BP, LDL-C and smoking were first estimated separately. Next, the overall benefit of achieving optimal control of all targets (including use of antithrombotic therapy) was modelled and the relevant ARRs calculated.
Statistics
Baseline characteristics at the index stroke event were described by means with standard deviations (SD) and proportions as appropriate. Estimated risks and ARRs are reported as median with interquartile range (IQR). We visually compared the distribution of estimated risk on current treatment and estimated risk with risk factor(s) at targets in density plots. We imputed missing data for clinical measurements at 3 months for prediction of CVD risk by means of single imputation using predictive mean matching, including all variables used in the analyses. Details and amount of missing data are shown in Table S3. All analyses were conducted using Stata version 16.1 or R statistical software V.4.0.2 (www.r-project.org, packages Hmisc, Survival, Cmprsk, Rms, Pec).
Results
Table 1 shows characteristics at index stay and Table 2 presents achieved risk factor levels 3 months poststroke. Mean LDL-C was 2.1 mmol/L (SD 0.8), mean % relative LDL-C reduction from index stay to 3 months was 24% (SD 33) and 43% reached the target at 3 months. Mean systolic BP was 140 mmHg (SD 19), 51% reached the BP target and 50% (55/109) of smokers quitted smoking at 3 months. Antithrombotic drugs were used by 98%, corresponding numbers for lipid-lowering and antihypertensive drugs were 89% and 73%. Detailed information on cardiovascular medications in use is shown in Table S4. In total, 80% (302/376) reported high adherence at 3 months defined as a score of 4 on Morisky Medication Adherence Scale 4 (MMAS4).2,33
 |
Table 1 Characteristics at the Index Stay (N = 465) |
 |
Table 2 Risk Factor Levels at the Index Stay and the 3-Month Visit (n = 465) |
In total, 52 cardiovascular events and 15 non-cardiovascular deaths were observed from 3 months poststroke during a follow-up of median 2.20 years (IQR 1.79 to 2.62), totally 991 patient-years (Figure 1). In total, 61% (n = 32) of the patients with a recurrent cardiovascular event had a non-fatal stroke, 31% (n = 16) experienced a non-fatal MI and 8% (n = 4) died due to cardiovascular causes.
Estimated Risk of Recurrent Events
The average observed 2-year risk in Nor-COAST was higher than the average predicted 2-year risk with the SMART-REACH model (Figure S1) (expected–observed ratio 0.54). After recalibration, the calibration curve showed adequate agreement between predicted and observed risk and modest discrimination (C-statistics 0.63, 95% CI 0.55 to 0.71) (Figure 2). Discrimination was slightly lower when excluding patients with cardioembolic stroke etiology (C-statistics 0.61, 95% CI 0.53 to 0.70, Figure S2). Sex-specific analyses showed C-statistics 0.65 (95% CI 0.56 to 0.73) for men and 0.57 (95% CI 0.41 to 0.74) for women (Figure S3).
Median estimated 10-year risk of recurrent events was 42% (IQR 32 to 54) (Table 3, Figures 3 and S4–S6). Median lifetime risk was 70% (IQR 63 to 76). Ten-year cardiovascular risk increased with age, while lifetime risk was highest in younger patients (Figure S7, Table S5 and S6). In total, 56% of the patients in the highest 10-year risk quartile had polyvascular disease (Table S5), and 22% were smoking; the corresponding proportions for patients in the lowest risk quartile were 2% and 5%, respectively.
 |
Table 3 Estimated Prognosis and Benefits of Optimal Guideline-Therapy |
Estimated Benefit from Optimization of Risk Factors
Figures S4–S6 shows the benefits of achieving targets for LDL-C, systolic BP and smoking cessation separately. Median 10-year ARR if patients with elevated LDL-C reached the target was 4% (IQR 2 to 7) and gain in CVD-free life-years was 0.8 years (IQR 0.4 to 1.6) (Figure S4B). Median 10-year ARR if patients with elevated BP reached the target was 8% (IQR 3 to 14) and 1.6 CVD-free life-years gained (IQR 0.6 to 3.1) (Figure S5B). Smoking cessation led to 14% (IQR 12 to 16) 10-year ARR and median 3.4 CVD-free life-years gained (IQR 2.4 to 4.3) (Figure S6).
If all targets were achieved, the overall median 10-year ARR was 6% (IQR 1 to 14), and lifetime ARR was 6% (IQR 1 to 15) (Table 3 and Figure 3). The population could gain median 1.4 (IQR 0.2 to 3.4) CVD-free life years. After optimization, the residual median 10-year risk had decreased to 32% (IQR 24 to 44), and lifetime CVD risk had decreased to 61% (IQR 49 to 70) with a CVD-free life expectancy of 82.2 (IQR 78.9 to 85.4) years. If all targets were reached, the 10-year risk would be <20% for 16% of the patients and <30% for 43%. Patient characteristics by quartiles of 10-year ARR are shown in Table S7. Treatment benefits in terms of gain in CVD-free life years were highest in younger patients with elevated risk factor levels (Table S8).
Discussion
In this observational study of patients with ischemic stroke, we found that a notable proportion suffered from a recurrent event the first 2 years poststroke and showed substantial variation in estimated future cardiovascular risk and treatment benefit from intensification of secondary prevention. We revealed a remaining preventive potential by reaching the guideline-recommended treatment targets and demonstrated that the SMART-REACH model generates prognostic risk information reasonably well in stroke patients.
Studies quantifying future cardiovascular risk in stroke populations are scarce. However, comparable findings of risk and potential benefit variations have been shown in patients with established CVD in general.6,19,34 The residual risk in Nor-COAST is quite high compared to other studies.6,19,34 However, Nor-COAST included solely patients with stroke, while other cohorts also included transient ischemic attacks.7,19 Moreover, the consecutive inclusion of stroke patients minimizes healthy participant bias35 and higher-risk patients are more likely to be included. Although high residual risk might be explained by non-modifiable factors such as age, already severely progressed atherosclerosis or genetic disposition, modifiable risk factors like inflammation or further reduction of BP and LDL-C are of importance.23,28,29 Mean risk factor levels in Nor-COAST are not far from targets and more in line with guideline recommendations compared to other populations,2–4 yielding less possibilities for benefit based on current cut-offs. However, BP and LDL-C are continuously related to CVD risk,28,29 and an individual patient could still benefit from further reduction.
The predicted 2-year risk corresponded adequately with the observed risk in Nor-COAST after recalibration. Discrimination was acceptable and in line with other prognostic tools already in clinical use,7,16,18 and previous validations of the SMART-REACH model have shown comparable results.19,34 Moreover, sex-specific analyses showed lower c-statistics for women; however, these results should be interpreted with caution due to lack of statistical power. Stroke is a heterogeneous condition with multiple possible etiologies where stroke classification is crucial. Performance of the model may be different in patients with cardioembolic stroke etiology, especially if the burden of atherosclerosis and associated risk factors is low or absent. Due to the limited sample size, the performance in this subgroup could not be evaluated. Still, the large overlap between underlying etiologies and other cardiovascular entities13–15 illustrates the need for optimal atherosclerotic risk factor control in general. Although some short-term risk prediction models developed separately for stroke patients already exist,16–18 the SMART-REACH19 model can be used in individuals with any type of atherosclerotic disease, also multiple manifestations, which often is the case in clinical practice. The SMART-REACH model is readily available via online calculators such as u-prevent.com. However, ideally the geographic correction factor should be applied when using the model in clinical practice for similar populations.
Strengths and Limitations
The strengths of this study include the multicenter design, valid registry data, an up-to-date time period and prospective consecutive inclusion of patients reflecting current clinical practice.35 Another strength is using a prediction tool that estimates both 10-year risk and lifetime risk adjusting for competing risks and remaining life-expectancy. As secondary prevention presumably is continued lifelong, it may be more intuitive to use a lifetime risk prediction model. Furthermore, adjusting for death of other causes avoids overestimating CVD risk and treatment benefit in older individuals.19 The observed 2-year event rate in Nor-COAST (Figure S8) corresponds reasonably well with event rates in a recent meta-analysis1 and the Nor-COAST population has characteristics in line with patients in the Norwegian stroke registry.2,35 Generalization at least to Norwegian stroke patients and comparable stroke populations is therefore plausible.
Not including the oldest patients is a significant limitation and performing external validation and recalibration based on 2-year predictions might be a weakness. However, previous studies have shown that lifetime estimates based on similar methods appear to be reliable for predictions up to at least 17 years.9 C-statistics for discrimination are moderate. However, demonstrating adequate calibration might be a more relevant measure since knowing that the predicted risk reflects the actual risk is important for clinical treatment decisions.8,36 We did not account for changes in risk factor levels over time. However, changes in risk factor levels after 3 months are not likely to affect predictive performance.37 We have previously published detailed data on how adherence to medications and risk factor control changes from discharge to 18 months poststroke in Nor-COAST,2 which showed that risk factor levels remain relatively unchanged. Risk factor levels also often deteriorate over time due to decrease in drug adherence and healthy lifestyle habits.2,5 Missing data for clinical measurements at the 3-month follow-up might, however, be a weakness. The relative effects of BP and LDL-C lowering are based on large meta-analyses synthesizing evidence from primary and secondary preventive settings and benefits might be smaller or larger depending on specific stroke characteristics. However, relative effect estimates are broadly similar across several subgroups of patients.28,29 Therefore, we consider these relative effects valid for our population. We did not account for disadvantages and harm of pharmacotherapy, like adverse reactions and costs. At last, risk prediction models include varying degrees of uncertainty and cannot replace good clinical judgment but help structure and guide clinicians in their medical decision-making process.8
Conclusions
Current risk factor control after ischemic stroke is suboptimal. The predicted future risk is high but with considerable individual variation and a corresponding variation in the benefit from intensification of secondary prevention. An available risk prediction tool such as the SMART-REACH model can be used to identify patients with the largest benefit from intensification of treatment and more intensive short-term or multidisciplinary follow-up. We believe the model can be a useful tool for more personalized surveillance of patients in both stroke units and other clinical settings like general practice. More research is needed to assess potential strategies for further lowering of the high residual cardiovascular risk in these patients, and selection of patients by risk stratification may help improve focus and efficiency in future trials.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We gratefully acknowledge all participants, the Nor-COAST research group and the dedicated study staff at participating hospitals. MMAS Research Morisky Widget Software US Copyright Office Number TX 8-816-517 is protected by US Copyright laws. Permission for use is required. A license agreement was made between St. Olav University Hospital and MMAS Research LLC. A license is available from MMAS Research LLC. E-mail: [email protected].
Funding
The Nor-COAST study was funded by the Norwegian Health Association and Norwegian University of Science and Technology (NTNU). The work of MNG was funded by Dam Foundation and the Liaison Committee between the Central Norway Regional Health Authority and NTNU.
Disclosure
The authors reports no conflicts of interest in this work. IS have been investigator in Boehringer-Ingelheim 1346.0023 and reports personal fees from Biogen, outside the submitted work.
References
1. Boulanger M, Béjot Y, Rothwell PM, Touzé E. Long-term risk of myocardial infarction compared to recurrent stroke after transient ischemic attack and ischemic stroke: systematic review and meta-analysis. J Am Heart Assoc. 2018;7(2):e007267. doi:10.1161/JAHA.117.007267
2. Gynnild MN, Aakerøy R, Spigset O, et al. Vascular risk factor control and adherence to secondary preventive medication after ischaemic stroke. J Intern Med. 2020;289(3):355–68. doi:10.1111/joim.13161
3. Brewer L, Mellon L, Hall P, et al. Secondary prevention after ischaemic stroke: the ASPIRE-S Study. BMC Neurol. 2015;15(1):216. doi:10.1186/s12883-015-0466-2
4. Heuschmann PU, Kircher J, Nowe T, et al. Control of main risk factors after ischaemic stroke across Europe: data from the stroke-specific module of the EUROASPIRE III survey. Eur J Prev Cardiol. 2015;22(10):1354–1362. doi:10.1177/2047487314546825
5. Norrving B, Barrick J, Davalos A, et al. Action plan for stroke in Europe 2018–2030. Eur Stroke J. 2018;3(4):309–336. doi:10.1177/2396987318808719
6. Kaasenbrood L, Boekholdt SM, van der Graaf Y, et al. Distribution of estimated 10-year risk of recurrent vascular events and residual risk in a secondary prevention population. Circulation. 2016;134(19):1419–1429. doi:10.1161/CIRCULATIONAHA.116.021314
7. Dorresteijn JA, Visseren FL, Wassink AM, et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart. 2013;99(12):866–872. doi:10.1136/heartjnl-2013-303640
8. Rossello X, Dorresteijn JA, Janssen A, et al. Risk prediction tools in cardiovascular disease prevention: a report from the ESC prevention of CVD programme led by the European Association of Preventive Cardiology (EAPC) in collaboration with the Acute Cardiovascular Care Association (ACCA) and the Association of Cardiovascular Nursing and Allied Professions (ACNAP). Eur Heart J Acute Cardiovasc Care. 2019;26(14):1534–1544. doi:10.1177/2048872619858285
9. Dorresteijn JAN, Kaasenbrood L, Cook NR, et al. How to translate clinical trial results into gain in healthy life expectancy for individual patients. BMJ. 2016;352:i1548. doi:10.1136/bmj.i1548
10. van der Leeuw J, Ridker PM, van der Graaf Y, Visseren FL. Personalized cardiovascular disease prevention by applying individualized prediction of treatment effects. Eur Heart J. 2014;35(13):837–843. doi:10.1093/eurheartj/ehu004
11. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319–1330. doi:10.1056/NEJMoa1709118
12. Giugliano Robert P, Pedersen Terje R, Saver Jeffrey L, et al. Stroke prevention with the PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibitor evolocumab added to statin in high-risk patients with stable atherosclerosis. Stroke. 2020;51(5):1546–1554. doi:10.1161/STROKEAHA.119.027759
13. Hoshino T, Sissani L, Labreuche J, et al. Prevalence of systemic atherosclerosis burdens and overlapping stroke etiologies and their associations with long-term vascular prognosis in stroke with intracranial atherosclerotic disease. JAMA Neurol. 2018;75(2):203–211. doi:10.1001/jamaneurol.2017.3960
14. Sirimarco G, Lavallée Philippa C, Labreuche J, et al. Overlap of diseases underlying ischemic stroke. Stroke. 2013;44(9):2427–2433. doi:10.1161/STROKEAHA.113.001363
15. Gongora-Rivera F, Labreuche J, Jaramillo A, Steg Philippe G, Hauw JJ, Amarenco P. Autopsy prevalence of coronary atherosclerosis in patients with fatal stroke. Stroke. 2007;38(4):1203–1210. doi:10.1161/01.STR.0000260091.13729.96
16. Kernan Walter N, Viscoli Catherine M, Brass Lawrence M, et al. The stroke prognosis instrument II (SPI-II). Stroke. 2000;31(2):456–462. doi:10.1161/01.STR.31.2.456
17. Ay H, Gungor L, Arsava EM, et al. A score to predict early risk of recurrence after ischemic stroke. Neurology. 2010;74(2):128–135. doi:10.1212/WNL.0b013e3181ca9cff
18. Weimar C, Diener HC, Alberts MJ, et al. The Essen stroke risk score predicts recurrent cardiovascular events: a validation within the REduction of Atherothrombosis for Continued Health (REACH) registry. Stroke. 2009;40(2):350–354. doi:10.1161/strokeaha.108.521419
19. Kaasenbrood L, Bhatt DL, Dorresteijn JAN, et al. Estimated life expectancy without recurrent cardiovascular events in patients with vascular disease: the SMART-REACH model. J Am Heart Assoc. 2018;7(16):e009217. doi:10.1161/jaha.118.009217
20. Thingstad P, Askim T, Beyer MK, et al. The Norwegian Cognitive impairment after stroke study (Nor-COAST): study protocol of a multicentre, prospective cohort study. BMC Neurol. 2018;18(1):193. doi:10.1186/s12883-018-1198-x
21. Varmdal T, Bakken IJ, Janszky I, et al. Comparison of the validity of stroke diagnoses in a medical quality register and an administrative health register. Scand J Public Health. 2016;44(2):143–149. doi:10.1177/1403494815621641
22. Govatsmark RES, Janszky I, Slørdahl SA, et al. Completeness and correctness of acute myocardial infarction diagnoses in a medical quality register and an administrative health register. Scand J Public Health. 2020;48(1):5–13. doi:10.1177/1403494818803256
23. Norwegian Guideline for Prevention of Cardiovascular Disease. The Norwegian directorate of health; March 5, 2018. Available from: https://www.helsedirektoratet.no/retningslinjer/forebygging-av-hjerte-og-karsykdom.
24. Harrell FE
25. Van Calster B, McLernon DJ, van Smeden M, Wynants L, Steyerberg EW. Calibration: the Achilles heel of predictive analytics. BMC Med. 2019;17(1):230. doi:10.1186/s12916-019-1466-7
26. Ducrocq G, Bhatt DL, Labreuche J, et al. Geographic differences in outcomes in outpatients with established atherothrombotic disease: results from the REACH registry. Eur J Prev Cardiol. 2014;21(12):1509–1516. doi:10.1177/2047487313501278
27. Steyerberg EW, Borsboom GJ, van Houwelingen HC, Eijkemans MJ, Habbema JD. Validation and updating of predictive logistic regression models: a study on sample size and shrinkage. Stat Med. 2004;23(16):2567–2586. doi:10.1002/sim.1844
28. Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi:10.1016/s0140-6736(10)61350-5
29. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi:10.1016/s0140-6736(15)01225-8
30. Antithrombotic Trialists C. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi:10.1016/S0140-6736(09)60503-1
31. Mons U, Müezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350(apr20 2):h1551. doi:10.1136/bmj.h1551
32. Gellert C, Schöttker B, Brenner H. Smoking and all-cause mortality in older people: systematic review and meta-analysis. Arch Intern Med. 2012;172(11):837–844. doi:10.1001/archinternmed.2012.1397
33. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi:10.1097/00005650-198601000-00007
34. de Vries TI, Eikelboom JW, Bosch J, et al. Estimating individual lifetime benefit and bleeding risk of adding rivaroxaban to aspirin for patients with stable cardiovascular disease: results from the COMPASS trial. Eur Heart J. 2019;40(46):3771–3778a. doi:10.1093/eurheartj/ehz404
35. Kuvås KR, Saltvedt I, Aam S, Thingstad P, Ellekjær H, Askim T. The risk of selection bias in a clinical multi-center cohort study. results from the Norwegian cognitive impairment after stroke (Nor-COAST) Study. Clin Epidemiol. 2020;12:1327–1336. doi:10.2147/clep.S276631
36. Cook Nancy R. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115(7):928–935. doi:10.1161/CIRCULATIONAHA.106.672402
37. Xu Z, Arnold M, Stevens D, et al. Prediction of cardiovascular disease risk accounting for future initiation of statin treatment. Am J Epidemiol. 2021. doi:10.1093/aje/kwab031
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


