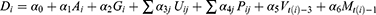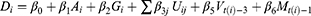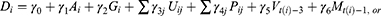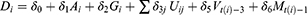Back to Journals » International Journal of General Medicine » Volume 16
Risk of Underlying Diseases and Effectiveness of Drugs on COVID-19 Inpatients Assessed Using Medical Claims in Japan: Retrospective Observational Study
Authors Mitsushima S , Horiguchi H, Taniguchi K
Received 2 November 2022
Accepted for publication 3 February 2023
Published 21 February 2023 Volume 2023:16 Pages 657—672
DOI https://doi.org/10.2147/IJGM.S394413
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Shingo Mitsushima,1 Hiromasa Horiguchi,2 Kiyosu Taniguchi3,4
1Center for Field Epidemic Intelligence, Research and Professional Development, National Institute of Infectious Diseases, Shinjuku-ku, Tokyo, Japan; 2Department of Clinical Data Management and Research, Clinical Research Center, National Hospital Organization Headquarters, Meguro-ku, Tokyo, Japan; 3Director-General, National Hospital Organization Mie National Hospital, Tsu, Mie, Japan; 4Research Director, The Tokyo Foundation for Policy Research, Minato-ku, Tokyo, Japan
Correspondence: Shingo Mitsushima, Center for Field Epidemic Intelligence, Research and Professional Development, National Institute of Infectious Diseases, 1-23-1 Toyama, Shinjuku-ku, Tokyo, 162-8640, Japan, Tel +81-3-5285-1111, Fax +81-3-5285-1150, Email [email protected]
Background: Results of earlier studies have demonstrated underlying diseases such as cancer, diabetes mellitus, immunodeficiency, hypertension and heart failure to be risk factors for severe outcomes and mortality. Furthermore, clinical trials have shown that drugs such as antiviral drugs, antibody cocktails, steroids and anti-inflammatory drugs can be expected to prevent severe COVID-19 outcomes and death.
Methods: This study, using inpatient records from the Medical Information Analysis Databank covering national hospital organizations in Japan, was conducted to evaluate the effects of underlying diseases and/or administered drugs on mortality. Subjects were all inpatients receiving oxygen administration and inpatients using respiratory ventilators, categorized by three age classes: all ages, patients 65 years old or older, and patients younger than 65 years old. We used logistic regression to analyze outcomes for underlying diseases, administered drugs, age, sex, the proportion of the mutated strains, and vaccine coverage.
Results: Patients with hypertension, except for younger inpatients, have a lower risk of mortality (estimated coefficient 0.67 among all inpatients (p < 0.01): 0.77 among inpatients with oxygen therapy (p = 0.02) and 0.57 among inpatients with respiratory ventilation w (p = 0.01)). Except for younger inpatients, antibody cocktail (casirivimab/imdevimab or sotrovimab) administration was associated with a higher probability of survival (estimated coefficient 0.27 among all inpatients (p < 0.01)). It raised the survival probability consistently, although other drugs might have reduced the probability of survival.
Conclusion: These findings suggest that antiviral drugs (remdesivir, estimated coefficient 1.44 (p < 0.01)), steroids (dexamethasone, estimated coefficient 1.85 (p < 0.01)), and anti-inflammatory drugs (baricitinib, estimated coefficient 1.62 (p < 0.01), and tocilizumab, estimated coefficient 2.73 (p < 0.01)) might not contribute to survival. These results have not been reported from earlier studies. More sophisticated estimation procedures, such as treatment effect models, are necessary to obtain conclusive results.
Keywords: antibody cocktail, anti-inflammatory drug, antiviral drug, hypertension, steroid, variant strains
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is responsible for coronavirus disease 2019 (COVID-19), emerged at the end of December 2019 in China and quickly spread worldwide. Human-to-human transmission was observed. This transmission is through spike protein receptor binding domain and receptor angiotensin-converting enzyme 2 (ACE2). ACE2 is related to the SARS-CoV-2 entry to the host cells and replication.1 Japan had one of the lowest fatality rates in the world in 2020.2
Results of earlier studies have shown underlying diseases such as cancer,3–7 diabetes mellitus,4,8–14 immunodeficiency,10 hypertension,4,8–11,15–17 and heart failure9,11,15 as risk factors for severe outcomes or mortality for COVID-19 patients. Vaccination against SARS-CoV-2 was expected to prevent severe or mortality of COVID-19. In Japan, vaccination for people 65 years old and older started in April 2021. The proportion of one or more dose vaccinations reached about 90% by the end of March 2022. Vaccination for people younger than 65 years old started in July 2021. The proportion of one or more dose vaccinations reached about 70% by the end of March 2022.18 Elderly people showed a higher proportion of one or more dose vaccinations than younger people, as an earlier study showed.19
Moreover, earlier studies and clinical trials have demonstrated that drugs used against COVID-19 such as antiviral drugs (remdesivir),20 antibody cocktails (casirivimab/imdevimab or sotrovimab),21,22 steroids (dexamethasone),23 and anti-inflammatory drugs (baricitinib and tocilizumab)24–26 can be expected to prevent severe COVID-19 outcomes and death. Other drugs have been used against COVID-19 such as statins,27,28 RNA-dependent RNA polymerase inhibitors (molnupiravir and favipiravir),29,30,34,35 protease inhibitors (nirmatrelvir/ritonavir),29,31 and JAK inhibitor (ruxolitinib),36 but none of those was evaluated for this study.
Japan’s National Hospital Organization (NHO), an organization of regional core hospitals and 140 medical facilities with about 52,000 beds, represents about 3.4% of all beds in Japan.32 One or more NHO hospitals are located in each prefecture. The NHO provides the Medical Information Analysis Databank (MIA), which collects data on medical insurance claims for outpatients and inpatients of NHO hospitals from 2010. The secondary use of MIA for epidemiological studies is available. MIA includes data related to patient characteristics and underlying diseases, medical interventions including oxygen administration and the use of respiratory ventilators, received therapy including administration of drugs, and outcomes such as discharge or death.33 The database does not include data related to a patient’s vaccination history or the causative strain. For this study, we analyzed data from 60 representative NHO hospitals with available data.
The object of this study was examination of risk factors among underlying diseases for severe COVID-19 outcomes and evaluation of the effectiveness of drugs against COVID-19 in Japan using MIA.
Methods
Data Source
This study used MIA data from the NHO for SARS-CoV-2 confirmed inpatients, including their age, sex, underlying diseases, hospitalized week, whether they received oxygen therapy and/or ventilation, administered drugs, and outcomes. Unfortunately, MIA includes no information about patient vaccine status or subtype of SARS-CoV-2. Therefore, we used data that include vaccine administration published by the Cabinet Secretariat (https://www.kantei.go.jp/jp/headline/kansensho/vaccine.html). Moreover, the prevalence situations of Alpha, Delta and Omicron variant strains, Alpha, Delta, and Omicron variant strains, were referred from a monitoring meeting in Tokyo (https://www.bousai.metro.tokyo.lg.jp/taisaku/saigai/1013388/index.html).
The study period was January 2020 through the end of March 2022, as of May 2022. The study area was the entirety of Japan.
Definitions of Variables
Data related to the patients’ physical condition included age and sex.
As underlying diseases, we examined cancer (C00-C96 in ICD10), hypertension (I10-I15), heart failure (I50, I51), and diabetes mellitus (DM) (E10-E14). It is noteworthy that a patient could have several of the considered underlying diseases. We examined effects of antiviral drug (remdesivir), antibody cocktails (casirivimab/imdevimab and sotrovimab), steroids (dexamethasone), and anti-inflammatory drugs (baricitinib and tocilizumab). Because the number of cases with antibody cocktails was insufficiently large, we did not divide it according to the drug name, either casirivimab/imdevimab or sotrovimab, and instead considered the effects of antibody cocktails overall. We selected these drugs from drugs of 15 types specified as COVID-19 treatments: remdesivir, casirivimab/imdevimab and sotrovimab, dexamethasone, baricitinib and tocilizumab. Some drugs that were shown to be ineffective in earlier studies were excluded from this examination.37–42 Among 9737 cases of administration, remdesivir was used for 18% of inpatients, antibody cocktails including it for 4%, dexamethasone for 54%, baricitinib for 10%, and tocilizumab for 5%. These were the top five drugs used for inpatients. Therefore, we limited our analyses to these major drugs. Some patients were administered multiple considered drugs during their hospitalization. Unfortunately, data about the timing of administration of the drug during their hospitalization were not available for this study.
Vaccine coverage was defined as the rate of completion of second-dose administration two weeks prior by age class: younger than 65 years old, or 65 years old or older.
Regarding variant strains, they were measured by percentage in the same week of admission. Omicron included BA.2 or later sublineage. Moreover, to check robustness, we used a dummy variable for data during waves 4–6 instead of the proportion of the mutated strains as an explanatory variable. By this specification, the Alpha variant strain emerged and then dominated in the fourth wave, defined as 1 March through 20 June, 2021 when the Alpha variant strain emerged and became dominant. Similarly, the Delta variant strain emerged and then dominated in the fifth wave defined as 21 June through 21 November, 2021. Omicron, BA.1 strain emerged and then became dominant in the sixth wave, defined as 22 November, 2021 to the end of study period.
The outcome was defined as death during hospitalization. Because our data are based on medical claims, we identified completely whether a patient had the considered underlying diseases and had been administered the considered drugs, or not. There were no missing data for underlying diseases or drugs. Moreover, we used the national averages as information about vaccine coverage and the mutant strains.
Patients
We presumed that all patients, as inpatients, had been infected by SARS-CoV-2. However, the criteria for hospitalization used for asymptomatic patients or patients with mild symptoms who did not require oxygen therapy were probably affected strongly by the scarcity of medical resources or social circumstances such as support for their stay at home and recuperation at home. Therefore, aside from pure medical criteria, we also limited scope of the subjects to inpatients who had received oxygen therapy or respiratory ventilation.
Statistical Analysis
To evaluate the effects of underlying diseases and/or pharmaceutical therapy, we used logistic regression to regress outcomes, whether death or not as dependent variable, on the patient physical condition, underlying diseases, pharmaceutical therapy, vaccine coverage, and prevalence in the variant strains as independent variables such as:
where subscript 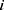 denotes the
denotes the 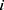 th inpatient, t(i) stands for the hospitalized week of the ith inpatient,
th inpatient, t(i) stands for the hospitalized week of the ith inpatient, 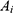 denotes the age on t(i),
denotes the age on t(i), 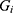 is a sex dummy,
is a sex dummy, 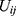 are dummy variables for the
are dummy variables for the 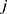 th underlying diseases on
th underlying diseases on 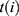 ,
, 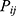 are dummy variables for the administered drug during their hospitalization period,
are dummy variables for the administered drug during their hospitalization period, 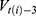 expresses the vaccine coverage on
expresses the vaccine coverage on 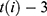 week, and
week, and 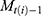 signifies the proportion of the mutated strain for
signifies the proportion of the mutated strain for 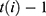 week. We assumed to take one week from infection to be hospitalized and two weeks for being immune after immunization.
week. We assumed to take one week from infection to be hospitalized and two weeks for being immune after immunization.
Moreover, because drug administration was based endogenously on the inpatient condition. Some bias might be attributable to simultaneous determination in the specification above. Therefore, we also regressed it as explanatory variables were, without administered drugs as
Finally, we regressed the outcome on the same explanatory variables, but using the wave period instead of the proportion of the variant strains: Alpha, Delta and Omicron such as
All statistical analyses were conducted using software (SE 17.0; Stata Corp.). We adopted 5% as significance level.
Ethical Considerations
Individual informed consent was not required to conduct this study because the dataset was provided as anonymized data by NHO.33 This study was approved by the Ethics Committee of Mie Hospital (Approval No. 2020-89). Specific permission to use MIA data was obtained from the NHO (Registration No. 1201003).
Results
Figure 1 presents the number of new COVID-19 hospitalizations from MIA data and the number of patients who had been administered drugs for treatment of COVID-19 during their hospitalization by the hospitalized week. The findings indicate that the most administered drug against COVID-19 in 2020 and 2021 was dexamethasone, but it was remdesivir in 2022. The numbers of patients who had been administered drugs closely paralleled the numbers of new COVID-19 hospitalizations.
Figure 2 shows the number of underlying diseases of SARS-CoV-2 infected inpatients in MIA data at their hospitalization. The number of all underlying diseases changed according to the increasing and decreasing of inpatients who had been infected by SARS-CoV-2. Throughout the study period, there were more inpatients with hypertension and diabetes mellitus than inpatients with cancer and heart failure.
Table 1 presents the estimation results obtained for all inpatients, inpatients receiving oxygen therapy and inpatients who used ventilators of all ages, 65 years old and older, or younger than 65 years old. Common results among these populations were odds of age greater than one, ie, rising risk of mortality by age, even in younger people.
 |
Table 1 Estimation Results of Logistic Regression for Death with the Administered Drug and Proportion of Mutated Strains |
Regarding underlying diseases, hypertension was associated with reduced risk of mortality, except for younger inpatients treated with oxygen therapy or a ventilator. That finding suggests that patients with hypertension have a higher probability of survival. Heart failure and cancer were associated with significantly elevated risk in some estimations. Most results for DM were not significant. However, it might be associated with higher survival among younger inpatients, including those without oxygen therapy or a ventilator and older inpatients with a ventilator.
Regarding the administered drugs, the odds of casirivimab/imdevimab or sotrovimab administration, except for younger inpatients, were significantly lower than one in some estimations, indicating that patients administered casirivimab/imdevimab or sotrovimab have a higher probability of survival. Odds of the other examined drugs are greater than one, suggesting that these drugs reduced patients’ probability of survival.
Results related to mutated strains were mixed. Among all inpatients, the proportions of mutated strains, whether Alpha, Delta or Omicron, were not different from those of the original strain. However, among inpatients of all ages who had received oxygen therapy, Delta and Omicron variant strains showed lower mortality than for the original strain. That finding was not shown by age class. By contrast, the Alpha variant strain was associated with higher mortality than the original strain among inpatients of all ages and those younger than 65 years old who had used a ventilator.
Table 2 presents estimation results excluding drugs from explanatory variables: DM among all inpatients of all ages was found to be significant. However, for hypertension among inpatients 65 years old or older who had received oxygen therapy, the proportion of Omicron among inpatients receiving oxygen therapy in all ages, and DM among inpatients 65 years old or older with ventilator use was not significant. Differences in the estimated coefficients of these variables were less than 10% in absolute terms.
 |
Table 2 Estimation Results of Logistic Regression for Death with Proportion of the Mutated Strains and with and without Administered Drugs |
Table 3 presents estimation results obtained using dummy variables during waves 4–6 instead of the proportions of the mutated strains. The results were almost identical to those presented in Table 1. Exceptions were baricitinib among inpatients receiving oxygen therapy in patients of all age. No variable shown in Table 1 is significantly different from the estimated sign. Therefore, using the period instead of the proportion of the mutated strains confirmed the estimated results shown in Table 1.
 |
Table 3 Estimation Results of Logistic Regression for Death with Administered Drugs and Periods of Waves When the Mutated Strains Were Dominant |
As shown in Table 1 and Table 3, no inference about casirivimab/imdevimab or sotrovimab could be made because of the small number of so-treated younger inpatients with a ventilator. The tables show estimation results excluding those for casirivimab/imdevimab or sotrovimab in the explanatory variables.
Discussion
Findings indicated that antibody cocktails (casirivimab/imdevimab or sotrovimab) raised the probability of survival, but other drugs might have reduced the probability of survival. These counterintuitive indications might be attributable to reverse causality: higher risk of mortality led to the use of these drugs. In other words, the selection of who had been administered these drugs, or not, is expected to be endogenous and determined by the patient condition. In this sense, drug administration might be inappropriate as an explanatory variable to explain outcomes. To resolve this difficulty, patients with almost identical conditions must be compared, with one patient coincidentally using the drug and another patient not using the drug. For such an experiment, we would be able to assign the drug randomly. However, when using these observational data after the fact, such experiments are impossible. Under these circumstances, propensity score matching provides a statistical experiment.43–45 That is expected to be a challenge for future study.
Similarly, findings indicate that patients with hypertension have a higher probability of survival than patients without cancer, hypertension, DM, or heart failure. At first glance, this result was inconsistent with those of earlier studies indicating hypertension as a risk factor for developing COVID-19.4,8–11,15–17 In 2020, COVID-19 patients who were taking angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin-receptor blockers (ARB) were suspected to be severely ill because the SARS-CoV-2 receptor is angiotensin-converting enzyme 2. However, some reports have described that taking ACE-I or ARB did not increase the risk of severe COVID-19 outcome or death.46,47 In fact, reports of some studies have suggested ACE-I or ARB as effective for preventing a severe COVID-19 outcome or death.48–52 Therefore, the risk posed by hypertension might be controversial. Our results might support the effectiveness of ACE-I or ARB because many patients are taking these medicines. This finding might partially explain the result that patients with hypertension tended to avoid a severe COVID-19 outcome or death. Furthermore, this finding also suggests that patients with hypertension, because they were characterized as high-risk patients, received more cautiously administered care than patients with no underlying disease. Consequently, they had a higher probability of survival. However, we defined hypertension based on the diagnosis. We did not depend on blood pressure test results. Therefore, this definition for hypertension might include patients with very mild hypertension. For that reason, their probability of survival was estimated as greater than for inpatients without hypertension. Whichever happened to be the case, more careful analysis is needed before one could conclude that hypertension was not a risk factor for COVID-19.
Patients with cancer have a significantly high risk of mortality among all inpatients and inpatients with oxygen therapy. This result is consistent with those of earlier studies.3–7 However, they have a low risk of mortality among inpatients receiving respiratory ventilation, although it was not significant. This might be true because inpatients with cancer, especially elderly inpatients, tend to avoid intensive care. Only inpatients with better condition might receive respiratory ventilation. Then the risk of mortality might be low. Inpatients with heart failure might have the same reason for low risk of mortality among inpatients receiving respiratory ventilation. Data from the MIA with more cases might provide detailed results such as indications of breakthrough infection, and detailed risk factors in each disease for mortality.53
The obtained results showed that vaccination coverage did not contribute to survival. Vaccine for COVID-19 was expected to reduce infection and severity when recipients were infected.54 Vaccination might not be expected to improve the probability for survival. For this study, we examined vaccine effects among all inpatients or inpatients receiving oxygen therapy or ventilator use. Therefore, the subjects were presumed to be severe cases. For that reason, vaccine effectiveness might not be confirmed by our data. Additionally, we used vaccine coverage instead of the vaccination history of the patients themselves because the latter was not available in our data. If the vaccine history of the patients was available, then the results might differ.
One earlier study examined social predictors of receiving vaccination.55 Even for COVID-19, such as study was conducted.56 If social factors affect vaccination and if vaccines were effective to prevent severe COVID-19, then the social backgrounds of inpatients might differ before and after the initiation of vaccination. For instance, because vaccination had started for old persons at first, the average age among new hospitalized patients can be expected to be lower. This changing age distribution of inpatients might affect the results. In general, younger inpatients have a higher probability of survival than older patients with the same condition other than age. Assuming those effects and knowing that some drugs had been approved later during the study period, the effects of drug administration can be expected to be biased upwardly by the changing age distribution attributable to vaccination initiation. Therefore, their effectiveness might be lower than the obtained result. Other social factors such as income, health insurance status or religion are not recorded in medical claim data. Therefore, we cannot examine the effects of these factors. Particularly, vaccination and treatment for COVID-19 required no payment for patients. All costs were paid by the government of Japan. Income and health insurance status were presumed to be independent of decisions about receiving vaccinations or visiting a doctor. No report describes a study of vaccination against COVID-19 in Japan examining that point. Moreover, decisions about drug administration and using oxygen therapy or respiratory ventilation were made by doctors. Therefore, these social factors other than age were presumed not to affect the results of this study.
We did not find the Delta and Omicron variant strain to have higher pathogenicity than the original or Alpha variant strain. One excess mortality study showed higher excess mortality during Delta and Omicron variant strain dominance in Japan.57 Our obtained result might be inconsistent with that study which found excess mortality. Nevertheless, it is noteworthy that this study examined the rate of mortality among inpatients, whereas the excess mortality study cited above examined the number of mortality cases attributable to all causes. Even if the mortality rate among inpatients was smaller, if the number of infected persons was much larger, then the number of mortalities could be larger. Therefore, our result might not be inconsistent with those of the excess mortality study.
Different timing of treatment initiation among patients might have affected the results. For example, patients with severe pneumonia but who had not been treated yet might have died before being administered the appropriate drug. If so, time-immortal bias might be severe.58 An example of this bias was death before operation, even though an operation had been planned. In such a case, these biases increase the risk of no-operation and therefore overestimate the benefits of the operation. However, because this study considered the administration of drugs, the period from decision-to-use to actual administration was not long, unlike the case of a surgical or other operation. We also examined these points for inpatients receiving oxygen therapy or respiratory ventilation. In these estimations, the inpatient condition was better-controlled than estimation among all inpatients: time-immortal bias was expected to be smaller, perhaps even negligible. Actually, results for drugs for severe patients (remdesivir, dexamethasone, baricitinib, and tocilizumab) showed that they did not contribute to survival. Therefore, time-immortal bias, which overestimates of treatment effectiveness, was not large. By contrast, antibody cocktails showed effectiveness. However, because these drugs were typically used for patients who were less severely ill, time-immortal bias might not be strongly applicable.
If test data such as oxygen saturation over time were used, then it would be possible to use survival analysis or a marginal structural model to evaluate drug effectiveness and to model the dynamics of development of the illness.59 However, our data did not include such time-varying data or test data. Therefore, we must limit the information at hospitalization, except for drug administration. We confirmed that estimation results were not affected heavily, whether drug administration was included among the explanatory variables, or not.
Limitations
First, we defined severity as the mortality rate among inpatients, or inpatients receiving oxygen therapy or ventilation. Therefore, the results might differ from those found for the case-fatality rate, which has been more commonly used to indicate the pathogen severity. In general, the case-fatality rate was defined as the number of cases of mortality among all infected patients, including moderate cases, with patients were not hospitalized, or asymptomatic cases. This study did not include those moderate cases or asymptomatic cases.
Second, although the selection of drugs and decisions to use drugs were endogenous by the patient situation and drugs available at that time, we treat them as exogenous variables of the estimation model. To address this difficulty, we must use a treatment effect model with propensity score matching.
Third, because no information was available to us about the vaccination histories of the patients and strains infecting the patients, we relied on the circumstances of the vaccine coverage or prevailing strain when the patients were presumed to have been infected. This indirect information and the consequent estimation results might have been affected if the patients’ vaccine history or infected strain had been available.
Fourth, because MIA is a database of medical claims, the data of MIA, especially data of the prior few weeks, might be revised slightly over a few months. For this study, we collected and analyzed MIA data from January 2020 to the end of March 2022 as of May 2022. Data and therefore estimation results might differ over time if the study period were extended. Further examinations must be undertaken with an extended period of study and a greater number of patients. Some ambiguity might result from the number of patients, which was insufficient to yield clear results. However, our MIA data were the widest dataset for inpatients including long-term outcomes and therapy in Japan. Therefore, we cannot expand the number of inpatients for improvement of the estimation, except by extending the study period. Of course, international comparisons through meta-analyses might overcome this difficulty, but such methods are beyond the scope of this study.
Fifth, we exclude camostat from analysis because it was shown to be ineffective in an earlier study.39 However, it was administered more than antibody cocktails. Therefore, we might analyze its effectiveness in the framework of this study. It might play a role as a negative control and might evaluate the precision of estimation procedures used for this study.
Conclusion
The obtained results demonstrated that patients with hypertension have a lower risk of mortality, except for younger patients, which suggests that hypertension might not pose a risk to survival. Results also show that only antibody cocktails (casirivimab/imdevimab or sotrovimab) raise the probability of survival consistently, although other drugs might reduce the probability of survival. In other words, other drugs such as antiviral drugs (remdesivir), steroids (dexamethasone), and anti-inflammatory drugs (baricitinib and tocilizumab) might not contribute to survival. These results have not been reported from any earlier study. More sophisticated estimation procedures such as treatment effect models must be used to obtain conclusive results.
Data Sharing Statement
The data in this study are available from the National Hospital Organization, but the availability of these data is restricted because of privacy reasons. These data were used under license for this study and are therefore not available to the public. Data are available from the authors with permission of the National Hospital Organization.
Acknowledgments
We acknowledge Mr. Masaya Nakadera and Mr. Masato Koizumi, who prepared database and all hospitals for submitting patients’ data. This study was supported by the Ministry of Health, Labour, and Welfare [grant number 20HA1005].
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Umakanthan S, Sahu P, Ranade AV, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. 2020;96(1142):753–758. doi:10.1136/postgradmedj-2020-138234
2. Ioannidis JPA. Infection fatality rate of COVID-19 inferred from seroprevalence data. Bull World Health Organ. 2021;99(1):19–33F. doi:10.2471/BLT.20.265892
3. Saini KS, Tagliamento M, Lambertini M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43–50. doi:10.1016/j.ejca.2020.08.011
4. Zhou Y, Yang Q, Chi J, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: a systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56. doi:10.1016/j.ijid.2020.07.029
5. Venkatesulu BP, Chandrasekar VT, Girdhar P, et al. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. JNCI Cancer Spectr. 2021;5(2):pkaa102. doi:10.1093/jncics/pkaa102
6. Salunke AA, Nandy K, Pathak SK, et al. Impact of COVID-19 in cancer patients on severity of disease and fatal outcomes: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(5):1431–1437. doi:10.1016/j.dsx.2020.07.037
7. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi:10.1016/S1470-2045(20)30096-6
8. Javanmardi F, Keshavarzi A, Akbari A, Emami A, Pirbonyeh N, Serra R. Prevalence of underlying diseases in died cases of COVID-19: a systematic review and meta-analysis. PLoS One. 2020;15(10):e0241265. doi:10.1371/journal.pone.0241265
9. Del Sole F, Farcomeni A, Loffredo L, et al. Features of severe COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020;50(10):e13378. doi:10.1111/eci.13378
10. Li J, Huang DQ, Zou B, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93(3):1449–1458. doi:10.1002/jmv.26424
11. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi:10.1016/j.jinf.2020.04.021
12. Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43(6):867–869. doi:10.1007/s40618-020-01236-2
13. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi:10.1038/s41586-020-2521-4
14. Killerby ME, Link-Gelles R, Haight SC, et al. Characteristics associated with hospitalization among patients with COVID-19 – Metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):790–794. doi:10.15585/mmwr.mm6925e1
15. Wu T, Zuo Z, Kang S, et al. Multi-organ dysfunction in patients with COVID-19: a systematic review and meta-analysis. Aging Dis. 2020;11(4):874–894. doi:10.14336/AD.2020.0520
16. Zhang J, Wu J, Sun X, et al. Association of hypertension with the severity and fatality of SARS-CoV-2 infection: a meta-analysis. Epidemiol Infect. 2020;148:e106. doi:10.1017/S095026882000117X
17. Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin Angiotensin Aldosterone Syst. 2020;21(2):1470320320926899. doi:10.1177/1470320320926899
18. The Cabinet Secretariat. Vaccination against SARS-CoV-2. Available from: https://www.kantei.go.jp/jp/headline/kansensho/vaccine.html.
19. Umakanthan S, Lawrence S. Predictors of COVID-19 vaccine hesitancy in Germany: a cross-sectional, population-based study. Postgrad Med J. 2022;98(1164):756–764. doi:10.1136/postgradmedj-2021-141365
20. Mozaffari E, Chandak A, Zhang Z, et al. Remdesivir treatment in hospitalized patients with Coronavirus Disease 2019 (COVID-19): a comparative analysis of in-hospital all-cause mortality in a large multicenter observational cohort. Clin Infect Dis. 2022;75(1):e450–e458. doi:10.1093/cid/ciab875
21. Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385(23):e81. doi:10.1056/NEJMoa2108163
22. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi:10.1056/NEJMoa2107934
23. Horby P, Lim WS, Emberson JR, et al; for RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704.
24. Marconi VC, Ramanan AV, de Bono S, et al; for COV-BARRIER Study Group. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled Phase 3 trial. Lancet Respir Med. 2021;9(12):1407–1418. doi:10.1016/S2213-2600(21)00331-3
25. Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30. doi:10.1056/NEJMoa2030340
26. Gordon AC, Mouncey PR; REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502.
27. Umakanthan S, Senthil S, John S, et al. The effect of statins on clinical outcome among hospitalized patients with COVID-19: a multi-centric cohort study. Front Pharmacol. 2022;13:742273. doi:10.3389/fphar.2022.742273
28. Bergqvist R, Ahlqvist VH, Lundberg M, et al. HMG-CoA reductase inhibitors and COVID-19 mortality in Stockholm, Sweden: a registry-based cohort study. PLoS Med. 2021;18(10):e1003820. doi:10.1371/journal.pmed.1003820
29. Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400(10359):1213–1222. doi:10.1016/S0140-6736(22)01586-0
30. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi:10.1056/NEJMoa2116044
31. Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–1408. doi:10.1056/NEJMoa2118542
32. Ministry of Health, Labour and Welfare. 医療施設調査 [Survey of medical institutions]. Japanese. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/iryosd/20a/.
33. Kanazawa N, Tani T, Imai S, Horiguchi H, Fushimi K, Inoue N. Existing data sources for clinical epidemiology: database of the National Hospital Organization in Japan. Clin Epidemiol. 2022;14:689–698. doi:10.2147/CLEP.S359072
34. Ivashchenko AA, Dmitriev KA, Vostokova NV, et al. AVIFAVIR for treatment of patients with moderate Coronavirus Disease 2019 (COVID-19): interim results of a Phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2021;73(3):531–534. doi:10.1093/cid/ciaa1176
35. Fujii S, Ibe Y, Ishigo T, et al. Early favipiravir treatment was associated with early defervescence in non-severe COVID-19 patients. J Infect Chemother. 2021;27(7):1051–1057. doi:10.1016/j.jiac.2021.04.013
36. Capochiani E, Frediani B, Iervasi G, et al. Ruxolitinib rapidly reduces acute respiratory distress syndrome in COVID-19 disease. Analysis of data collection from RESPIRE protocol. Front Med. 2020;7:466. doi:10.3389/fmed.2020.00466
37. Cao B, Wang Y, Wen D, et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi:10.1056/NEJMoa2001282
38. Quinn TM, Gaughan EE, Bruce A, et al. Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: phase 1b/2a experimental study to investigate safety, pharmacokinetics and pharmacodynamics. EBioMedicine. 2022;76:103856. doi:10.1016/j.ebiom.2022.103856
39. Gunst JD, Staerke NB, Pahus MH, et al. Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial. EClinicalMedicine. 2021;35:100849. doi:10.1016/j.eclinm.2021.100849
40. Duvignaud A, Lhomme E, Onaisi R, et al. Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE). Clin Microbiol Infect. 2022;28(7):1010–1016. doi:10.1016/j.cmi.2022.02.031
41. Terada-Hirashima J, Suzuki M, Tsujimoto Y, et al. Impact of inhaled ciclesonide on asymptomatic or mild COVID-19: a randomized trial. Drug Discov Ther. 2022;16(5):225–232. doi:10.5582/ddt.2022.01068
42. López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426–1435. doi:10.1001/jama.2021.3071
43. Abadie A, Imbens GW. Large sample properties of matching estimators for average treatment effects. Econometrica. 2006;74:235–267. doi:10.1111/j.1468-0262.2006.00655.x
44. Abadie A, Imbens GW. On the failure of the bootstrap for matching estimators. Econometrica. 2008;76:1537–1557.
45. Abadie A, Imbens GW. Bias-corrected matching estimators for average treatment effects. J Bus Econ Stat. 2011;29:1–11. doi:10.1198/jbes.2009.07333
46. Cohen JB, Hanff TC, William P, et al. Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9(3):275–284. doi:10.1016/S2213-2600(20)30558-0
47. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382(25):2441–2448. doi:10.1056/NEJMoa2008975
48. Alhaddad MJ, Almulaify MS, Alshabib AA, et al. Relation between renin–angiotensin–aldosterone system inhibitors and COVID-19 severity. Cureus. 2022;14(3):e22903. doi:10.7759/cureus.22903
49. Kurdi A, Mueller T, Weir N. An umbrella review and meta-analysis of renin–angiotensin system drugs use and COVID-19 outcomes. Eur J Clin Invest. 2023;53(2):e13888. doi:10.1111/eci.13888
50. Guo X, Zhu Y, Hong Y. Decreased mortality of COVID-19 with renin–angiotensin–aldosterone system inhibitors therapy in patients with hypertension: a meta-analysis. Hypertension. 2020;76(2):e13–e14. doi:10.1161/HYPERTENSIONAHA.120.15572
51. Peng M, He J, Xue Y, Yang X, Liu S, Gong Z. Role of hypertension on the severity of COVID-19: a review. J Cardiovasc Pharmacol. 2021;78(5):e648–e655. doi:10.1097/FJC.0000000000001116
52. Chen R, Yang J, Gao X, et al. Influence of blood pressure control and application of renin–angiotensin–aldosterone system inhibitors on the outcomes in COVID-19 patients with hypertension. J Clin Hypertens. 2020;22(11):1974–1983. doi:10.1111/jch.14038
53. Sharafeldin N, Bates B, Song Q, et al. Outcomes of COVID-19 in patients with cancer: report from the national COVID cohort collaborative (N3C). J Clin Oncol. 2021;39(20):2232–2246. doi:10.1200/JCO.21.01074
54. Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385(4):320–329. doi:10.1056/NEJMoa2107058
55. Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–2159. doi:10.1016/j.vaccine.2014.01.081
56. Umakanthan S, Bukelo MM, Bukelo MJ, Patil S, Subramaniam N, Sharma R. Social environmental predictors of COVID-19 vaccine hesitancy in India: a population-based survey. Vaccines. 2022;10(10):1749. doi:10.3390/vaccines10101749
57. Kurita J, Sugawara T, Ohkusa Y. Pathogenicity of the omicron variant strain comparison with delta variant strain and seasonal influenza in Japan. Available from: https://jxiv.jst.go.jp/index.php/jxiv/preprint/view/59.
58. Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492–499. doi:10.1093/aje/kwm324
59. Williamson T, Ravani P. Marginal structural models in clinical research: when and how to use them? Nephrol Dial Transplant. 2017;32(suppl_2):ii84–ii90. doi:10.1093/ndt/gfw341
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.