Back to Journals » Journal of Inflammation Research » Volume 16
Risk Genetic Variants (IL-10) for Osteoporosis in Han Population from Northwest China
Authors Rong K, Lang Y, Zhou Y, Ni L, Wang L, Wang L, Zhang Y, Wen F, Wang Z, Chen P
Received 15 November 2022
Accepted for publication 4 February 2023
Published 14 March 2023 Volume 2023:16 Pages 1091—1102
DOI https://doi.org/10.2147/JIR.S396914
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Kai Rong, Yi Lang, Yubo Zhou, Liangtao Ni, Lei Wang, Long Wang, Yaowu Zhang, Fengli Wen, Zhan Wang, Pingbo Chen
Department of Traumatology, Traditional Chinese Medicine Hospital Affiliated to Xinjiang Medical University, Wulumuqi, Xinjiang, People’s Republic of China
Correspondence: Pingbo Chen, Department of Traumatology, Traditional Chinese Medicine Hospital Affiliated to Xinjiang Medical University, No. 116, Huanghe Street, Wulumuqi, Xinjiang, People’s Republic of China, Tel/Fax +86-13899907800, Email [email protected]
Background: Osteoporosis (OP) is a common metabolic bone disease characterized by loss of bone mass. IL-10 is considered to be a powerful immune and inflammatory suppressor. This study aimed to assess association between genetic loci in IL-10 and susceptibility to OP.
Methods: Association analysis between IL-10 genetic loci and OP risk through SNPStats online software. FPRP analysis (false-positive report probability) verified whether the positive results were noteworthy findings. Linkage disequilibrium (LD) and haplotype analysis were completed by Haploview 4.2 and SNPStats. Multi-factor dimensionality reduction (MDR) was used to assess interaction of SNP–SNP in susceptibility to OP.
Results: Allele “G” of IL-10-rs1554286 (OR = 1.21, p = 0.013), allele “C” of IL-10-rs1518111 (OR = 1.22, p = 0.011), allele “C” of IL-10-rs3024490 (OR = 1.20, p = 0.018), and allele “G” of IL-10-rs1800871 (OR = 1.21, p = 0.015) were risk factors for OP. In females, smoking, drinking, or aging ≤ 60 years old participants, the above genetic loci are also significantly associated with the increased risk of OP. FPRP analysis showed that all positive results are noteworthy findings. There are significant differences in serum levels of uric acid, mean hemoglobin concentration, or mean hemoglobin among different genotypes of IL-10 gene loci. MDR showed that four loci model composed rs1554286, rs1518111, rs3021094, and rs1800871 is the best model for predicting OP risk.
Conclusion: IL-10-rs1554286, -rs1518111, -rs3021094, and -rs1800871 are risk factors for susceptibility to OP.
Keywords: osteoporosis, IL-10, genetic loci, Han population from northwest China
Introduction
Osteoporosis is a common bone metabolic disease, which is characterized by reduced bone mass and destruction of bone microstructure.1 Patients with osteoporosis have fewer bone matrix, lower bone mineral density, lower bone strength, and increased brittleness, thereby increasing the risk of fracture. OP can be divided into POP (primary osteoporosis) and SOP (secondary osteoporosis). The cause of POP is, generally, growth of age, and the secondary causes of OP include smoking, excessive drinking, type I diabetes, hyperthyroidism, etc.2,3 The study found that the quality of life of patients with OP decreased significantly.4 Patients with osteoporotic fractures often suffer from limited activity and physical pain. In particular, with osteoporotic hip fractures, in severe cases disability or death may even occur.4,5 The social burden caused by osteoporotic fractures is increasing globally.6–8 Therefore, early identification of osteoporosis and thus preventing the occurrence of osteoporotic fractures is an urgent need.
Johnston et al emphasized in their report that 20% of osteoporosis is affected by environmental factors, while 80% depends on genetic factors.9 A twin study showed that most of the differences in bone mineral density (BMD) are genetically determined,10 and osteoporosis is mainly diagnosed using BMD.11 With the development of the human genome project, more and more genes related to the occurrence and development of osteoporosis have been identified.12 Nevertheless, the genetic factors that can elucidate osteoporosis have not been fully clear.13 Therefore, it is necessary to identify the susceptibility genes associated with the occurrence and development of osteoporosis in a specific population, which is of great significance for the early prevention of osteoporosis.
A number of studies have shown that the IL-10 gene family (IL-10, IL-20, IL-22, IL-26, etc.) play important roles in bone and joint diseases including osteoporosis.14–16 Recent studies have reported that IL-10 is independently associated with disease activity in rheumatoid arthritis patients accompanied by osteoporosis.17 In addition, several studies have found evidence that IL-10 genetic polymorphisms are associated with OP risk (Taiwan population,18 Korean postmenopausal women,19 etc.), which have provided new ideas for early prevention of osteoporosis in specific populations. However, whether there is a correlation between OP susceptibility and IL-10 genetic polymorphism in Chinese Han population has not been reported in detail and needs to be supplemented.
The study will use “case-control” study design to explore the association between IL-10 genetic loci and susceptibility to OP in Han population from northwest China. This study will help to further understand the pathogenesis of OP in Han population from northwest China at the genetic level and provide new ideas and theoretical basis for the early prevention and treatment of osteoporosis.
Materials and Methods
Subject Information
All participants (786 OP patients + 719 controls) were recruited from Traditional Chinese Medicine Hospital Affiliated to Xinjiang Medical University. All participants were Han population from northwest China without any genetic relationship. The bone mineral density (BMD) at the lumbar spine (L1-L4) and hip joint were measured using dual-energy X-ray bone densitometry, and a T-score will be obtained. We recruited patients with osteoporosis according to the WHO criteria for osteoporosis diagnosis (T-score ≤-2.5). Patients with a history of osteoarthritis, hip fracture, other bone metabolism-related diseases, or kidney/liver disease will be excluded from the case group. In addition, patients with a history of taking anti-osteoporosis drugs and hyperthyroidism or hypothyroidism will be excluded. During the same period, we recruited healthy individuals at the health examination center of the same hospital according to the following inclusion criteria:1 the control group and case group were matched in age and sex;2 No history of hip fracture;3 No diseases or metabolic disorders related to bone metabolism disorders. Demographic information and environmental exposure factors of all participants were obtained by reviewing medical records or face-to-face questionnaire survey.
Participants were informed of the purpose and content of this study through oral notification or informed consent. After obtaining their informed consent, we collected 10mL serum for subsequent DNA extraction. Our study has been approved by the ethics committee of the Traditional Chinese Medicine Hospital Affiliated to Xinjiang Medical University before the beginning.
Methods for Testing Laboratory Indicators
Overall, 3–5mL fasting venous blood was extracted from all participants in the morning and injected into a vacuum blood collection tube containing anticoagulant EDTA-K2 5.4mg and aprotinin. Evenly shake the blood sample and send it to the laboratory in time and complete the test of relevant blood indexes within 2 hr. Blood indicators were measured with BeckmanDHX automatic blood analyzer (uric acid, platelet count, platelet distribution width (PDW), mean platelet volume (MPV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and red blood cell count, etc.). In addition, 3–5 mL of venous blood was pumped into an anticoagulant tube containing heparin. After being evenly shaken, uric acid was detected by automatic sample examination system (HITACHI, Japan). Test of blood samples is strictly carried out by professional medical test technicians in accordance with the operating instructions and quality control.
Selection of SNPs
First, we used e!GRCh37online searching tool (http://asia.ensembl.org/Homo_sapiens/Info/Index) and found that the physical position of the IL-10 was on the Chromosome 1: 206,767,602–206,774,541. After downloading genetic variants (IL-10) file from e!Ensemble online software (https://asia.ensembl.org/Homo_sapiens/Gene/Variation_Gene/Table?db=core;g=ENSG00000136634;r=1:206767602-206774541), we found that there are a total of 9773 genetic variants in IL-10. We also used the online converter window of e!GRCh37 (VCF to PED: http://grch37.ensembl.org/Homo_sapiens/Tools/VcftoPed) to download the related files of IL-10 genetic variants after choosing CHB and CHS population. In order to narrow the scope of the study, we set specific conditions on the Haploview software (Tagger r2>0.8, Min Genotype>75%, MAF>0.05, and HWE>0.01) to screen IL-10 genetic variants of the downloaded files. Finally, five candidate genetic loci in IL-10 were randomly selected for subsequent study (rs1554286, rs1518111, rs3021094, rs3024490, and rs1800871).
Genotyping
After the extraction and purification of genomic DNA, specific amplification and extension primers were designed over MassARRAY Assay Design software (Supplemental Table 1). Genotyping was performed by the MassARRAY ® -IPLEX SNP genotyping technology.
We randomly selected 5% DNA samples for repeated experiments, which will improve the reliability and repeatability of the experimental results.
Data Analysis
In our study, continuous variables including age, bone mineral density (BMD) T score, and clinical indicators were represented by “mean ± SD.” Sex, BMI, smoking/drinking status, and other categorical variables were expressed in terms of frequency. All statistical analyses were performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). The prediction of the potential function of candidate SNPs was constructed by HaploReg v4.1 (https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php). In this study, the associations between susceptibility to OP and candidate genetic loci were assessed using SNPStats online software (https://www.snpstats.net/start.htm?q=snpstats/start.htm). We investigate the impact of candidate genetic loci on OP risk through odds ratios (OR) and 95% confidence intervals (CI). All results were adjusted by the confounding factors (such as age, gender, smoking, or drinking) to avoid influences of confounding factors. False-positive report probability (FPRP) analysis detected whether positive result is noteworthy at a prior probability level of 0.25 and an FPRP threshold of 0.2.20 Haploview 4.2 software and SNPStats online software were used to perform haplotype analysis of candidate SNPs and evaluation of linkage disequilibrium (LD). Finally, the associations between interaction of SNP–SNP and OP risk were evaluated by multi-factor dimensionality reduction (MDR). In all statistical analyses of this study, p<0.05 indicated that it was statistically significant.
Results
Subject Information
The basic information of 786 patients with OP and 719 healthy controls included in this study can be seen in Table 1. The mean age of case group and control group was 57.68 ± 11.65 and 57.48 ± 11.52 years, respectively. The results showed that the difference in age, gender, and smoking/drinking status between case and control groups is not significant, which indicated that the two groups of subjects were matched in the variables mentioned above. In addition, there were statistical differences in clinical indicators between the two groups, including uric acid, platelet count, platelet distribution width (PDW), mean platelet volume (MPV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and red blood cell distribution width (RDW-CV).
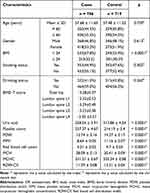 |
Table 1 Characteristics of Patients with OP and Healthy Individuals |
Genotyping and Information About Candidate SNPs
The genotyping of five IL-10 genetic polymorphisms (rs1554286 G/A, rs1518111 C/T, rs3021094 G/T, rs3024490 C/A, rs1800871 G/A) have been successfully completed. The candidate IL-10 genetic polymorphisms are all intronic variants. Table 2 shows that all candidate genetic polymorphisms we randomly selected are in accordance with Hardy-Weinberg equilibrium (HWE p > 5%). The HaploReg online software predicted that five candidate SNPs may be regulated by a variety of factors (Table 2).
 |
Table 2 The Basic Information and HWE About the Candidate SNPs of IL-10 |
Association Between IL-10 Polymorphisms and Susceptibility to OP (Overall Analysis)
Table 3 shows that four IL-10 genetic polymorphisms were identified to be significantly associated with susceptibility to OP under multiple genetic models (rs1554286, rs1518111, rs3024490, rs1800871). Specifically, allele “G” or genotype “GG” of IL-10-rs1554286 is significantly associated with increasing OP risk (G: OR (95% CI) = 1.21 (1.04–1.41), p = 0.013; GG: OR (95% CI) = 1.52 (1.08–2.14), p = 0.016). And IL-10-rs1554286 is associated with susceptibility to OP under dominant (p = 0.037), recessive (p = 0.042) and log-additive (p = 0.012) genetic models. The allele “C” or genotype “CC” of IL-10-rs1518111 is significantly associated with increasing OP risk (C: OR (95% CI) = 1.22 (1.05–1.42), p = 0.011; CC: OR (95% CI) = 1.57 (1.11–2.21), p = 0.010). Associations between IL-10-rs1518111 and susceptibility to OP can be observed in dominant (p = 0.040), recessive (p = 0.025), and log-additive (p = 0.010) genetic models. The allele “C” or genotype “CC” of IL-10-rs3024490 is significantly associated with increasing OP risk (C: OR (95% CI) = 1.20 (1.03–1.40), p = 0.018; CC: OR (95% CI) = 1.50 (1.06–2.12), p = 0.022). Associations between IL-10-rs3024490 and susceptibility to OP can also be observed in dominant (p = 0.046) and log-additive (p = 0.016) genetic models. The allele “G” or genotype “GG” of IL-10-rs1800871 is significantly associated with increasing OP risk (G: OR (95% CI) = 1.21 (1.04–1.41), p = 0.015; GG: OR (95% CI) = 1.54 (1.09–2.17), p = 0.015). And IL-10-rs3024490 is associated with susceptibility to OP under dominant (p = 0.047), recessive (p = 0.034) and log-additive (p = 0.013) genetic models.
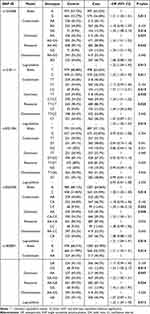 |
Table 3 Genetic Loci in IL10 Associated with Susceptibility to OP |
Additionally, we found no evidence that IL-10-rs3021094 was associated with susceptibility to OP.
Stratified Analysis of Association Between IL-10 Gene Polymorphisms and Susceptibility to OP
We have made stratified analysis to determine whether the association between candidate genetic polymorphisms and OP risk is dependent on potential risk factors for OP (age, gender, smoking, alcohol status). We have found evidence that the candidate IL-10 SNPs have associations with susceptibility to OP among female, smoking, drinking participants, or participants aging ≤60 years old. The details are as follows:
The stratified analysis showed that (Supplemental Tables 2 and 3) allele “G” of IL-10-rs1554286, allele “C” of IL-10-rs1518111, allele “C” of IL-10-rs3024490 and allele “G” of IL-10-rs1800871 are all risk genetic factors for risk of susceptibility to OP among female, smoking, drinking participants, and participants aging ≤60 years old. In addition, IL-10-rs1554286, -rs1518111, -rs3024490, and -rs1800871 were also significantly associated with an increased risk of OP in above-mentioned subgroups under multiple genetic models.
No association between IL-10-rs3021094 and susceptibility to OP has been found in stratified analysis. We have divided participants according to BMI value to explore the association between candidate genetic loci and susceptibility to OP but did not find any positive results (Supplemental Table 4).
FPRP Analysis for Positive Results
Statistical powers for the positive results range from 91.7% to 100.0% in overall analysis. Although some statistical powers in stratified analysis are less than 85%, prior probability of all positive results is less than 0.2 at the prior probability level of 0.25 and FPRP threshold of 0.2 (Supplemental Table 5). Taken together, all associations between genetic loci and susceptibility to OP found in this study are noteworthy.
Association Analysis Between Genetic Polymorphism and Clinical Characteristics
The evaluation of differences in clinical characteristics of OP patients under different genotypes of candidate genetic polymorphism has been completed (Table 4 and Supplemental Table 6). Under genotype “GG” of rs1554286, the levels of uric acid (p = 0.025) and mean corpuscular hemoglobin concentration (p = 0.024) are significantly lower than levels under other genotypes. Similarly, the “CC” genotype of rs1518111 has the lowest levels of uric acid (p = 0.043) and mean corpuscular hemoglobin concentration (p = 0.018) compared with other genotypes. The levels of uric acid (p = 0.045), mean corpuscular hemoglobin concentration (p = 0.022), and mean corpuscular hemoglobin (p = 0.036) under different genotypes of rs3024490 Hemoglobin are significantly different, and these characteristic levels are lowest under the “CC” genotype of rs3024490. Similarly, the “GG” genotype of rs1800871 has the lowest levels of uric acid (p = 0.043), mean corpuscular hemoglobin (p = 0.034), and mean corpuscular hemoglobin concentration (p = 0.019) compared with other genotypes.
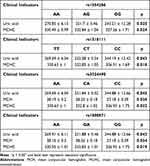 |
Table 4 Difference Analysis of Clinical Indicators of Patients Under Different Genotypes of Candidate Genetic Polymorphism |
SNP-SNP Interaction and OP Risk
As is shown in Supplemental Figure 1, the dendrogram describes the interaction between the four candidate genetic loci. The color of the lines in the dendrogram represents the level of redundancy or synergy. As is shown in (Supplemental Figure 1A), the closer the color of lines red the stronger the synergy between genetic loci, the closer the color of lines to blue the more redundant they are. It follows that the interaction between the five candidate genetic loci is redundant. In addition, information gain (IG) was used to evaluate attribute interactions. As shown in (Supplemental Figure 1B), the IG value of rs1518111 was the highest. MDR analysis showed (Table 5) that the five loci and four loci model all have the highest test accuracy (0.517) and perfect CVC (10/10). However, due to the small sample size in this study, the four-site model consisting of rs1554286, rs1518111, rs3021094, and rs1800871 can be considered as the best model to predict the risk of osteoporosis.
 |
Table 5 IL-10 SNP–SNP Interaction Models Analyzed by MDR Method |
Haplotype Analysis
The result of linkage disequilibrium showed that (Figure 1) the five candidate genetic loci in IL-10 (rs1554286, rs1518111, rs3021094, rs3024490, and rs1800871) composed one LD block. However, the results of haplotype analysis showed that there is no haplotype associated with the susceptibility to OP (Supplemental Table 7).
Discussion
This study has investigated the association between IL-10 loci and OP susceptibility in Han population from northwest China. At the same time, the association between candidate genetic loci and potential risk factors of OP was evaluated through hierarchical analysis. We have found strong evidence that four candidate IL-10 genetic polymorphisms (rs1554286, rs1518111, rs3024490, and rs1800871) were associated with OP susceptibility: allele “G” of IL-10-rs1554286, allele “C” of IL-10-rs1518111, allele “C” of IL-10-rs3024490, and allele “G” of IL-10-rs1800871 are all significantly associated with increased risk for OP. In addition, the above genetic loci are also significantly associated with the increased risk of OP in stratified analysis (female, smoking, drinking, or aging ≤60 years old participants). FPRP analysis showed that all positive results are noteworthy findings. More importantly, FPRP analysis showed that all positive results found in this study are noteworthy findings.
IL-10 is considered to be a potent immune and inflammatory suppressor and plays an important role in the pathogenesis of inflammation, autoimmune diseases, and immune escape of tumor cell antigens. This determines that IL-10 has important and broad clinical application prospects in autoimmune and gene therapy of inflammatory diseases. In recent years, a correlation between IL-10 genetic polymorphism and various disease risks has been reported, including tuberculosis,21 periodontitis,22 and bacterial sepsis.23 In addition, the correlation between IL-10 genetic polymorphism and bone mineral density and OP risk has also been reported in many populations, including South Korea,19 Turkey,16 Taiwan,18 and Chinese postmenopausal women.24 Studies have reported the association of IL-10-rs1554286, -rs1518111, -rs3024490, and -rs1800871 with susceptibility to Behcet‘s disease,25 tuberculosis,26 systemic lupus erythematosus27 or breast cancer.28 However, no association analysis between the above genetic polymorphisms and susceptibility to OP of any population has been reported. We have found strong evidence that the above four IL-10 genetic polymorphisms are associated with increased OP risk for the first time.
Recent animal experiments and related clinical studies have confirmed that IL-10 can be used as an important anti-inflammatory medium to inhibit bone resorption and reduce bone loss.29,30 Güret al found that the IL-10 level in blood of postmenopausal women with osteoporosis was significantly lower than that of postmenopausal normal women.31 The above studies suggest that the IL-10 level in patients with osteoporosis is lower than that in the normal population. Studies have found that IL-10 gene polymorphism affects the level of IL-10 and evidence related to BMD reduction and osteoporosis risk.24 Combined with previous studies and the results of this study, we speculate that the presence of allele “G” of IL-10-rs1554286, allele “C” of IL-10-rs1518111, allele “C” of IL-10-rs3024490, or allele “G” of IL-10-rs1800871 will reduce the level of IL-10, thereby increasing the risk of OP. However, the above is only a speculation, and further functional verification experiments are necessary, which will further confirm the results of this study.
In addition, our results showed that the levels of UC, MCH, or MCHC in the population carrying homozygous or heterozygous mutant genotypes of IL-10-rs1554286 (AG/GG), -rs1518111 (CT/CC), -rs3024490 (CA/CC), and -rs1800871 (GA/GG) were significantly lower than those in the population carrying wild genotypes. Many studies have confirmed that serum uric acid and bone mineral density are positively correlated.32–34 Uric acid protects bone metabolism.33 Low levels of MCH or MCHC suggest anemia,35,36 and anemia patients should be alert to osteoporosis.37,38 It can be seen that people with low levels of UC MCH or MCHC are more likely to develop osteoporosis. Combined with the results of this study, we speculated that homozygous or heterozygous mutations of IL-10-rs1554286, -rs1518111, -rs3024490, and -rs1800871 may affect the level of UC, MCH, or MCHC and thus affect the susceptibility of osteoporosis. However, the above speculation requires functional verification experiments to further verify it.
In any case, this study is the first to explore the association between IL-10 genetic polymorphisms and susceptibility to OP in Han population from Northwest China, and found noteworthy results. However, the shortcomings of this study cannot be ignored: it is necessary to expand the sample size in future studies and carry out validation tests in different regions or populations with different genetic backgrounds. In addition, we believe it is of great interest to conduct necessary functional studies to support the speculations based on results obtained from this study. The above study will further explore the molecular mechanism of IL-10 in the occurrence and development of OP and provide a more reliable theoretical basis for the treatment of osteoporosis at the gene level.
Conclusion
In summary, this study showed that IL-10-rs1554286, -rs1518111, -rs3024490, and -rs1800871 are associated with increased risk of OP. This study found for the first time that IL-10 genetic loci are significantly associated with susceptibility to OP among Han population from Northwest China. Our study has laid a reliable theoretical foundation for the study of the mechanism of IL-10 in the occurrence and development of osteoporosis.
Data Sharing Statement
The data sets for this study were uploaded and deposited on Zenodo, DOI (10.5281/zenodo.6951352).
Ethics Approval and Consent to Participate
This study was conducted under the standard approved by Experimental Animal Ethics Committee of Xinjiang Medical University (IACUC-20211104-31). And conformed to the ethical principles for medical research involving humans of the World Medical Association Declaration of Helsinki. All participants signed informed consent forms before participating in this study.
Author Contributions
Author Pingbo Chen and author Kai Rong have given substantial contributions to the conception or the design of the manuscript, author Yi Lang, author Yubo Zhou, and author Liangtao Ni to acquisition of the data, author Lei Wang, author Long Wang, and author Yaowu Zhang analysis of the data, author Fengli Wen, and author Zhan Wang to interpretation of the data. Author Kai Rong has participated in drafting the manuscript, and author Pingbo Chen has revised it critically. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2022D01C555).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Ebeling PR, Nguyen HH, Aleksova J, Vincent AJ, Wong P, Milat F. Secondary Osteoporosis. Endocr Rev. 2022;43(2):240–313. doi:10.1210/endrev/bnab028
2. Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22(9):2395–2411. doi:10.1007/s00198-011-1713-z
3. Sobh MM, Abdalbary M, Elnagar S, et al. Secondary osteoporosis and metabolic bone diseases. J Clin Med. 2022;11:9. doi:10.3390/jcm11092382
4. Yong EL, Logan S. Menopausal osteoporosis: screening, prevention and treatment. Singapore Med J. 2021;62(4):159–166. doi:10.11622/smedj.2021036
5. Collaborators GDaIIaP. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1789–1858. doi:10.1016/S0140-6736(18)32279-7
6. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475. doi:10.1359/jbmr.061113
7. Cooper C, Campion G, Melton LJ. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2(6):285–289. doi:10.1007/BF01623184
8. Si L, Winzenberg TM, Jiang Q, Chen M, Palmer AJ. Projection of osteoporosis-related fractures and costs in China: 2010–2050. Osteoporos Int. 2015;26(7):1929–1937. doi:10.1007/s00198-015-3093-2
9. Johnston CC
10. Hopper JL, Green RM, Nowson CA, et al. Genetic, common environment, and individual specific components of variance for bone mineral density in 10- to 26-year-old females: a twin study. Am J Epidemiol. 1998;147(1):17–29. doi:10.1093/oxfordjournals.aje.a009361
11. Morris JA, Kemp JP, Youlten SE, et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet. 2019;51(2):258–266. doi:10.1038/s41588-018-0302-x
12. Zhu X, Bai W, Zheng H. Twelve years of GWAS discoveries for osteoporosis and related traits: advances, challenges and applications. Bone Res. 2021;9(1):23. doi:10.1038/s41413-021-00143-3
13. Teerlink CC, Jurynec MJ, Hernandez R, et al. A role for the MEGF6 gene in predisposition to osteoporosis. Ann Hum Genet. 2021;85(2):58–72. doi:10.1111/ahg.12408
14. Ni S, Shan F, Geng J. Interleukin-10 family members: biology and role in the bone and joint diseases. Int Immunopharmacol. 2022;108:108881. doi:10.1016/j.intimp.2022.108881
15. Kany S, Vollrath JT, Relja B. Cytokines in Inflammatory Disease. Int J Mol Sci. 2019;20:23. doi:10.3390/ijms20236008
16. Tural S, Alayli G, Kara N, Tander B, Bilgici A, Kuru O. Association between osteoporosis and polymorphisms of the IL-10 and TGF-beta genes in Turkish postmenopausal women. Hum Immunol. 2013;74(9):1179–1183. doi:10.1016/j.humimm.2013.03.005
17. Qiu J, Lu C, Zhang L, Zhou X, Zou H. Osteoporosis in patients with rheumatoid arthritis is associated with serum immune regulatory cellular factors. Clin Rheumatol. 2022;41(9):2685–2693. doi:10.1007/s10067-022-06212-0
18. Tu MY, Han KY, Lan YW, et al. Association of TGF-β1 and IL-10 gene polymorphisms with osteoporosis in a study of Taiwanese osteoporotic patients. Genes. 2021;12:6. doi:10.3390/genes12060930
19. Park BL, Han IK, Lee HS, et al. Association of interleukin 10 haplotype with low bone mineral density in Korean postmenopausal women. J Biochem Mol Biol. 2004;37(6):691–699. doi:10.5483/bmbrep.2004.37.6.691
20. He J, Zou Y, Liu X, et al. Association of common genetic variants in pre-microRNAs and neuroblastoma susceptibility: a two-center study in Chinese children. Mol Ther Nucleic Acids. 2018;11:1–8. doi:10.1016/j.omtn.2018.01.003
21. Shah JA, Warr AJ, Graustein AD, et al. REL and BHLHE40 variants are associated with IL-12 and IL-10 responses and tuberculosis risk. J Immun. 2022;208(6):1352–1361. doi:10.4049/jimmunol.2100671
22. Stolf CS, Sacramento CM, Paz HES, et al. IL10 promoter rs6667202 polymorphism is functional in health but not in grade c periodontitis patients: a pilot study. J Periodontal Res. 2022;57(1):85–93. doi:10.1111/jre.12940
23. Vivas MC, Villamarín-Guerrero HF, Sanchez CA. Interleukin-10 (IL-10) 1082 promoter polymorphisms and plasma IL-10 levels in patients with bacterial sepsis. Rom J Intern Med. 2021;59(1):50–57. doi:10.2478/rjim-2020-0033
24. Chen HY, Chen WC, Hsu CM, Tsai FJ, Tsai CH. Tumor necrosis factor alpha, CYP 17, urokinase, and interleukin 10 gene polymorphisms in postmenopausal women: correlation to bone mineral density and susceptibility to osteoporosis. Eur J Obstet Gynecol Reprod Biol. 2005;122(1):73–78. doi:10.1016/j.ejogrb.2005.02.003
25. Shahriyari E, Vahedi L, Roshanipour N, Jafarabadi MA, Khamaneh A, Laleh MG. Exploring the association of IL-10 polymorphisms in Behcet’s disease: a systematic review and meta-analysis. J Inflamm. 2019;16:26. doi:10.1186/s12950-019-0230-2
26. He S, Yang S, Zhao Q, et al. Association of IL4, IL6, and IL10 polymorphisms with pulmonary tuberculosis in a Tibetan Chinese population. Oncotarget. 2018;9(23):16418–16426. doi:10.18632/oncotarget.23995
27. Lv TT, Wu J, Li J, et al. Association of interleukin-10 gene single nucleotide polymorphisms with susceptibility to systemic lupus erythematosus in a Chinese population. Gene. 2018;642:549–554. doi:10.1016/j.gene.2017.11.072
28. Moghimi M, Ahrar H, Karimi-Zarchi M, et al. Association of IL-10 rs1800871 and rs1800872 polymorphisms with breast cancer risk: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2018;19(12):3353–3359. doi:10.31557/APJCP.2018.19.12.3353
29. Al-Rasheed A, Scheerens H, Rennick DM, Fletcher HM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10. J Dent Res. 2003;82(8):632–635. doi:10.1177/154405910308200812
30. Cohen SL, Moore AM, Ward WE. Interleukin-10 knockout mouse: a model for studying bone metabolism during intestinal inflammation. Inflamm Bowel Dis. 2004;10(5):557–563. doi:10.1097/00054725-200409000-00009
31. Gür A, Denli A, Nas K, et al. Possible pathogenetic role of new cytokines in postmenopausal osteoporosis and changes during calcitonin plus calcium therapy. Rheumatol Int. 2002;22(5):194–198. doi:10.1007/s00296-002-0223-x
32. Yao X, Chen L, Xu H, Zhu Z. The association between serum uric acid and bone mineral density in older adults. Int J Endocrinol. 2020;2020:3082318. doi:10.1155/2020/3082318
33. Yan DD, Wang J, Hou XH, et al. Association of serum uric acid levels with osteoporosis and bone turnover markers in a Chinese population. Acta Pharmacol Sin. 2018;39(4):626–632. doi:10.1038/aps.2017.165
34. Lee HN, Kim A, Kim Y, Kim GT, Sohn DH, Lee SG. Higher serum uric acid levels are associated with reduced risk of Hip osteoporosis in postmenopausal women with rheumatoid arthritis. Medicine. 2020;99(24):e20633. doi:10.1097/MD.0000000000020633
35. Rattanawan C, Cheloh S, Maimahad A, Tayeh M. Prevalence and associated factors of Anemia among Muslim students, Nakhon Si Thammarat, Thailand: a cross-sectional study. Inquiry. 2021;58:469580211013476. doi:10.1177/00469580211013476
36. Hamali HA, Mobarki AA, Saboor M, et al. Prevalence of Anemia Among Jazan University Students. Int J Gen Med. 2020;13:765–770. doi:10.2147/IJGM.S275702
37. Cui R, Zhao Z, Fei Z, Li Y, Gao W. Anemia is related to osteoporosis in Chinese type 2 diabetic patients. Arch Osteoporos. 2021;16(1):161. doi:10.1007/s11657-021-01030-4
38. Korkmaz U, Korkmaz N, Yazici S, et al. Anemia as a risk factor for low bone mineral density in postmenopausal Turkish women. Eur J Intern Med. 2012;23(2):154–158. doi:10.1016/j.ejim.2011.11.009
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.