Back to Journals » International Journal of General Medicine » Volume 15
Risk Factors of Pregnancy Failure in Infertile Patients Undergoing Assisted Reproductive Technology
Authors Yi H , Yang M, Tang H , Lin M
Received 1 November 2022
Accepted for publication 21 December 2022
Published 30 December 2022 Volume 2022:15 Pages 8807—8817
DOI https://doi.org/10.2147/IJGM.S394236
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Honggan Yi,1,2 Man Yang,1,2 Haiyu Tang,1,2 Mei Lin1,2
1Reproductive Medicine Center, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Precision Medicine and Clinical Translational Research of Hakka Population, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China
Correspondence: Honggan Yi, Reproductive Medicine Center, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, No. 63 Huangtang Road, Meijiang District, Meizhou, 514031, People’s Republic of China, Tel +86 753-2131-883, Email [email protected]
Background: Infertile couples need to use assisted reproductive technology (ART) to give birth. However, pregnancy failure after ART is not uncommon. At present, the results of studies on the causes of pregnancy failure after ART are inconsistent.
Methods: A retrospective cohort study involving 715 embryo transfer cycles was conducted at the Reproductive Medicine Center of Meizhou People’s Hospital, from December 2015 to June 2022. According to the pregnancy, they were divided into clinical pregnancy group and pregnancy failure group. The relationship between demographic characteristics and pregnancy status between the two groups was analyzed.
Results: The pregnancy failure rate after ART was 49.7% (355/715). There were statistically significant distribution differences of maternal age, paternal age, COH protocols, and number of embryos transferred between clinical pregnancy and pregnancy failure groups (all P< 0.01). Multiple logistic regression analysis shows that high maternal age (> 35 years old vs ≤ 35 years old: OR 2.173, 95% CI: 1.386– 3.407, P=0.001), and GnRH-a short protocol (GnRH-a short protocol vs GnRH-a long protocol: OR 2.139, 95% CI: 1.127– 4.058, P=0.020) may increase risk of pregnancy failure in ART pregnancies, while two embryos transferred (two embryos transferred vs one embryo transferred: OR 0.563, 95% CI: 0.377– 0.839, P=0.005) may reduce risk of pregnancy failure. In addition, high maternal age, GnRH antagonist protocol, and GnRH-a short protocol may increase risk of implantation failure, while two embryos transferred may reduce risk of implantation failure. And high maternal age may increase risk of biochemical pregnancy.
Conclusion: The risk of pregnancy failure increased in ART cycles with maternal age > 35 years old and GnRH-a short protocol, while reduced with two embryos transferred.
Keywords: assisted reproductive technology, risk factors, pregnancy failure, infertility, miscarriage
Introduction
Infertility is medically defined as the failure to achieve pregnancy of a couple after 12 months of regular sexual intercourse without any form of contraception.1 Infertility affects more than 6 million people in the United States, equivalent to 10% of the reproductive-age population.2 In China, the prevalence of infertility among couples with childbearing age is about 25%.3 The reasons of infertility include female factors and male factors,4 and the psychological stress caused by the fertility status and the side effects of some hormones and drugs may also increase the risk of infertility.5,6 Assisted reproductive technology (ART) is one of the most effective methods for the treatment of infertility patients, and also an important means to achieve eugenic inheritance.7
In recent decades, ART has been widely used in the fertility treatment of infertility patients, especially in the prevention of some serious genetic diseases. Regardless, ART treatment does not guarantee pregnancy and live birth. Pregnancy failure is one of the most important limitations in ART treatment.8 Pregnancy failure rate is an important indicator to evaluate the success rate of ART treatment for infertility. According to the US Centers for Disease Control and Prevention, the clinical pregnancy rate of ART procedures that progressed to the embryo-transfer stage is 54.7%, but the corresponding live birth rate is only 45.0%, the pregnancy failure rate is as high as 18.5%.9 The live birth rate is about 42% in China.10 Pregnancy failure causes great psychological pressure and economic burden to infertile couples. A major problem in reproductive medicine is implantation failure, which refers to the inability to conceive after transferring high-quality embryo/embryos.11 Pregnancy loss is defined as the natural termination of the entire gestational cycle, including biochemical pregnancy, embryonic arrest, spontaneous abortion, and stillbirth.12
In order to meet the increasing demand for ART, it is very important to improve the success rate of clinical pregnancy after the implementation of ART. Therefore, the factors affecting the success rate of clinical pregnancy after ART have become the focus of research and discussion in the field of ART at home and abroad. It is of great significance to analyze the related influencing factors of pregnancy failure in ART for clinical prevention and treatment. Many studies only analyzed risk factors for pregnancy failure after fresh or frozen cycles,13–15 whereas taking pregnancy failure for the whole ART into consideration is essential for a more appropriate evaluation. Based on this, this study selected infertility patients who underwent ART in our hospital in the past 6 years as the research objects to study the related influencing factors (patients’ baseline and treatment characteristics) of pregnancy failure.
Materials and Methods
By June 2022, the Reproductive Medicine Center of Meizhou People’s Hospital (Meizhou Academy of Medical Sciences) had carried out a total of 715 embryo transfer cycles with pregnancy outcomes. In this study, cycles with a final pregnancy outcome were analyzed. The study was performed under the guidance of the Declaration of Helsinki and approved by the Ethics Committee of Meizhou People’s Hospital (Clearance No.: 2016-A-53).
Detailed information on parental characteristics and ART procedures was collected from the electronic medical records of Reproductive medicine centers. Pregnancy outcomes were obtained from the follow-up database. Pregnancy was defined as positive serum human chorionic gonadotropin (hCG) levels on day 14 after oocyte retrieval, and clinical pregnancy was defined as the presence of gestational sac on ultrasound 3–4 weeks after hCG positive. The risk factors of pregnancy failure investigated in this study were maternal age, maternal body mass index (BMI, kg/m2), paternal age, type of infertility, controlled ovarian hyperstimulation (COH) regimen, total gonadotropin (Gn) dose, ovarian hyperstimulation syndrome (OHSS), fertilization methods, number of embryos transferred, endometrial thickness, and so on.
Maternal age and paternal age were divided into four subgroups (<30 years, 30–35 years, 36–40 years, and >40 years), respectively. Maternal BMI was divided into four subgroups based on the Chinese criteria:16,17 <18.5 kg/m2, 18.5–23.9 kg/m2, 24.0–27.9 kg/m2, ≥28.0 kg/m2. Primary infertility was defined as the failure to achieve pregnancy of a couple after 12 months of regular sexual intercourse without any form of contraception when a woman has never conceived, while secondary infertility was the incapability to conceive in a couple who have had at least one successful clinical pregnancy previously. COH protocol was divided into six subgroups: GnRH-a long protocol, GnRH antagonist protocol, long term follicular protocol, GnRH-a short protocol, GnRH-a prolonged protocol, and other protocol. Three subgroups of number of embryos transferred were 1 embryo transferred, 2 embryos transferred, and 3 embryos transferred. Fertilization methods including in vitro fertilisation (IVF), intracytoplasmic sperm injection (ICSI), IVF and ICSI were performed simultaneously (IVF+ICSI).
Statistical Analyses
Demographics and clinical characteristics of pregnancies conceived through ART were calculated by χ2 test. The distributions of continuous variables were evaluated by Student’s t-test or the Mann–Whitney U-test. Logistic regression analysis was applied to assess the associations between the risk factors and pregnancy outcome. Data analysis was performed using SPSS 21.0 (IBM Inc., USA).
Results
Demographics and Clinical Characteristics of Infertility Patients Treated with ART
The mean of the maternal age was 32.45±5.12 years old, there were 225 cases (31.5%) under the age of 30, 287 cases (40.1%) between the ages of 30 and 35, 157 cases (22.0%) between the ages of 36 and 40, and 46 cases (6.4%) beyond the age of 40. The mean of the paternal age was 35.04±6.03 years old, there were 133 patients (18.6%) under the age of 30, 281 cases (39.3%) between the ages of 30 and 35, 165 cases (23.1%) between the ages of 36 and 40, and 136 cases (19.0%) beyond the age of 40. There were 74 (10.3%) cases with maternal BMI <18.5 kg/m2, 459 (64.2%) cases with 18.5–23.9 kg/m2, 127 (17.8%) cases with 24.0–27.9 kg/m2, and 55 (7.7%) cases with ≥28.0 kg/m2. There were 332 (46.4%) cases with primary infertility and 383 (53.6%) cases with secondary infertility. GnRH-a long protocol was the most common COH protocol, followed by GnRH antagonist protocol, long term follicular protocol, GnRH-a short protocol, and GnRH-a prolonged protocol. There were 7 (1.0%) patients developed ovarian hyperstimulation syndrome (OHSS). There were 614 (85.9%) patients receiving IVF and 93 (13.0%) patients receiving ICSI. Two embryos were transferred in 566 (79.2%) cases, 1 embryo was transferred in 137 (19.2%) cases, and 3 embryos were transferred in 12 (1.7%) cases. In this study, in 715 cycles with assisted reproductive technology, clinical pregnancy occurred in 360 (50.3%) cycles and pregnancy failure occurred in 355 (49.7%) cycles. Among the pregnancy failures, there were 300 (84.5%) implantation failures and 55 (15.5%) biochemical pregnancies (Table 1).
 |
Table 1 Demographics and Clinical Characteristics of Infertility Patients Treated with Assisted Reproductive Technology (ART) |
Comparison of Demographic and Clinical Characteristics Between Clinical Pregnancy and Pregnancy Failure Groups Among Infertility Patients Performed ART
Table 2 shows the results of differences in demographic and clinical characteristics between clinical pregnancy and pregnancy failure among infertility patients performed ART. There were statistically significant differences in the distribution of maternal age (P<0.001) and paternal age (P<0.001) between clinical pregnancy and pregnancy failure groups. In addition, there were statistically significant distribution differences of COH protocols, and number of embryos transferred between clinical pregnancy and pregnancy failure groups (all P<0.01). Specifically, the proportions of the COH protocols from high to low ranked as GnRH-a long protocol (43.4%), GnRH antagonist protocol (38.3%), GnRH-a short protocol (9.3%), long term follicular protocol (7.3%), and GnRH-a prolonged protocol (1.4%) in pregnancy failure group, while GnRH-a long protocol (54.2%), GnRH antagonist protocol (28.9%), long term follicular protocol (10.0%), GnRH-a short protocol (5.0%), and GnRH-a prolonged protocol (1.4%) in clinical pregnancy group. In terms of the number of embryos transferred, two embryos were mainly transferred in both groups, but the proportion of one embryo transferred in the pregnancy failure group was higher than that in the clinical pregnancy group (24.2% vs 14.2%, P<0.001). The pregnancy failure group had lower endometrial thickness (10.67±2.07 mm vs 11.13±1.98 mm, P=0.002) than clinical pregnancy group. There were no differences found in maternal BMI, infertility type, total Gn dose, OHSS, and fertilization methods.
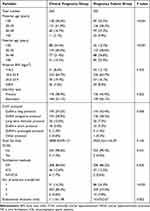 |
Table 2 Comparison of Demographic and Clinical Characteristics Between Clinical Pregnancy and Pregnancy Failure Groups Among Infertility Patients Performed ART |
Logistic Regression Analysis of Risk of Pregnancy Failure in ART Pregnancies
To gain insight into the independent influence of clinical characteristics on pregnancy failure, logistic regression analysis was performed. The univariate analyses performed indicated that high maternal age, high paternal age, GnRH antagonist protocol, GnRH-a short protocol, and two embryos transferred may have some effect on pregnancy failure in ART pregnancies. The multiple regressions performed showed that high maternal age (>35 years old vs ≤35 years old: OR 2.173, 95% CI: 1.386–3.407, P=0.001), and GnRH-a short protocol (GnRH-a short protocol vs GnRH-a long protocol: OR 2.139, 95% CI: 1.127–4.058, P=0.020) may increase risk of pregnancy failure in ART pregnancies, while two embryos transferred (two embryos transferred vs one embryo transferred: OR 0.563, 95% CI: 0.377–0.839, P=0.005) may reduce risk of pregnancy failure in ART pregnancies (Table 3).
 |
Table 3 Logistic Regression Analysis of Risk of Pregnancy Failure in Assisted Reproductive Technology (ART) Pregnancies |
Independent Risk Factors for Different Types of Pregnancy Failure
In this study, pregnancy failure including implantation failure, and biochemical pregnancy. Multiple logistic regression analysis showed that high maternal age (>35 years old vs ≤35 years old: OR 2.355, 95% CI: 1.405–3.945, P=0.001), GnRH antagonist protocol (GnRH antagonist protocol vs GnRH-a long protocol: OR 1.672, 95% CI: 1.122–2.491, P=0.012) and GnRH-a short protocol (GnRH-a short protocol vs GnRH-a long protocol: OR 2.272, 95% CI: 1.120–4.612, P=0.023) may increase risk of implantation failure, while two embryos transferred (two embryos transferred vs one embryo transferred: OR 0.445, 95% CI: 0.286–0.694, P<0.001) may reduce risk of implantation failure (Table 4). Multiple logistic regression analysis showed that high maternal age (>35 years old vs ≤35 years old: OR 3.367, 95% CI: 1.300–8.718, P=0.012) may increase risk of biochemical pregnancy (Table 4).
 |
Table 4 Independent Risk Factors for Different Types of Pregnancy Failure |
Discussion
This retrospective study showed that maternal age, COH regimen, and number of embryos transferred significantly affected pregnancy failure in the ART population. To be specific, high maternal age and GnRH-a short protocol may increase risk of pregnancy failure, while two embryos transferred may reduce risk of pregnancy failure in ART pregnancies. And high maternal age, GnRH antagonist protocol, and GnRH-a short protocol may increase risk of implantation failure, while two embryos transferred may reduce risk of implantation failure. In addition, high maternal age may increase risk of biochemical pregnancy. To our knowledge, this was the first retrospective cohort study to explore independent risk factors for pregnancy failure and extensively distinguish risk factors for different types of pregnancy failure from Meizhou, China.
Maternal age is one of the common risk factors for pregnancy failure in infertility patients with ART. Studies showed that the risk factors of pregnancy failure in infertile patients undergoing ART are related to maternal age.18,19 The age of the woman is one of the most important determinants of pregnancy success after natural conception and ART.20 In this study, maternal age >35 years old showed significant increase pregnancy failure rate after ART, compared with those ≤35 years old. Our result is consistent with other previous studies. The reason is that with the increase of maternal age, the formation of ovarian germ cells decreases, the quality of oocytes decreases, and eventually leads to ovarian reproductive failure.21 Some studies have suggested that decreased fecundity and fertilization rates in elderly women are also associated with decreased follicle reserve22 and increased aneuploidy.23,24 The proportion of fetuses born to women aged ≥35 years old with chromosomal abnormalities is significantly increased, as is the probability of spontaneous abortion.25,26 In terms of mechanism, advanced maternal age associated sirtuin family member 1 (SIRT1) deficiency compromises trophoblast epithelial–mesenchymal transition (EMT) through an increase in vimentin acetylation, damage trophoblast invasion and migration, promote premature placental senescence.27
Controlled ovulation stimulation is the basic step of in vitro fertilization and embryo transfer. In present study, GnRH-a short protocol may increase risk of pregnancy failure in ART pregnancies, and GnRH antagonist protocol and GnRH-a short protocol may increase risk of implantation failure. Studies showed that the normal fertilization rate in GnRH antagonist protocol group was lower than that in GnRH-a long protocol group.28,29 Studies found that the implantation and pregnancy rates in the GnRH-a short protocol group were significantly lower than those in the GnRH-a long protocol group.30–32 The cumulative live birth rate (cLBR) of the GnRH antagonist protocol group was significantly lower than that in the long-protocol group.33 Other studies have found that GnRH-a short protocol and GnRH antagonist protocol do not affect implantation rate and pregnancy rate.34–37 Therefore, further controlled randomized prospective studies with large sample sizes are needed to verify these inconsistent results in the future.
In our finding, two embryos transferred may reduce risk of pregnancy failure in ART pregnancies and implantation failure. Previous study has also shown that single embryo transferred has lower live birth and pregnancy rates than double embryo transferred.38 The pregnancy rate of single embryo transferred is lower than that of multiple embryos transferred, but multiple embryos transferred is easy to cause multiple pregnancy. Therefore, attention should be paid to controlling the number of transferred embryos while reducing the early pregnancy failure rate and improving the live birth rate, so as to achieve a good pregnancy outcome.39 Study has shown that the transfer of two high-quality embryos in elderly patients can achieve the ideal pregnancy rate and minimize the occurrence of multiple pregnancies.40
Previous studies have generally suggested a positive association between advanced paternal age and deterioration in semen quality.41,42 Paternal age more than 46 years is associated with a lower percentage of pregnancy and live birth among couples performed IVF.43 Paternal age <50 years can significantly reduce the rate of miscarriage and increase the success rate of ART.44 Paternal age was associated with chromosomal aberrations-related miscarriage in couples performed ART.45 Advanced paternal age has a significant negative influence on the outcome of ART.46 The results of a British study showed that paternal age over 50 years decreased the success rate of ART.47 However, some studies have shown that paternal age was not associated with adverse IVF outcomes.48,49 In this study, paternal age was not associated with pregnancy failure after ART in infertile patients. The results of studies on the effect of paternal age on the outcome of ART are inconsistent and the effect of paternal age needs further clarification.
In this study, maternal BMI was not associated with pregnancy failure after ART in infertile patients. Study showed that obesity was associated with lower clinical pregnancy rate50 and miscarriage.51 Maternal obesity is associated with slower embryo development.52 Placental leptin and adiponectin play an important role in placental development, maternal obesity is associated with the down-regulation of placental leptin and adiponectin, which is not conducive to placental development.53 In contrast, compared with the results in normal-weight women, the rates of clinical pregnancy and ongoing pregnancy were reduced in underweight women after ART.54 In addition, in another review, overweight was not associated with lower clinical pregnancy rate after ART.55 BMI was not a predictor of ART outcome in infertile patients.56 And study has found that BMI has different effects on ART pregnancy and live birth rates depending on race and ethnicity.57 The different results may be due to differences in the number of cases included in different studies, as well as differences in the definition of BMI classification.
Our results are strengthened by a cohort with a certain number cycles and breadth of available patient and cycle characteristic data. We controlled for some factors that potentially affect pregnancy failure, implantation failure, and biochemical pregnancy. However, there are some limitations in this study. First, the present study did not collect and analyze factors on the whole process of ART, which may have other potential factors leading to pregnancy failure. Second, the relatively small number of cycles with biochemical pregnancy limited the ability of this study to assess the influencing factors of biochemical pregnancy. Third, as a retrospective study, this study did not analyze all the reported possible influencing factors and could not provide a more comprehensive assessment of influencing factors. So, multicenter, large-sample, prospective trials are needed for further study.
Conclusions
In summary, the factors associated with pregnancy failure after assisted reproductive technology were identified through a cohort study from Meizhou, China. The risk of pregnancy failure increased in ART cycles with maternal age >35 years old and GnRH-a short protocol, while reduced with two embryos transferred. Hopefully, the efficiency of the clinical evaluation of pregnancy failure risk may be improved when the maternal age, COH protocol, and number of embryos transferred are taken into account. Further research is needed to validate our results and investigate the mechanism on the reported associations.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of Medicine, Meizhou People’s Hospital (Huangtang Hospital), Meizhou Academy of Medical Sciences. All participants signed informed consent in accordance with the Declaration of Helsinki.
Acknowledgments
The author would like to thank other colleagues whom were not listed in the authorship of Reproductive Medicine Center, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences for their helpful comments on the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Guangdong Provincial Key Laboratory of Precision Medicine and Clinical Translation Research of Hakka Population (Grant No.: 2018B030322003), and Science and Technology Program of Meizhou (Grant No.: 2019B0202001).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Carson SA, Kallen AN. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76. doi:10.1001/jama.2021.4788
2. Pisarska MD, Chan JL, Lawrenson K, Gonzalez TL, Wang ET. Genetics and epigenetics of infertility and treatments on outcomes. J Clin Endocrinol Metab. 2019;104(6):1871–1886. doi:10.1210/jc.2018-01869
3. Zhou Z, Zheng D, Wu H, et al. Epidemiology of infertility in China: a population-based study. BJOG. 2018;125(4):432–441. doi:10.1111/1471-0528.14966
4. Passet-Wittig J, Greil AL. Factors associated with medical help-seeking for infertility in developed countries: a narrative review of recent literature. Soc Sci Med. 2021;277:113782. doi:10.1016/j.socscimed.2021.113782
5. Simionescu G, Doroftei B, Maftei R, et al. The complex relationship between infertility and psychological distress (Review). Exp Ther Med. 2021;21(4):306. doi:10.3892/etm.2021.9737
6. Szkodziak F, Krzyżanowski J, Szkodziak P. Psychological aspects of infertility. A systematic review. J Int Med Res. 2020;48(6):030006052093240. doi:10.1177/0300060520932403
7. Hilbert SM, Gunderson S. Complications of Assisted Reproductive Technology. Emerg Med Clin North Am. 2019;37(2):239–249. doi:10.1016/j.emc.2019.01.005
8. Szamatowicz M. Assisted reproductive technology in reproductive medicine - possibilities and limitations. Ginekol Pol. 2016;87(12):820–823. doi:10.5603/GP.2016.0095
9. Sunderam S, Kissin DM, Zhang Y, et al. Assisted reproductive technology surveillance - United States, 2018. MMWR Surveill Summ. 2022;71(4):1–19. doi:10.15585/mmwr.ss7104a1
10. Hu L, Bu Z, Huang G, Sun H, Deng C, Sun Y. Assisted reproductive technology in china: results generated from data reporting system by CSRM from 2013 to 2016. Front Endocrinol. 2020;11:458. doi:10.3389/fendo.2020.00458
11. Cohen AM, Ye XY, Colgan TJ, Greenblatt EM, Chan C. Comparing endometrial receptivity array to histologic dating of the endometrium in women with a history of implantation failure. Syst Biol Reprod Med. 2020;66(6):347–354. doi:10.1080/19396368.2020.1824032
12. Hu L, Du J, Lv H, et al. Influencing factors of pregnancy loss and survival probability of clinical pregnancies conceived through assisted reproductive technology. Reprod Biol Endocrinol. 2018;16(1):74. doi:10.1186/s12958-018-0390-6
13. Healy M, Patounakis G, Zanelotti A, et al. Does premature elevated progesterone on the day of trigger increase spontaneous abortion rates in fresh and subsequent frozen embryo transfers? Gynecol Endocrinol. 2017;33(6):472–475. doi:10.1080/09513590.2017.1291612
14. Pontré JC, Ryan JP, Tan A, Hart RJ. The interval transfer of a frozen-thawed embryo is more successful than a fresh embryo transfer for women undergoing IVF with recurrent implantation failure after cleavage stage embryo biopsy. Aust N Z J Obstet Gynaecol. 2019;59(1):134–139. doi:10.1111/ajo.12798
15. Tehraninejad ES, Kashani NG, Hosseini A, Tarafdari A. Autologous platelet-rich plasma infusion does not improve pregnancy outcomes in frozen embryo transfer cycles in women with history of repeated implantation failure without thin endometrium. J Obstet Gynaecol Res. 2021;47(1):147–151. doi:10.1111/jog.14445
16. He W, Li Q, Yang M, et al. Lower BMI cutoffs to define overweight and obesity in China. Obesity. 2015;23(3):684–691. doi:10.1002/oby.20995
17. Tang J, Zhu X, Chen Y, et al. Association of maternal pre-pregnancy low or increased body mass index with adverse pregnancy outcomes. Sci Rep. 2021;11(1):3831. doi:10.1038/s41598-021-82064-z
18. Peng S, Sun H, Zheng J, Zeng N, Peng F. Risk factors of pregnancy failure in elderly infertility patients undergoing human assisted reproductive technology. Am J Transl Res. 2021;13(6):7306–7311. PMID: 34306498.
19. Rehman R, Mahmood H, Syed F, Syed H, Gul H. Intracytoplasmic sperm injection and advanced maternal age: success or treatment failure? Pak J Pharm Sci. 2019;32(4):1495–1499. PMID: 31608867.
20. Wang X, Lin Y, Liu Z, Huang X, Chen R, Huang H. Analysis of the causes and influencing factors of fetal loss in advanced maternal age: a nested case-control study. BMC Pregnancy Childbirth. 2021;21(1):538. doi:10.1186/s12884-021-04027-6
21. Guimarães RM, Ribeiro LM, Sasaki LP, Nakagawa HM, Cabral IO. Oocyte morphology and reproductive outcomes - case report and literature review. JBRA Assist Reprod. 2021;25(3):500–507. doi:10.5935/1518-0557.20210001
22. Ziebe S, Loft A, Petersen JH, et al. Embryo quality and developmental potential is compromised by age. Acta Obstet Gynecol Scand. 2001;80(2):169–174. doi:10.1034/j.1600-0412.2001.080002169.x
23. Mikwar M, MacFarlane AJ, Marchetti F. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutat Res Rev Mutat Res. 2020;785:108320. doi:10.1016/j.mrrev.2020.108320
24. Franasiak JM, Forman EJ, Hong KH, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656–663.e651. doi:10.1016/j.fertnstert.2013.11.004
25. Zhang X, Wu H, Gu Z, Yu Z, Lan L, Huang Q. Chromosomal copy number variation analysis in pregnancy products from recurrent and sporadic miscarriage using next-generation sequencing. Reprod Sci. 2022;29(10):2927–2936. doi:10.1007/s43032-022-00969-0
26. Wu H, Huang Q, Zhang X, Yu Z, Zhong Z. Analysis of genomic copy number variation in miscarriages during early and middle pregnancy. Front Genet. 2021;12:732419. doi:10.3389/fgene.2021.732419
27. Xiong L, Ye X, Chen Z, et al. Advanced maternal age-associated SIRT1 deficiency compromises trophoblast epithelial-mesenchymal transition through an increase in vimentin acetylation. Aging Cell. 2021;20(10):e13491. doi:10.1111/acel.13491
28. Yang J, Zhang X, Ding X, Wang Y, Huang G, Ye H. Cumulative live birth rates between GnRH-agonist long and GnRH-antagonist protocol in one ART cycle when all embryos transferred: real-word data of 18,853 women from China. Reprod Biol Endocrinol. 2021;19(1):124. doi:10.1186/s12958-021-00814-0
29. Grow D, Kawwass JF, Kulkarni AD, Durant T, Jamieson DJ, Macaluso M. GnRH agonist and GnRH antagonist protocols: comparison of outcomes among good-prognosis patients using national surveillance data. Reprod Biomed Online. 2014;29(3):299–304. doi:10.1016/j.rbmo.2014.05.007
30. Ou J, Xing W, Li T, Li Y, Xu Y, Zhou C. Short versus long gonadotropin-releasing hormone analogue suppression protocols in advanced age women undergoing IVF/ICSI. Gynecol Endocrinol. 2016;32(8):622–624. doi:10.3109/09513590.2016.1147546
31. Ou J, Xing W, Li Y, Xu Y, Zhou C, Sun Q-Y. Short versus long gonadotropin-releasing hormone analogue suppression protocols in IVF/ICSI cycles in patients of various age ranges. PLoS One. 2015;10(7):e0133887. doi:10.1371/journal.pone.0133887
32. Xing W, Lin H, Li Y, Yang D, Wang W, Zhang Q. Is the GnRH Antagonist Protocol Effective at Preventing OHSS for Potentially High Responders Undergoing IVF/ICSI? PLoS One. 2015;10(10):e0140286. doi:10.1371/journal.pone.0140286
33. Zhang W, Xie D, Zhang H, et al. Cumulative live birth rates after the first ART cycle using flexible GnRH antagonist protocol vs. standard long GnRH agonist protocol: a retrospective cohort study in women of different ages and various ovarian reserve. Front Endocrinol. 2020;11:287. doi:10.3389/fendo.2020.00287
34. Madani T, Ashrafi M, Yeganeh LM. Comparison of different stimulation protocols efficacy in poor responders undergoing IVF: a retrospective study. Gynecol Endocrinol. 2012;28(2):102–105. doi:10.3109/09513590.2011.579206
35. Wang R, Lin S, Wang Y, Qian W, Zhou L, Sun Q-Y. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: a systematic review and meta-analysis. PLoS One. 2017;12(4):e0175985. doi:10.1371/journal.pone.0175985
36. Xiao JS, Su CM, Zeng XT, Tian X. Comparisons of GnRH antagonist versus GnRH agonist protocol in supposed normal ovarian responders undergoing IVF: a systematic review and meta-analysis. PLoS One. 2014;9(9):e106854. doi:10.1371/journal.pone.0106854
37. Eldar-Geva T, Zylber-Haran E, Babayof R, et al. Similar outcome for cryopreserved embryo transfer following GnRH-antagonist/GnRH-agonist, GnRH-antagonist/HCG or long protocol ovarian stimulation. Reprod Biomed Online. 2007;14(2):148–154. doi:10.1016/s1472-6483(10)60781-x
38. McLernon DJ, Harrild K, Bergh C, et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ. 2010;341:c6945. doi:10.1136/bmj.c6945
39. Lebovitz O, Haas J, James KE, Seidman DS, Orvieto R, Hourvitz A. The expected cumulative incidence of live birth for patients starting IVF treatment at age 41 years or older. Reprod Biomed Online. 2018;37(5):533–541. doi:10.1016/j.rbmo.2018.08.014
40. Luke B, Brown MB, Wantman E, et al. Application of a validated prediction model for in vitro fertilization: comparison of live birth rates and multiple birth rates with 1 embryo transferred over 2 cycles vs 2 embryos in 1 cycle. Am J Obstet Gynecol. 2015;212(5):676.e671–677. doi:10.1016/j.ajog.2015.02.005
41. Bertoncelli Tanaka M, Agarwal A, Esteves SC. Paternal age and assisted reproductive technology: problem solver or trouble maker? Panminerva Med. 2019;61(2):138–151. doi:10.23736/S0031-0808.18.03512-7
42. Brandt JS, Cruz Ithier MA, Rosen T, Ashkinadze E. Advanced paternal age, infertility, and reproductive risks: a review of the literature. Prenat Diagn. 2019;39(2):81–87. doi:10.1002/pd.5402
43. Marsidi AM, Kipling LM, Kawwass JF, Mehta A. Influence of paternal age on assisted reproductive technology cycles and perinatal outcomes. Fertil Steril. 2021;116(2):380–387. doi:10.1016/j.fertnstert.2021.03.033
44. Murugesu S, Kasaven LS, Petrie A, et al. Does advanced paternal age affect outcomes following assisted reproductive technology? A systematic review and meta-analysis. Reprod Biomed Online. 2022;45(2):283–331. doi:10.1016/j.rbmo.2022.03.031
45. Wang Z, Liu X, Xu J, et al. Paternal age, body mass index, and semen volume are associated with chromosomal aberrations-related miscarriages in couples that underwent treatment by assisted reproductive technology. Aging. 2020;12(9):8459–8472. doi:10.18632/aging.103151
46. Vogiatzi P, Pouliakis A, Sakellariou M. Male age and progressive sperm motility are critical factors affecting embryological and clinical outcomes in oocyte donor ICSI cycles. Reprod Sci. 2022;29(3):883–895. doi:10.1007/s43032-021-00801-1
47. Morris G, Mavrelos D, Odia R, Viñals Gonzalez X. Paternal age over 50 years decreases assisted reproductive technology (ART) success: a single UK center retrospective analysis. Acta Obstet Gynecol Scand. 2021;100(10):1858–1867. doi:10.1111/aogs.14221
48. Tiegs AW, Sachdev NM, Grifo JA, McCulloh DH, Licciardi F. Paternal age is not associated with pregnancy outcomes after single thawed euploid blastocyst transfer. Reprod Sci. 2017;24(9):1319–1324. doi:10.1177/1933719116687660
49. Lai SF, Li RH, Yeung WS, Ng EH. Effect of paternal age on semen parameters and live birth rate of in-vitro fertilisation treatment: a retrospective analysis. Hong Kong Med J. 2018;24(5):444–450. doi:10.12809/hkmj177111
50. Xiong Y, Wang J, Huang S, et al. Association between maternal prepregnancy body mass index and pregnancy outcomes following assisted reproductive technology: a systematic review and dose-response meta-analysis. Obes Rev. 2021;22(6):e13219. doi:10.1111/obr.13219
51. Cozzolino M, García-Velasco JA, Meseguer M, Pellicer A, Bellver J. Female obesity increases the risk of miscarriage of euploid embryos. Fertil Steril. 2021;115(6):1495–1502. doi:10.1016/j.fertnstert.2020.09.139
52. Bartolacci A, Buratini J, Moutier C, et al. Maternal body mass index affects embryo morphokinetics: a time-lapse study. J Assist Reprod Genet. 2019;36(6):1109–1116. doi:10.1007/s10815-019-01456-3
53. Nogues P, Dos Santos E, Jammes H, et al. Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta. Clin Epigenetics. 2019;11(1):20. doi:10.1186/s13148-019-0612-6
54. Tang S, Huang J, Lin J, Kuang Y. Adverse effects of pre-pregnancy maternal underweight on pregnancy and perinatal outcomes in a freeze-all policy. BMC Pregnancy Childbirth. 2021;21(1):32. doi:10.1186/s12884-020-03509-3
55. Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women. Hum Reprod. 2012;27(2):457–467. doi:10.1093/humrep/der416
56. Fuchs Weizman N, Defer MK, Montbriand J, Pasquale JM, Silver A, Librach CL. Does body mass index impact assisted reproductive technology treatment outcomes in gestational carriers. Reprod Biol Endocrinol. 2020;18(1):35. doi:10.1186/s12958-020-00602-2
57. Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R. Racial and ethnic disparities in assisted reproductive technology pregnancy and live birth rates within body mass index categories. Fertil Steril. 2011;95(5):1661–1666. doi:10.1016/j.fertnstert.2010.12.035
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
