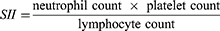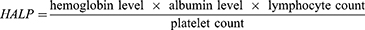Back to Journals » International Journal of General Medicine » Volume 16
Risk Factors of Gastrointestinal Perforation with a Poor Prognosis
Authors Yuan W, Zhou X, Cai Z, Qiu J, Li X, Tong G
Received 19 June 2023
Accepted for publication 9 October 2023
Published 16 October 2023 Volume 2023:16 Pages 4637—4647
DOI https://doi.org/10.2147/IJGM.S426676
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hossam El-Din Shaaban
Wenqing Yuan,1,* Xiaozhuan Zhou,2,* Zhigao Cai,1 Junyu Qiu,1 Xi Li,2 Gangling Tong1,3
1Department of Gastrointestinal Surgery, Longchuan County People’s Hospital, Heyuan, 517300, People’s Republic of China; 2Department of Gastroenterology, Peking University Shenzhen Hospital, Shenzhen, 518036, People’s Republic of China; 3Department of Oncology, Peking University Shenzhen Hospital, Shenzhen 518036, China and Shenzhen Key Laboratory of Gastrointestinal Cancer Translational Research, Cancer Institute of Shenzhen-PKU-HKUST Medical Center, Shenzhen, 518036, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xi Li, Department of Gastroenterology, Peking University Shenzhen Hospital, Shenzhen, Guangdong Province, 518036, People’s Republic of China, Tel/Fax +86-75583923333, Email [email protected] Gangling Tong, Department of Oncology, Peking University Shenzhen Hospital, Shenzhen, Guangdong Province, 518036, People’s Republic of China, Tel +86-75583923333, Fax +86-75583061340, Email [email protected]
Background: Despite medical progress, mortality from gastrointestinal perforation was relatively high. Our study’s objective was to identify risk factors associated with a poor prognosis for gastrointestinal perforation.
Methods: Patients diagnosed with gastrointestinal perforation at the Longchuan County People’s Hospital between January 2019 and February 2022 were the subject of a retrospective analysis of their laboratory data. Patients were grouped based on length of hospital stay, septic shock, and mortality.
Results: A total of 240 patients participated in our study. Using univariate and multivariate analysis, we identified several risk factors for gastrointestinal perforation associated with a dismal prognosis. Lower digestive tract perforation (OR=2.418, 95% CI 1.119– 5.227, P=0.025), low total protein (OR=0.934, 95% CI 0.879– 0.992, P=0.026) and low hemoglobin (OR=0.985, 95% CI 0.971– 0.999, P=0.039) were linked to a longer length of stay, especially hemoglobin (OR=0.978, 95% CI 0.966– 0.991, P=0.001) in upper digestive tract. High ratio of neutrophils to lymphocytes (NLR) (OR=1.043, 95% CI 1.012– 1.076, P=0.007), high lymphocyte-to-monocyte ratio (LMR) (OR=2.158, 95% CI 1.495– 3.115, P< 0.001) and low prognostic nutrition index (PNI) (OR=0.814, 95% CI 0.751– 0.833, P< 0.001) predicted septic shock. In upper digestive tract, PLR (OR=1.001, 95% CI 1.000– 1.002, P=0.067), LMR (OR=2.160, 95% CI 1.440– 3.240, P< 0.001) and PNI (OR=0.843, 95% CI 0.767– 0.926, P< 0.001) were risk factors for septic shock, and total protein (OR=0.796, 95% CI 0.686– 0.923, P=0.003) was a risk factor for septic shock in lower digestive tract. High NLR (OR=1.056, 95% CI 1.019– 1.093, P=0.003), high LMR (OR=1.760, 95% CI 1.177– 2.632, P=0.006) and low PNI (OR=0.832, 95% CI 0.754– 0.918, P< 0.001) were the risk factors of mortality. In subgroup analysis of perforation site, albumin (OR=0.820, 95% CI 0.719– 0.934, P=0.003) and LMR (OR=1.506, 95% CI 1.069– 2.123, P=0.019) were risk factors for mortality in upper digestive tract and PNI (OR=0.636, 95% CI 0.445– 0.908, P=0.013) was a risk factor for mortality in lower digestive tract.
Conclusion: Our research found that the perforation site, total protein, albumin, hemoglobin, NLR, LMR, PLR and PNI were risk factors for gastrointestinal perforation with a poor prognosis.
Keywords: gastrointestinal perforation, length of stay, septic shock, mortality, prognosis
Introduction
Gastrointestinal perforation is the complete penetration of the digestive tract, which may be caused by inflammation,1 ulcer,2 medication,3 diverticulum,4 connective tissue disorders, neoplasm,5 iatrogenic procedure6 and so on. Leakage of alimentary contents into the peritoneal cavity may result in peritoneal infection, sepsis, septic shock, and even life-threatening complications following gastrointestinal perforation. Despite advances in surgery and emergency medicine, gastrointestinal perforation has a 10% to 50% mortality rate.7–10 Acute abdominal pain may be the most frequent clinical manifestation of gastrointestinal perforation. However, some perforations, particularly colonic perforations, may progress slowly, delaying the initiation of appropriate treatment. Timely recognition of gastrointestinal perforation, particularly in cases with a poor prognosis, is crucial for reducing complications and mortality.
Some studies have identified risk factors for severe gastrointestinal perforation, such as aging, diabetes, and the use of nonsteroidal anti-inflammatory drugs or glucocorticoids.11–13 Numerous studies have linked inflammatory response and nutritional status to the prognosis of gastrointestinal perforation. Aydin O demonstrated that platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) were positively associated with peptic ulcer perforation mortality, whereas lymphocyte count values were negatively associated with peptic ulcer perforation mortality.14 In Mller MH’s meta-analysis, a low albumin level was positively associated with peptic ulcer perforation mortality.15 Recent research has focused on the relationships between systemic inflammation and nutrition parameters, such as the systemic immune-inflammation index (SII), prognostic nutritional index (PNI), and hemoglobin, albumin, lymphocyte, and platelet (HALP) scores. They were calculated with the formula as follow.
Liu found SII outperformed NLR and PLR in predicting severe acute pancreatitis.16 In Jiang’s systematic review, decreased PNI was associated with poor oesophageal cancer overall survival.17 In acute pancreatitis with a high PNI, Akkuzu’s study found no local complications or fatalities.18 High HALP score was associated with improved survival in several carcinomas, including colorectal cancer,19 small cell lung cancer,20 renal cell carcinoma,21 and so on. However, SII, PNI, and HALP scores were not utilized in studies of gastrointestinal perforation. We reviewed patients with gastrointestinal perforation to identify risk factors of poor prognosis with common laboratory data and combined parameters related to inflammatory and nutritional status, which may be useful to primary hospital practitioners. Ours was the first study to investigate the relationship between SII, PNI, and HALP score and poor prognosis in gastrointestinal perforation.
Materials and Methods
Patients with gastrointestinal perforation admitted to Longchuan County People’s Hospital between January 2019 and February 2022 were identified. Such demographic data as age and gender were recorded. Laboratory data, including total protein, albumin, and routine blood test were reviewed. In addition, the location of the perforation, length of stay, septic shock, and prognosis for survival were recorded. Patients were divided into two groups based on length of hospital stay, incidence of septic shock, and mortality. Written informed consent was obtained from all the participants prior to the enrollment of this study. Our study was also approved by our institutional review board (Longchuan County People’s Hospital) and conducted in accordance with the Declaration of Helsinki’s principles.
Using SPSS 25.0 and Stata/SE 12.0, statistical analysis was conducted. Frequency (n/N) and percent (%) were used to express discrete variables. Continuous variables were expressed as median and quartile for nonparametric data or as mean ± standard deviation for data with a normal distribution. We analyzed univariate relationships with the Fisher’s exact test for categorical data, the Mann–Whitney U-test for nonparametric data, and the Student’s t-test for data with a normal distribution, selecting those parameters P < 0.1 for multivariate analysis. In the multivariate analysis, logistic regression analysis was used to identify independent risk factors for gastrointestinal perforation in terms of length of hospital stay, septic shock, and survival prognosis, and then a nomogram was generated. Using the receiver operator characteristic (ROC) curve, the C-index of the nomogram was calculated. The significance level was defined as a P value of <0.05.
Results
Patients
The study included 240 patients with gastrointestinal perforation. Among them, 180 patients were male. There were 185 patients diagnosed with upper gastrointestinal perforation, 51 with lower gastrointestinal perforation, and 4 with unknown perforation site. The average age of patients involved was 63.5 years old. The range of length of stay was from 0 to 59 days, with a mean of 9 days. Twenty-six patients experienced septic shock, and 21 patients’ dead eventually.
Risk Factors for Prolonged Length of Stay
A hospitalization was deemed to be prolonged if it was longer than or equal to the median length of stay. Table 1 displays patients’ information associated with length of stay.
 |
Table 1 Included Patients’ Information Divided According to Length of Stay |
Univariate analyses identified variables with a potential independent correlation with prolonged length of stay and revealed that sex (P=0.043), the site of perforation (P=0.026), total protein (P=0.001), albumin (P=0.004), and hemoglobin (P=0.001) were associated with prolonged length of stay. Multivariate regression analyses including factors P<0.1 at univariate analyses and the significant clinical factor PLR disregarding P>0.1 at univariate analyses revealed that lower digestive tract, low total protein, and low hemoglobin were risk factors for prolonged length of stay, with OR values of 2.418 (95% CI 1.119–5.227, P=0.025), 0.934 (95% CI 0.879–0.992, P=0.026) and 0.985 (95% CI 0.971–0.999, P=0.039). Figure 1 depicts the risk factor nomogram of length of stay for patients with gastrointestinal perforation. Figure 2 depicts the ROC curve for discriminating length of stay, with a C-index of 0.693.
 |
Figure 1 Nomogram of prolonged length of stay. |
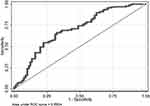 |
Figure 2 The ROC curve of length of stay. |
In subgroup analysis of perforation site, hemoglobin was a risk factor for prolonged length of stay in upper digestive tract, with OR values of 0.978 (95% CI 0.966–0.991, P=0.001). There was not risk factor for prolonged length of stay in lower digestive tract.
Risk Factors for Septic Shock
Table 2 depicts patients’ information associated with septic shock.
 |
Table 2 Included Patients’ Information Classified by Septic Shock |
Univariate analyses identified variables with a potential independent correlation with septic shock and identified sex (P<0.001), age (P<0.001), total protein (P<0.001), albumin (P<0.001), neutrophils (P=0.025), lymphocyte (P=0.003), basophils (P=0.004), monocyte (P=0.001), hemoglobin (P<0.001), PLR (P=0.039), PNI (P=0.001) and HALP score (P=0.004) as these variables. Multivariate regression analyses including factors P<0.1 at univariate analyses and the significant clinical factor NLR and LMR disregarding P>0.1 at univariate analyses revealed that high NLR, high LMR, and low PNI were risk factors for septic shock, with OR values of 1.043 (95% CI 1.012–1.076, P=0.007), 2.158 (95% CI 1.495–3.115, P<0.001) and 0.814 (95% CI 0.751–0.833, P<0.001). Figure 3 depicts the risk factors nomogram of septic shock for patients with gastrointestinal perforation. Figure 4 depicts the ROC curve for discriminating septic shock, with a C-index of 0.845.
 |
Figure 3 Nomogram representing septic shock. |
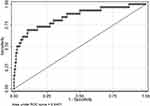 |
Figure 4 The ROC curve for septic shock discrimination. |
In subgroup analysis of perforation site, PLR, LMR and PNI were risk factors for septic shock in upper digestive tract, with OR values of 1.001 (95% CI 1.000–1.002, P=0.067), 2.160 (95% CI 1.440–3.240, P<0.001) and 0.843 (95% CI 0.767–0.926, P<0.001). In lower digestive tract, total protein was a risk factor for septic shock, with OR values of 0.796 (95% CI 0.686–0.923, P=0.003).
Risk Factors for Mortality
Patients information associated with mortality is depicted in Table 3.
 |
Table 3 Included Patients’ Information Divided According to Mortality |
Variables with a potential independent correlation with mortality were identified by univariate analyses, indicating total protein (P=0.001), albumin (P<0.001), lymphocyte (P=0.008), monocyte (P=0.019), hemoglobin (P=0.033) and PNI (P=0.001). Multivariate regression analyses including factors P<0.1 at univariate analyses and the significant clinical factor NLR, PLR, and LMR disregarding P>0.1 at univariate analyses revealed that NLR, LMR, and PNI were the risk factors of mortality, with OR values of 1.056 (95% CI 1.019–1.093, P=0.003), 1.760 (95% CI 1.177–2.632, P=0.006) and 0.832 (95% CI 0.754–0.918, P<0.001), respectively. The mortality nomogram for patients with gastrointestinal perforation is shown in Figure 5. The ROC curve for distinguishing mortality is plotted in Figure 6, and the C-index was 0.858.
 |
Figure 5 Nomogram of mortality. |
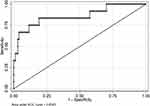 |
Figure 6 The ROC curve for discriminating mortality. |
In subgroup analysis of perforation site, albumin and LMR were risk factors for mortality in upper digestive tract, with OR values of 0.820 (95% CI 0.719–0.934, P=0.003) and 1.506 (95% CI 1.069–2.123, P=0.019). In lower digestive tract, PNI was a risk factor for mortality, with OR values of 0.636 (95% CI 0.445–0.908, P=0.013).
Discussion
Acute gastrointestinal perforation is associated with a high mortality rate. It is crucial to identify severe gastrointestinal perforations with a poor prognosis. To evaluate the mortality of critically ill patients, scoring systems such as Acute Physiology And Chronic Health Evaluation II (APACHE II) and Simplified Acute Physiology Score (SAPS II) exist. However, it is disease-independent. The Mannheim peritonitis index (MPI) is comprised of clinical information to evaluate outcome of peritonitis.22 In our study, we identified several common blood indices that aid in the diagnosis of severe gastrointestinal perforation.
There were few studies that examined the length of hospital stay for patients with gastrointestinal perforation. In Kayano’s study, hospitalization duration was shorter in the high psoas muscle index (PMI) group than in the low PMI group.23 As a nutrient storage system, muscles utilized amino acids to combat inflammation during a bacterial invasion. Similar to Kayano’s study, but with different nutrient indices for total protein and hemoglobin, our outcome was comparable. Low levels of total protein and hemoglobin were associated with an extended length of stay, especially low hemoglobin in upper digestive tract perforation. It was widely acknowledged that malnutrition suppressed immune function, which may result in severe infection and protracted hospitalization.24,25 Anemic patients frequently coexisted with malnutrition, cancer, and other chronic conditions, and had longer hospital stays.26 Wang discovered that the group with an upper gastrointestinal tract had a longer length of stay than the group with a lower gastrointestinal tract,27 while our outcome was contrary. In Wang’s study, it may have been caused by more severe conditions in the intensive care unit, whereas the majority of our patients were treated in the general ward. And only 33 patients participated in Wang’s study. In addition, there was no significant difference in septic shock between upper and lower gastrointestinal perforation in our study. In Wang’s study, however, the use of norepinephrine was more in upper gastrointestinal perforation than in lower gastrointestinal perforation, which may have contributed to longer hospitalization duration. The OR value of NLR was 0.970 (95% CI 0.939–1.002, P=0.065) in our study, which implied NLR maybe another risk factor of prolonged length of stay. Increased inflammation was associated with severe infection, extending the length of treatment. In COVID-19, a low PNI was associated with hospitalization lengthening.28 SII played a significant role in predicting the length of stay in spinal deformity in adults.29 There was no research examining the correlation between HALP score and length of hospital stay. In our univariate analyses, a P<0.1 correlation between low PNI and length of stay was observed. However, the OR values were 1.043 (95% CI 0.921–1.182, P=0.506) in multivariate regression analyses. In univariate analyses, the SII and HALP score P values were 0.436 and 0.163, respectively. In our study, PNI, SII, and HALP scores were not risk factors for length of stay in patients with gastrointestinal perforation.
Perforation of the gastrointestinal tract may cause septic shock. In patients with gastrointestinal perforation, CRP and PCT were independent risk factors for septic shock according to Chen’s warning scoring system.30 Zhou discovered an association between increased SOFA score and sepsis of gastrointestinal perforation.31 High NLR, high LMR, and low PNI were identified as risk factors for septic shock in our study. CRP, PCT, NLR, and LMR were common inflammatory response indices. Increased inflammation was closely associated with an immune response that was out of control, which may result in septic shock. Based on the physiological relationship between neutrophilia, lymphopenia, and mononucleosis in systemic inflammation, NLR and LMR were more sensitive than single white blood cell count.32 In subgroup analysis, there were inflammatory response indices PLR and LMR detected significantly in upper digestive tract but not lower digestive tract. It might be due to lower digestive tract perforation tended to be inflammatory and upper digestive tract varied in inflammation. Severe inflammation was related to septic shock in upper digestive tract perforation. Nutritional reserve was essential for defending against bacterial invasion, and patients with low PNI and total protein were susceptible to septic shock. Li also demonstrated that neonatal PNI decreased with increasing severity of sepsis.33 P<0.05 indicated a correlation between HALP score and septic shock in our univariate analyses, but the OR values were 1.013 (95% CI 0.933–1.099, P=0.764) in multivariate regression analyses. Other HALP score studies focused on carcinoma and did not include sepsis. Pricop suggested SII for sepsis prediction in odontogenic infection.34 In Ma’s study, high-level SII sepsis patients tended to develop septic shock.35 In our study, the P value of SII in univariate analyses was 0.277. In our study, neither SII nor HALP score was associated with septic shock in patients with gastrointestinal perforation.
The mortality rate associated with gastrointestinal perforation was relatively high. In Shimoyama’s study, NLR and PLR were independently linked to gastrointestinal perforation mortality.32 We also discovered that high levels of the inflammatory factors NLR and LMR increased the mortality associated with gastrointestinal perforation, especially in upper digestive tract. As stated previously, uncontrolled inflammatory response led to the destruction of tissue and even death. In a study involving 146 patients with colorectal perforation, Matsuoka T. found a correlation between decreased albumin level and mortality.36 Typically, serum albumin was used to assess nutritional status. In inflammatory responses, hypoalbuminosis frequently foretold a dismal prognosis. The PNI, a combination of albumin and lymphocytes, was associated with mortality from gastrointestinal perforation, according to our findings. In Wang’s study, hepatocellular carcinoma patients with high PNI and low SII had longer recurrence-free survival and overall survival.37 In univariate analyses, however, SII was not a significant predictor of survival in our patients with gastrointestinal perforation with P value >0.1. Low HALP score was associated with decreased survival in patients with solid tumors, according to a meta-analysis by Xu.38 In our study, a P<0.1 correlation between HALP score and mortality was observed in univariate analyses, but the OR values were 1.050 (95% CI 0.945–1.166, P=0.364) in multivariate regression analyses. In our study, SII and HALP scores were weak predictors of poor gastrointestinal perforation.
There were a few limitations to our research. Firstly, the study was conducted at a single location. Secondly, because our study was retrospective, it was challenging to obtain missing information such as other inflammatory markers, method of perforation and co-morbidities. Then, because the mean age of the included patients was over 60 years, our study was not representative. Lastly, there were insufficient patients for subgroup analysis of perforation site, which leaded to different results especially in lower digestive tract. In the future, we will conduct a large-scale, multi-center study to confirm our findings.
In conclusion, the perforation site, total protein, albumin, hemoglobin, NLR, LMR, PLR and PNI were poor prognostic factors for gastrointestinal perforation. In practice, we could assign significance to these parameters in order to distinguish perforations of the gastrointestinal tract with a poor prognosis.
Disclosure
The authors report no conflicts of interest in the work.
References
1. Bona D, Incarbone R, Chella B, et al. Heartburn and multiple-site foregut perforations as primary manifestation of Crohn’s disease. Dis Esophagus. 2005;18(3):199–201. doi:10.1111/j.1442-2050.2005.00468.x
2. Bertleff MJ, Lange JF. Perforated peptic ulcer disease: a review of history and treatment. Dig Surg. 2010;27(3):161–169. doi:10.1159/000264653
3. Fujita T, Kutsumi H, Sanuki T, et al. Adherence to the preventive strategies for nonsteroidal anti-inflammatory drug- or low-dose aspirin-induced gastrointestinal injuries. J Gastroenterol. 2013;48(5):559–573. doi:10.1007/s00535-013-0771-8
4. Wang YJ, Wang T, Xia SL, et al. Perforation of Meckel`s diverticulum in a very low birth weight neonate with severe pneumoperitoneum and review of literature. Turk J Pediatr. 2019;61(3):460–465. doi:10.24953/turkjped.2019.03.025
5. Yang XF, Pan K. Diagnosis and management of acute complications in patients with colon cancer: bleeding, obstruction, and perforation. Chin J Cancer Res. 2014;26(3):331–340. doi:10.3978/j.issn.1000-9604.2014.06.11
6. Hoffman A, Atreya R, Rath T, et al. Current endoscopic resection techniques for gastrointestinal lesions: endoscopic mucosal resection, submucosal dissection, and full-thickness resection. Visc Med. 2021;37(5):358–371. doi:10.1159/000515354
7. Pouli S, Kozana A, Papakitsou I, et al. Gastrointestinal perforation: clinical and MDCT clues for identification of aetiology. Insights Imaging. 2020;11(1):31. doi:10.1186/s13244-019-0823-6
8. Chen H, Zhang H, Li W, et al. Acute gastrointestinal injury in the intensive care unit: a retrospective study. Ther Clin Risk Manag. 2015;11:1523–1529. doi:10.2147/TCRM.S92829
9. Chongxi R, Jinggang J, Yan S, et al. Spontaneous colonic perforation in adults: evaluation of a pooled case series. Sci Prog. 2020;103(3):36850420945462. doi:10.1177/0036850420945462
10. Søreide K, Thorsen K, Søreide JA. Strategies to improve the outcome of emergency surgery for perforated peptic ulcer. Br J Surg. 2014;101(1):e51–e64. doi:10.1002/bjs.9368
11. Xie F, Yun H, Bernatsky S, et al. Brief report: risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 2016;68(11):2612–2617. doi:10.1002/art.39761
12. Gisbert JP, Legido J, García-Sanz I, et al. Helicobacter pylori and perforated peptic ulcer prevalence of the infection and role of non-steroidal anti-inflammatory drugs. Dig Liver Dis. 2004;36(2):116–120. doi:10.1016/j.dld.2003.10.011
13. Amini A, Lopez RA. Duodenal perforation. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
14. Aydin O, Pehlivanlı F. Is the platelet to lymphocyte ratio a potential biomarker for predicting mortality in peptic ulcer perforation? Surg Infect (Larchmt). 2019;20(4):326–331. doi:10.1089/sur.2018.288
15. Møller MH, Adamsen S, Thomsen RW, et al. Preoperative prognostic factors for mortality in peptic ulcer perforation: a systematic review. Scand J Gastroenterol. 2010;45(7–8):785–805. doi:10.3109/00365521003783320
16. Liu X, Guan G, Cui X, et al. Systemic Immune-Inflammation Index (SII) can be an early indicator for predicting the severity of acute pancreatitis: a retrospective study. Int J Gen Med. 2021;14:9483–9489. doi:10.2147/IJGM.S343110
17. Jiang Y, Xu D, Song H, et al. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta-analysis. BMJ open. 2021;11(9):e048324. doi:10.1136/bmjopen-2020-048324
18. Akkuzu MZ, Altıntaş E, Yaraş S, et al. Controlling Nutritional Status (CONUT) Score and Prognostic Nutritional Index (PNI) are good candidates for prognostic markers for acute pancreatitis. Medicina. 2022;59(1):70. doi:10.3390/medicina59010070
19. Yalav O, Topal U, Unal AG, et al. Prognostic significance of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients undergoing curative resection for colorectal cancer. Ann Ital Chir. 2021;92:283–292.
20. Shen XB, Zhang YX, Wang W, et al. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) score in patients with small cell lung cancer before first-line treatment with etoposide and progression-free survival. Med Sci Monit. 2019;25:5630–5639. doi:10.12659/MSM.917968
21. Peng D, Zhang CJ, Tang Q, et al. Prognostic significance of the combination of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urol. 2018;18(1):20. doi:10.1186/s12894-018-0333-8
22. Billing A, Fröhlich D, Schildberg FW. Prediction of outcome using the Mannheim peritonitis index in 2003 patients. Peritonitis Study Group. Br J Surg. 1994;81(2):209–213. doi:10.1002/bjs.1800810217
23. Kayano H, Nomura E, Abe R, et al. Low psoas muscle index is a poor prognostic factor for lower gastrointestinal perforation: a single-center retrospective cohort study. BMC Surg. 2019;19(1):181. doi:10.1186/s12893-019-0629-y
24. Stratton RJ, King CL, Stroud MA, et al. “Malnutrition Universal Screening Tool” predicts mortality and length of hospital stay in acutely ill elderly. Br J Nutr. 2006;95(2):325–330. doi:10.1079/BJN20051622
25. Wang X, Naito Y, Nakatani H, et al. Prevalence of undernutrition in surgical patients and the effect on length of hospital stay. J Anesth. 2022;36(1):89–95. doi:10.1007/s00540-021-03013-8
26. Lin RJ, Evans AT, Chused AE, et al. Anemia in general medical inpatients prolongs length of stay and increases 30-day unplanned readmission rate. South Med J. 2013;106(5):316–320. doi:10.1097/SMJ.0b013e318290f930
27. Wang H, Liu X, Bi H, et al. Clinical analysis of septic shock caused by acute upper and lower gastrointestinal perforation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32(8):943–946. doi:10.3760/cma.j.cn121430-20200417-00312
28. Fernandes AL, Reis BZ, Murai IH, et al. Prognostic nutritional index and oxygen therapy requirement associated with longer hospital length of stay in patients with moderate to severe COVID-19: multicenter Prospective cohort analyses. Front Nutr. 2022;9:802562. doi:10.3389/fnut.2022.802562
29. Arora A, Wague A, Srinivas R, et al. Risk factors for extended length of stay and non-home discharge in adults treated with multi-level fusion for lumbar degenerative pathology and deformity. Spine Deform. 2022;11(3):685–697.
30. Chen P, Gao J, Li J, et al. Construction and efficacy evaluation of an early warning scoring system for septic shock in patients with digestive tract perforation: a retrospective cohort study. Front Med. 2022;9:976963. doi:10.3389/fmed.2022.976963
31. Ye-Ting Z, Dao-Ming T. Systemic Inflammatory Response Syndrome (SIRS) and the pattern and risk of sepsis following gastrointestinal perforation. Med Sci Monit. 2018;24:3888–3894. doi:10.12659/MSM.907922
32. Shimoyama Y, Umegaki O, Agui T, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are superior to other inflammation-based prognostic scores in predicting the mortality of patients with gastrointestinal perforation. JA Clin Rep. 2017;3(1):49. doi:10.1186/s40981-017-0118-1
33. Li T, Qi M, Dong G, et al. Clinical value of prognostic nutritional index in prediction of the presence and severity of neonatal sepsis. J Inflamm Res. 2021;14:7181–7190. doi:10.2147/JIR.S343992
34. Pricop M, Ancusa O, Talpos S, et al. The predictive value of systemic immune-inflammation index and symptom severity score for sepsis and systemic inflammatory response syndrome in odontogenic infections. J Pers Med. 2022;12(12):2026. doi:10.3390/jpm12122026
35. Ma K, Zhang Y, Hao J, et al. Correlation analysis of systemic immune inflammatory index, Serum IL-35 and HMGB-1 with the severity and prognosis of sepsis. Pak J Med Sci. 2023;39(2):497–501. doi:10.12669/pjms.39.2.6651
36. Matsuoka T, Yamamoto R, Matsumura K, et al. Perioperative clinical parameters associated with short-term mortality after colorectal perforation. Eur J Trauma Emerg Surg. 2022;48(4):3017–3024. doi:10.1007/s00068-021-01719-8
37. Wang D, Hu X, Xiao L, et al. Prognostic Nutritional Index and Systemic immune-inflammation index predict the prognosis of patients with HCC. J Gastrointest Surg. 2021;25(2):421–427. doi:10.1007/s11605-019-04492-7
38. Xu H, Zheng X, Ai J, et al. Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: a systematic review and meta-analysis of 13,110 patients. Int Immunopharmacol. 2023;114:109496. doi:10.1016/j.intimp.2022.109496
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.