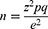Back to Journals » International Journal of General Medicine » Volume 15
Risk Factors for the Development of Tuberculosis Among HIV-Positive Adults Under Highly Active Antiretroviral Therapy at Government Hospitals in Amhara Region, Ethiopia
Authors Tegegne AS , Minwagaw MT
Received 21 January 2022
Accepted for publication 10 March 2022
Published 15 March 2022 Volume 2022:15 Pages 3031—3041
DOI https://doi.org/10.2147/IJGM.S358517
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Awoke Seyoum Tegegne,1 Molalign Tarekegn Minwagaw2
1Department of Statistics, Bahir Dar University, Bahir Dar, Ethiopia; 2Department of Public Health, Amhara Community Health Institution, Bahir Dar, Ethiopia
Correspondence: Awoke Seyoum Tegegne, Department of Statistics, Bahir Dar University, Po. Box 79, Bahir Dar, Ethiopia, Tel +251 918779451, Fax + 251 2205927, Email [email protected]
Background: Tuberculosis is one of the leading infectious diseases for people living with HIV. Therefore, the purpose of this study was to investigate factors affecting the development of TB among HIV-positive adults under treatment in government hospitals of Amhara Region, Ethiopia.
Methods: A hospital-based retrospective study design was conducted among 700 HIV-positive adults under HAART in 17 government hospitals in the Amhara region, Ethiopia.
Results: Age of the patients (AOR = 1.122, 95% CI:1.013, 2.234), baseline CD4 cell count (AOR = 0.888, 95% CI: 0.714, 0.945), patients living without their partner (AOR = 1.212, 95% CI: 1.051, 1.123), females under treatment (AOR = 0.786, 95% CI; 0.564, 0.845), non-opportunistic diseases (AOR = 0.865, 95% CI: 0.731, 0.938), patients not disclosed their HIV status (AOR = 1.241, 95% CI: 1.087, 2.341), rural patients (AOR = 1.135, 95% CI: 1.032, 1.453, patient with no education (AOR = 1.125, 95% CI: 1.056, 1.546), low adherence patients (AOR = 1.225, 95% CI: 1.191, 2.453), bedridden patients (AOR = 1.223, 95% CI: 1.131, 1.521), ambulatory patients (AOR = 1.156, 95% CI:1.091, 1.267), non-smoker patients (AOR = 0.854, 95% CI: 0.686, 0.935) significantly affected on the variable of interest. Similarly, alcohol intake, drug toxicity and baseline clinical WHO stages significantly affected for the development of tuberculosis in HIV-positive patients under treatment.
Conclusion: In this study, baseline CD4 cell count, female patients, non-opportunistic diseases, and non-smoking status were negatively associated with the development of TB, whereas age of patients, living without partners, patients with no education, patients with low adherence, bedridden and ambulatory patients were positively associated to the development of TB in HIV patients. The findings obtained in this study are important for both service providers and patients. More attention should be given to those positively associated variables to response variables. The regional health bureau should open TB/HIV co-infection subsections like ART sections in each hospital.
Keywords: TB development, HIV-positive people, HAART, binary logistic regression model
Introduction
Currently, about 1.1 million HIV-positive people have also developed Tuberculosis (TB).1 Of these, about 80% of them belong to Sub-Saharan Africa.1 Among the people living with the human immune deficiency virus (HIV), about 380,000 of them are dying because of the development of TB in addition to HIV.2 On the other hand, an estimated number of 36 million people lived with HIV globally in 2017.3 Approximately, one-third of these people are being co-infected with TB.3
Ethiopia is one of the Sub-Saharan African countries with the high development of TB among people living with HIV.4 This accounts for the country to be 10th in the world and 4th in Africa, after Nigeria, South Africa, and the Democratic Republic of Congo.4 About 85% of the country’s population is living in rural areas with low living standards, where TB is highly distributed.5
Amhara region, one of the regions in Ethiopia, has about 30% of the people living with HIV, followed by the Oromia region (26%).6 Research conducted previously declared that TB development is high in areas where there is a great prevalence of HIV but with poor adherence.7 Hence, people living with the human immune deficiency virus (PLWHIV) in the region are also extra vulnerable to TB development.8 HIV is the first and TB is the second important reason for death from infectious illness in the region.9
Even though studies have been conducted on the development of TB in HIV-infected people in Ethiopia, risk factors for the development of TB in HIV patients with comprehensive data at the regional level are not conducted in the study area.10 Studies including the large mass of data with many treatment sites may reveal evidence-based information as compared to data taken from a single treatment site.11
As far as our knowledge is concerned, there is a shortage of regional wide research done recently on factors affecting the development of TB among HIV-infected adults under HAART in the study area.12 Therefore, this research has been conducted with the objective to investigate factors affecting the development of TB among HIV patients under treatment followed in 17 government hospitals in the region. The current investigation also aimed to triangulate whether previously identified factors in developed countries also worked in the study area (low-income country). It is anticipated that the finding in this investigation will be used as the source of information for the knowledge that promotes HIV/TB program planners, decision-makers, and project implementers.
Methods and Materials
Study Population and Study Area
A hospital-based retrospective longitudinal design was conducted in 17 government hospitals on 700 randomly selected HIV-positive people who had started their treatment in the Amhara region, Ethiopia. The study was conducted in the period between Oct. 2018 and June 2021. The selection frame comprised 6500 HIV-positive people whose follow-ups were at each of the 17 government hospitals after the initiation of HAART.
Inclusion and Exclusion Criteria
All HIV-infected adults (age > 15 years) who enrolled for HAART in the 1st five months of 2018, who initiated their treatment for at least 6 months after the initiation of HAART, having a full record of clinical and socio-demographic variables were included in this study. On the other hand, those patients under 15 years of age, patients with no full recorded information were excluded in the current study.
Variables Under Study
The variable of interest under the current investigation was the development/occurrence of TB among HIV-positive adults under HAART. The response variable is dichotomous in nature, whether HIV- positive adults develop HIV/TB co-infection or not during their treatment period. The predictor variables under current investigation were the age of patients in years, gender (male, female), the status of employment (employed, not employed), educational status (educated, non-educated), marital status (living with partners, living with partners), WHO clinical stages (stage I, stage II, Stage III, Stage IV), baseline CD4 count, functional status (working, bedridden, ambulatory), smoking status (yes, no), Alcohol intake (yes, no), adherence to medication status (adherent, non-adherent), disclosure of the disease status to people living with them (yes, no), opportunistic with other diseases (yes, no), drug toxicity (yes, no) and the existence of mental depression during initiation of drugs (yes, no). WHO clinical stages stand for the status of the viruses. It can be Stage I, Stage II, Stage III, and Stage IV. Stage IV is the worst stage in which more attention should be given at this stage. Hence, the number of viruses at Stage IV is by far more than that of Stage I and stage II.
Sample Size Estimation
The sample size was determined using Cochran’s formula, considering 95% confidence level, 5% error tolerance for the proportion of TB development.13 The formula used in this regard was
where n is the required sample size in the whole target population, z is the selected critical value of the desired confidence level, p is the estimated proportion of an attribute that is presented in the population, q= 1-p, and e is the desired level of precision. After calculation of the sample size for the whole population, a proportional random sampling technique was used in the demonstration of random samples from the study population in each of the governmental hospitals in the region.
Samples were randomly selected considering the charts in each hospital belonging to each patient. The study considered all HIV-infected adults fulfilled inclusion criteria, irrespective of their behavioral group in the study time. Hence, a total of 700 randomly selected patients were considered for this investigation.
Data Collection Procedures
The health staff who worked in the ART section of each of the 17 hospitals were randomly selected to collect related information from February 2021 to June 2021. Variables included in the current investigation were clearly defined and given to data collectors.
Molecular Tests of Active TB
Molecular tests were used for TB diagnosis/tests in patients’ follow-up time of their medication adherence. Molecular tests have the potential to reach in tests of TB who are undiagnosed or not reported each visiting time and to improve the quality of care among TB patients receive by providing accurate, quick results, including rapid drug-susceptibility testing. Currently, a wide array of molecular tests for TB detection is being developed and evaluated, and while some tests are intended for reference laboratory use, others are being aimed at the point-of-care and peripheral health care settings.13 Hence, an emergence of molecular tests was designed, manufactured, and rolled out in the current investigation in tests of TB.
During their follow-ups, when patients came to hospitals to take pills, the clinical treatment outcomes like CD4 cell count, viral suppression, WHO stages, weights of patients, and HIV/TB co-infection were tested for each patient at every visiting time. Similarly, TB status was also tested/detected using molecular tests. This was done by the health staff (laboratory technicians). All the recorded data on HIV/AIDS patients between October 2018 and May 2021 (a total of 18 visits or assessments) were taken into consideration during data analysis. Hence, laboratory molecular tests, supplemental forms, TB drugs record forms, and patients’ cards were examined during data collection. The quality of data was pre-tested using a standard data collection tool and trained data collectors. Two experts were assigned for monitoring and supervise all activities related to data collection.
Data Analysis
SAS software version 9.4 was used for data analysis. Assessment of normality as well the existence of outliers and missingness of observation were conducted using descriptive Statistics. A multi covariate regression model was used by considering all variables with a P-value less than 0.25 in the bivariate analysis. Then, the binary logistic regression model was used to investigate factors affecting on the development of TB in HIV-positive people. The crude odds ratio (COR) and adjusted odds ratio (AOR) and its 95% level of confidence were computed in the data analysis.
Result
Baseline Characteristics of Participants
Out of a total of 700 HIV-positive people, 53.6% of them were males, 54.6% of them were living without their partners, 68.6% of them were educated, 63.1% were virally unsuppressed, and 77.3% of them were urban residents. The majority of them (81.4%) were at working status.
Among participants included in this investigation, about 32% of them did not disclose their HIV status to family members. In this investigation, about 35%, 33%, and 16.9% were at WHO clinical stages IV, III, and II respectively, and about 6% of the patients were HIV/TB co-infected at the enrolment stage (baseline HIV/TB co-infection). About 2.4%, 3.9%, and 4% of the patients were alcohol consumers, smokers, and opportunistic with other diseases respectively. The baseline characteristics of the participants on categorical variables are indicated in Table 1.
 |
Table 1 Baseline Socio-Demographic, Economic and Clinical Variables (n=700) |
Similar to the categorical variables, continuous variables were also summarized in this investigation. In this regard, the average (median) weight of the patients was 58kg (IQR: 52, 64), and the average age in years of all patients was 35.67 years (st.dev= 10.7 years). The average (median) baseline CD4 cell count for all patients was 134 cells /mm3 (IQR: 113,180).
Clinical Variables During HAART
The clinical outcome variables during treatment (follow-ups) were also recorded at each visiting time. The clinical outcome variables recorded at the end of the study time are indicated in Table 2. As it is indicated in Table 2, about 67.3% of the patients were virally unsuppressed, 77.3% of the patients were at working status, 60.7% of the patients under treatment were non-adherent, 23% of the patients did not disclose to people living with them, 56% of them faced opportunistic with other infectious diseases and 55.5% of the patients faced drug toxicity. Among the participants under this investigation, about 39.6% of the HIV patients developed HIV/TB co-infection at the end of the study period. Hence, the baseline HIV/TB co-infection (6%) was increased to be 39.6% at the last visits (end of the study period). Different factors played a significant role in the increase of HIV/TB co-infection and these factors are discussed in Table 3.
 |
Table 2 Clinical Outcome Variables After HAART (End of Study Period) |
 |
Table 3 Multivariate Data Analysis for TB/HIV Co-Infection |
Among those patients who disclosed their disease status, a considerable number of them (65%) declared social support was given to them by families and communities around them. Similarly, an incidence of mental depression among the participants was also inventoried using Beck’s depression inventory scale at each visit and the result showed that 27 (3.7%) of them were mentally depressed.
The nature of the missingness pattern in the current investigation was tested using a logistic regression model which is known to be monotone (dropouts). The pattern indicates that there were no missing observations in the first two visits and the number of dropouts increased linearly as follow-up times/visits increased. The result, in this regard, revealed that dropouts were not affected by the previous outcomes (β = 0.5017, p = 0.862). Hence, the missingness pattern was Missed Completely at Random (MCAR).
In data analysis, missed values were handled using multiple imputation techniques. The association between covariates and the variable of interest was assessed using the Chi-square test of association.
The potential predictor variables from univariate analysis were selected using stepwise selection criteria for multivariate analysis considering p-values less than 0.25 for variables to be significant. The result of multivariate data analysis for this investigation was indicated in Table 3. The significant predictors of the variable of interest under multivariate analysis are interpreted as follows.
Age had a significant effect on the development of TB among HIV-infected patients. As age enlarged by one year, the average odds of TB development was also increased by 12.2%, keeping the other covariates constant (AOR= 1.122, 95% CI: 1.013, 2.234, p-value< 0.001).
As, baseline CD4 cell count of HIV-positive people rise up by one cell/mm3, the expected/average odds of developing TB on HIV-positive people was decreased by 11.2%, considering the other covariates the same (AOR= 0.888, 95% CI: 0.714, 0.945, p-value< 0.001).
Marital status had a significant effect on the development of TB in HIV-positive patients. Hence, the expected odds of developing TB for HIV patients living without partners was increased by 21.2% than patients living with partners, given the other covariates constant (AOR=1.212, 95% CI: 1.051, 1.323, p-value= 0.021).
The sex of patients also significantly affected the variable of interest. Comparing female patients with males, the expected odds of developing TB for female HIV-patients was decreased by 21.4% as compared to males (AOR= 0.786, 95% CI: 0.564, 0.845, p-value= 0.021), keeping the others constant.
Opportunistic with other diseases significantly affected the development of HIV/TB co-infection status. The expected odds of developing TB for patients not opportunistic with other diseases was reduced by 13.5% than patients who were unscrupulous with other diseases, keeping the other covariates constant (AOR= 0.865, 95% CI: 0.731, 0.938, p-value= 0.031).
The expected odds of developing TB for patients who did not disclose their disease status to people living with them was increased by 24% as compared to patients who disclosed their disease status (AOR=1.241, 95% CI: 1.087, 1.341, p-value= 0.002).
The expected odds of developing TB for HIV-patients living in rural areas was increased by 13.5% as compared to urban HIV patients, keeping the other variables constant (AOR=1.135, 95% CI: 1.056, 1.246, p-value= 0.048).
Education significantly affected the variable of interest under investigation. The expected odds of developing TB for non-educated patients was increased by 12.5% as compared to educated patients, keeping the other covariates constant (AOR=1.125, 95% CI: 1.056, 1.546, p-value= 0.012).
Medication adherence played a significant for the development of TB in HIV-positive adults. Hence, the expected odds of developing TB for medication non-adherent patients was increased by 22.5% as compared to medication adherent patients (AOR=1.225, 95% CI: 1.191, 2.453, p-value= 0.014).
The functional status of HIV patients played a significant contribution to the possibility of developing TB in HIV-positive people. Hence, the expected odds of developing TB for bedridden functional status of HIV patients was increased by 22.3% as compared to working status, keeping the other variables constant (AOR= 1.223, 95% CI: 1.131, 1.521, p-value= 0.023). Similarly, the expected odds of developing TB for ambulatory functional status of HIV patients was increased by 15.6% as compared to working status, keeping the other variables constant (AOR= 1.156, 95% CI: 1.091, 1.267, p-value= 0.001).
The expected odds of developing TB for non-smoker HIV patients was reduced by 14.6% than smokers, considering the others the same (AOR= 0.854, 95% CI: 0.686, 0.935, p-value= 0.001). Similarly, the expected odds of developing TB for patients who did not drink alcohol was reduced by 12.6% than patients who drank alcohol, considering the others the same (AOR= 0.874, 95% CI: 0.735, 0.972, p-value= 0.002).
Similar to the above significant variables, WHO stages and drug toxicity also affected significantly for the development of TB on HIV-positive patients in this investigation.
Discussions
As the age of patients increased, the possibility of being HIV/TB co-infection for HIV patients is also increased. The potential reason for this result might be the fact that aged people are less likely to be medication adherent and this leads to drug-resistant co-infection by the other diseases and TB is one of such occasions. This is supported by previous research.14 The increasing stage of the age of patients also leads to decreasing rate of CD4 cell count which further leads to being exposed to other infectious diseases. The result obtained in this study is also supported by one of the previous researches.15
Patients who started their medication with a large number of baseline CD4 cell count are less likely to be exposed to other infectious diseases, because of the reason that they may have a large number of white blood cells, and this further leads to the destruction of the viral loads. Hence, patients who started their treatment with a large number of CD4 cell counts are less likely to be exposed to TB in HIV-positive people. As CD4 cell count reduced, the body’s defense device for various opportunistic infections also reduced. Another previous study states that patients with at most CD4 cell count of 200 cells/mL were twice more likely to develop TB than those patients who started their treatment with at least 200 cells/mL.16 The result in this regard is also supported by previous research17 and contradicted with other research.17 The possible reason for contradicted results may be the sample size, study area, the medication adherence status, and the other unexplained factors. Hence, this result needs further investigation.
Marital status significantly affected the development of TB status in HIV-positive people. HIV patients living with their partners are more likely to be medication adherent and this leads to less likelihood of being exposed to other co-infections. Patients living together might help each other to take medication on time and this helps to take the prescribed medication on time. This further leads to be less likelihood for TB development in HIV-positive adults. This result is similar to another research17 and contradicted with other previously conducted studies.18 The contradicted result states that patients living with their partner make sexual intercourse frequently and this leads the patient to be exposed to another infectious disease. In this regard, the result needs further investigation.
The sex of patients significantly affected the development of TB in HIV-positive people in this investigation. Hence, female HIV patients are less likely to be exposed to the development of TB. This result is supported by previously conducted research.18 The potential reason might be that female HIV patients are more likely to be medication adherents because of their experience on pills taken for family planning as compared to males.
HIV-positive patients with other opportunistic diseases accelerate the development of TB as compared to HIV patients with no other opportunistic disease. Dual infection of opportunistic disease with TB has an important impact on HIV disease. Opportunistic disease accelerates the progression of HIV/TB co-infection by increasing viral replication and reducing CD4 cell counts further. This finding is similar to another research.19
Patients disclosing their disease to their family members may help to be medication adherent (to take pills on time). Disclosed the disease also important to take pills at a prescribed time without fear of the others living together. This further leads to the patients not being exposed to the other infectious diseases. Hence, patients who disclosed their HIV disease status are less likely to be HIV/TB co-infection. The result is similar to other previous investigations.20
The residence area of patients is also played significant role for the development of TB in HIV patients. Most of the time, rural patients started their HIV medication after the destruction of CD4 cell count and they cannot be easily recovered their HIV status; rather they are exposed to other infectious diseases. One such infectious disease is TB and this further leads to HIV/TB co-infection. This result is similar to findings obtained from previously conducted research.21
The current study revealed that education is one of the predictors for the development of TB in HIV patients. Educated HIV patients might have better knowledge on how HIV patients have long lived with the virus and they have good knowledge about how to use medication. Hence, educated HIV patients are less likely to be exposed to the development of TB as compared to non-educated patients. The current result with regard is the same as another study done in South-west Ethiopia.21 However, it is contradicted by the other study done in Eastern Ethiopia.22 This could need further investigation in the future.
Non-adherence to HAART poses a challenge to TB treatment since it increases the risk of drug resistance, death, relapse, and prolonged infectiousness. The result is consistent with another research.23
Patients who are ambulatory at the commencement of HAART are more likely to develop TB as compared to those who are at working status during the initial stages of HAART. Hence, this study indicated that bedridden and ambulatory status patients were more exposed to the development of TB as compared to working status. This result is also supported by the previous study.23
Smoking cigarettes and drinking alcohol significantly affected the development of TB status in this investigation. Research conducted previously obtained the same result and states that cigarette smoking and alcohol consumption are the risk factors for the development of TB among HIV patients.23
Drug toxicity has a significant effect on the development of TB among HIV patients. Patients who faced drug toxicity may not have progressive treatment outcomes or improvements due to treatment. This further leads for the patients to be exposed to other drug-resistant infectious diseases like TB.24 The extent of drug toxicity significantly differs between developed and developing countries due to various reasons. One of the reasons for drug toxicities related to HAART in the developing country, in sub-Saharan Africa in particular, is the regimens contain older and more toxic agents like Stavudine (d4t), Zidovudine (AZT), and Nevirapine (NVP).24 A patient who faced drug toxicity may be forced to stop medication adherence and this further leads to exposure to other infectious diseases like TB.
The other significant variable under current investigation was the status of the HIV WHO clinical stages. Patients at the 1st and 2nd WHO clinical stages are less likely to develop TB as compared with those patients at the 4th WHO clinical stages. Consistent findings have been declared in previous research.25 It is known that the development of TB in HIV-positive people is one of the indications for the stage of HIV to be the worst (4th WHO clinical stage).26
Conclusions
Among the predictors included in the current investigation, age of patients, baseline CD4 cell count, marital status, sex of patients, the existence of other opportunistic diseases, disclosure status of HIV, residence area, level of education, level of adherence, functional status of the patients, smoking and drinking status and HIV WHO clinical stages were significantly affected for the development of TB in HIV-patients. This study revealed that CD4 cell count was negatively associated with the development of TB in HIV-positive adults under treatment. Similarly, the level of medication adherence and disclosing the HIV disease statuses were negatively associated with the development of TB. However, the age of patients, alcohol intake, smoking cigarettes, and the existence of other opportunistic diseases were positively associated with the development of TB in HIV-positive people. Under current investigation, the HIV/TB co-infection was increased from 6% at the baseline to 39.6% at the last visiting time (at the end of the study period).
As a recommendation, counseling and guidance shall be delivered to patients with low adherence status, aged patients, patients who came from rural areas, HIV-positive people with other opportunistic diseases, and non-educated patients. A counseling service should be given to smokers and patients who are taking alcohol. More attention should be also given to patients with WHO clinical stage IV, patients not disclosed the disease status, and ambulatory and bedridden functional statuses.
This study was not without limitations; secondary data obtained from charts of patients in each hospital had limited variables there. Including more variables like diabetes, rheumatoid diseases, environmental factors, and concomitant immunosuppressive therapy may give additional information about HIV/TB co-infection. Hence, research can be conducted in the future, including such variables, considering this as one research gap.
Data Sharing Statement
The data used for the current investigation is available within the corresponding author.
Ethical Approval and Consent to Participate
The data used in the current investigation were secondary and there was no chance of obtaining respondents to obtain consent to participate form from participants, because this fact, informed consent has been waived. To secure the confidentiality and compliance with the Declaration of Helsinki within the manuscript of patient-related data, the name of patients was not given to investigators, rather id number and important variables related to the current investigation were given to researchers. The waiver was done by Bahir Dar University Ethical approval committee, Ethiopia with reference number: RCS/1412/2021. Hence, the Bahir Dar University Ethical Committee approved and waived this study.
Consent for Publication
The manuscript submitted to this journal is not published anywhere or didn’t consider for publication by any other journal.
Acknowledgments
All the health staff at each government hospital is gratefully acknowledged for the data they supplied for our health research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared that there are no conflicts of interest in this work.
References
1. Moghaddam HT, Moghadam ZE, Khademi G, et al. Tuberculosis: past, present and future. Int J Pediatr. 2016;4(1):1243.
2. Trinh Q, Nguyen HL, Nguyen VN, et al. Tuberculosis and HIV co-infection—focus on the Asia-Pacific region. Int J Infect Dis. 2015;32:170–178. doi:10.1016/j.ijid.2014.11.023
3. Platt L, French CE, McGowan CR, et al. Prevalence and burden of HBV co‐infection among people living with HIV: a global systematic review and meta‐analysis. J Viral Hepat. 2020;27(3):294–315. doi:10.1111/jvh.13217
4. Mohammed H, Assefa N, Mengistie B. Prevalence of extrapulmonary tuberculosis among people living with HIV/AIDS in sub-Saharan Africa: a systemic review and meta-analysis. HIV/AIDS. 2018;10:225.
5. Asgedom SW, Teweldemedhin M, Gebreyesus H. Prevalence of multidrug-resistant tuberculosis and associated factors in Ethiopia: a systematic review. J Pathog. 2018;2018:1–8. doi:10.1155/2018/7104921
6. Gelaw YA, Assefa Y, Magalhaes RJ, et al. TB and HIV epidemiology and collaborative service: evidence from Ethiopia, 2011–2015. HIV/AIDS. 2020;12:839.
7. Martinson NA, Hoffmann CJ, Chaisson RE. Epidemiology of tuberculosis and HIV: recent advances in understanding and responses. Proc Am Thorac Soc. 2011;8(3):288–293. doi:10.1513/pats.201010-064WR
8. Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8(1):e1000391. doi:10.1371/journal.pmed.1000391
9. Yirdaw KD, Jerene D, Gashu Z, et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLoS One. 2014;9(8):e104557. doi:10.1371/journal.pone.0104557
10. Tesfaye B, Alebel A, Gebrie A, et al. The twin epidemics: prevalence of TB/HIV co-infection and its associated factors in Ethiopia; A systematic review and meta-analysis. PLoS One. 2018;13(10):e0203986. doi:10.1371/journal.pone.0203986
11. Clark DM. Realizing the mass public benefit of evidence-based psychological therapies: the IAPT program. Annu Rev Clin Psychol. 2018;14:159–183. doi:10.1146/annurev-clinpsy-050817-084833
12. GebreEyesus F, Assefa Y, Magalhaes RJ, et al. Levels of adherence and associated factors among children on ART over time in Northwest, Ethiopia: evidence from a multicenter follow-up study. HIV/AIDS. 2021;13:829.
13. Gezae KE, Abebe HT, Gebretsadik LG. Incidence and predictors of LTFU among adults with TB/HIV co-infection in two governmental hospitals, Mekelle, Ethiopia, 2009–2016: survival model approach. BMC Infect Dis. 2019;19(1):1–9. doi:10.1186/s12879-019-3756-2
14. Naidoo P, Peltzer K, Louw J, et al. Predictors of tuberculosis (TB) and antiretroviral (ARV) medication non-adherence in public primary care patients in South Africa: a cross sectional study. BMC Public Health. 2013;13(1):1–10. doi:10.1186/1471-2458-13-396
15. Rogerson SJ, Wijesinghe RS, Meshnick SR. Host immunity as a determinant of treatment outcome in Plasmodium falciparum malaria. Lancet Infect Dis. 2010;10(1):51–59. doi:10.1016/S1473-3099(09)70322-6
16. Okonko IO, Ejike IU, Innocent-Adiele C, et al. HIV coinfections with tuberculosis among HIV-1 infected individuals in old Cross River State, Nigeria. J Immunoassay Immunochem. 2020;41(3):245–256. doi:10.1080/15321819.2020.1717527
17. Nagu TJ, Aboud S, Mwiru R, et al. Tuberculosis associated mortality in a prospective cohort in Sub Saharan Africa: association with HIV and antiretroviral therapy. Int J Infect Dis. 2017;56:39–44. doi:10.1016/j.ijid.2017.01.023
18. Koneru S, Kocharla L, Higgins GC, et al. Adherence to medications in systemic lupus erythematosus. J Clin Rheumatol. 2008;14(4):195–201.
19. Ugochukwu EF. HIV/TB co-infection in Nigerian children. Niger Med J. 2010;51(3):120.
20. Gao L, Zhou F, Li X, et al. HIV/TB co-infection in mainland China: a meta-analysis. PLoS One. 2010;5(5):e10736. doi:10.1371/journal.pone.0010736
21. Fenta A, Demeke G, Bitew A, et al. Prevalence and associated factors of TB co-morbidity among HIV sero-positive individuals in Shegaw Motta District Hospital, Ethiopia. Int J Gen Med. 2020;13:1529. doi:10.2147/IJGM.S278758
22. Ranganath T, Kishore SG, Reddy R, et al. Risk factors for non-adherence among people with HIV-associated TB in Karnataka, India: a case–control study. Indian J Tuberc. 2022;69:65–72.
23. Wessels J. Nutritional status of patients with tuberculosis and TB/HIV co-infection at Standerton TB specialised hospital, Mpumalanga. University of the Free State; 2017.
24. Shankar EM, Vignesh R, Ellegård R, et al. HIV- Mycobacterium tuberculosis co-infection: a ‘danger-couple model’ of disease pathogenesis. Pathog Dis. 2014;70(2):110–118. doi:10.1111/2049-632X.12108
25. Buck W, Olson D, Kabue MM, et al. Risk factors for mortality in Malawian children with human immunodeficiency virus and tuberculosis co-infection. Int J Tuberc Lung Dis. 2013;17(11):1389–1395. doi:10.5588/ijtld.13.0030
26. Bayabil S, Seyoum A. Joint modeling in detecting predictors of CD4 cell count and status of tuberculosis among people living with HIV/AIDS under HAART at Felege Hiwot teaching and specialized Hospital, North-West Ethiopia. HIV/AIDS. 2021;13:527.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.