Back to Journals » Infection and Drug Resistance » Volume 15
Risk Factors for Radiological Progression Within Admissive One Week in the Hospitalized COVID-19 Omicron Variant-Infected Patients
Authors Zhu FF, Gu BB , Jin YJ, Yao L, Zhou L, Zou D, Ding J, Zhou T, Shen XH, Chen C
Received 4 September 2022
Accepted for publication 29 November 2022
Published 6 December 2022 Volume 2022:15 Pages 7127—7137
DOI https://doi.org/10.2147/IDR.S388696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Feng-Feng Zhu,1,* Bin-Bin Gu,1,* Yu-Jia Jin,2,* Lin Yao,3 Lin Zhou,3 Di Zou,1 Jian Ding,1 Teng Zhou,1 Xing-Hua Shen,1 Cheng Chen2
1Intensive Care Unit, The Fifth People’s Hospital of Suzhou, The Affiliated Infectious Disease Hospital of Soochow University, Suzhou, 215000, People’s Republic of China; 2Department of Respiratory and Critical Medicine, The First Affiliated Hospital of Soochow University, Suzhou, 215000, People’s Republic of China; 3Department of Pulmonary, The Fifth People’s Hospital of Suzhou, The Affiliated Infectious Disease Hospital of Soochow University, Suzhou, 215000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xing-Hua Shen, Soochow University Affiliated Infectious Disease Hospital, 10 Guangqian Road, Suzhou, 215000, People’s Republic of China, Tel +8613606217315, Email [email protected] Cheng Chen, The First Affiliated Hospital of Soochow University, 899 Pinghai Road, Suzhou, 215000, People’s Republic of China, Tel +8613771775292, Email [email protected]
Purpose: Recently, the SARS-CoV-2 Omicron variant was identified as responsible for a novel wave of COVID-19 worldwide. We perform a retrospective study to identify potential risk factors contributing to radiological progression in the COVID-19 patients due to the Omicron variant infection. These findings would provide guiding information for making clinical decisions that could improve the Omicron infection prognosis and reduce disease-related death.
Methods: This is a retrospective cohort study from a single center in China. According to the radiological change within admissive one week, enrolled cases were divided into two groups: the progressive (1w-PD) and the stable or improved disease (1w-non-PD). Separate analyses were performed on patients stratified into subgroups using the Mann–Whitney U-test, the Fisher exact test, or the Chi-squared test and a multivariable logistic regression analysis.
Results: Both the 1w-non-PD and 1w-PD cohorts displayed comparable asymptomatic infection, have similar underlying disease, impairment in respiratory function, coagulation dysfunction, tissue injury, SARS-CoV-2 viral load, and disease severity. However, the 1w-PD cohort was more inclined to cluster in populations presented with age between 41 and 65, higher CURB-65 scores, undetectable SARS-CoV-2 IgG, and lung affection. Based on the multiple logistic regression analysis, complicated bilateral and ground-glass opacities (GGOs) like pneumonia at admission were independent risk factors to radiological progression within admissive one week.
Conclusion: This study provided preliminary data regarding disease progression in Omicron-infected patients that indicated the development of pneumonia in the context of Omicron infection was worthy of potential risk factors.
Keywords: COVID-19, Omicron, pneumonia, radiology, risk
Introduction
Recently, the SARS-CoV-2 variant of concern, Omicron, was identified as responsible for a novel wave of COVID-19 worldwide.1 In particular, this variant is the most heavily mutated one among all the variants of concern (VOC) so far, and this paves the way for enhanced transmissibility and partial resistance to immunity induced by COVID-19 vaccines.2
Various concerns have been raised regarding the source of emergence, the effect of mutations in the response to vaccinations, the influence of mutations on the modulation of host immunity, the spreading potency, and lethality.3 As the number of infected and severe cases is across the globe, there is a pressing need to investigate the clinical, radiological and laboratory characteristics in Omicron-infected patients. More importantly, it is essential to identify the population at the highest risk of unfavorable outcomes if they were infected with Omicron.
To identify potential risk factors contributing to the progression of the COVID-19 disease due to the Omicron variant infection, we performed a retrospective study based on 146 Omicron patients in Suzhou, China. These findings would provide guiding information for making clinical decisions that could improve the Omicron infection prognosis and reduce disease-related death.
Methods
Patients
All of COVID-19-infected cases were enrolled from February 13 to March 10, 2022 who hospitalized at The Fifth People’s Hospital of Suzhou. Correspondingly, all patients had the SARS-CoV-2 Omicron Variant, which was confirmed by S gene target failure (SGTF). All cases were defined according to the diagnostic and treatment guideline for COVID-19 pneumonia issued by the National Health and Family Planning Commission of P.R. China (Version 8–9).
Data Collection
The data of all of the patients were collected from an electronic case report form and included the following: demographic characteristics (age and sex), comorbidities, clinical symptoms, laboratory tests (blood routine test, arterial blood gas analysis, and blood chemistry), severity of disease, SARS-CoV-2 viral load, images of the lung (chest CT), as well as the CURB-65 score.
Study Design
This was a retrospective case-control study. We tested whether the cases had increased severity in the chest CT within one week after admission, which was regarded as primary endpoint. According to the radiological change within admissive one week, they were divided into two groups: the progressive (1w-PD) and the stable or improved disease (1w-non-PD). Separate analyses were performed on patients stratified into these subgroups. The Ethics Committee of The Fifth People’s Hospital of Suzhou approved this study (2022–009).
Definition
The available chest CT images of each patient were reviewed by a senior radiologist blinded to the clinical data. The radiological progression of COVID-19 was defined as meeting any one or both of following features: 1) enlarger involvement; 2) new finding lung lesion. We also defined the interval from onset of diseases to the date of the first COVID-19 RNA-negative result before discharge as the Omicron variant RNA shedding duration.
Statistical Analysis
Data were described as the median (interquartile range, IQR) or number (%). Comparisons of the features between the different cohorts were performed using the Mann–Whitney U-test, the Fisher exact test, or the Chi-squared test to compare proportions. To identify risk factors, we performed a multivariable logistic regression analysis adjusted for baseline covariates. The receiver operating characteristic (ROC) curves were calculated to the value of the candidate variables to indicate the exacerbation of COVID-19. Statistical analyses were performed using SPSS, version 24.0 for Windows. The probabilities were two-tailed, and a two-tailed P value of <0.05 was considered significant.
Results
Overview in Omicron Infected-Patients
The chest CT scan was performed in all patients of the cohorts. Among the 146 patients, 16 did not receive repeated chest CTs within one week after admission. Of the remaining 130 cases, 17 patients (13.08%) were found worsen in the chest CT scan, and 113 (86.92%) achieved stable or improved chest CT scan within admissive one week (Figure 1). In a further investigation, these participants were stratified by the one-week progression (1w-PD cohort, n = 17) and the one-week non-progression (1w-non-PD cohort, n = 113).
 |
Figure 1 Flow chart detailing the selection of the patients in this study. |
In terms of treatment in window of investigation, all cases received the Chinese traditional medicine and medical surveillance. Low molecular weight heparin calcium (LMWHC) was administrated in 64.71% cases in 1w-PD and 13.27% cases in 1w-non-PD cohort, respectively. Thymosin was administrated in 52.94% cases in 1w-PD and 23.89% cases in 1w-non-PD cohort, respectively. Neutralization antibody was injected in 23.53% cases in 1w-PD and 14.16% cases in 1w-non-PD cohort, respectively. In addition, no cases received corticosteroid treatment, antibiotics, and anti-virus therapy.
Demographic Feature in Omicron Infected-Patients
As shown in Table 1, the proportion of males in the 1w-PD and the 1w-non-PD cohorts were comparable (52.94% vs 53.10%, respectively, P = 0.990). The median age of the 1w-PD cohort was 57.0 years old, which was older than that in the 1w-non-PD cohort (36.0 years old, P = 0.02). Precisely, of the patients in the 1w-PD vs the 1w-non-PD cohort, 5.88% vs 7.08% were ≤18 years old, 23.53% vs 59.29% were 19–40 years old, 47.06% vs 22.12% were 41–65 years old, and 25.53% vs 11.50% were >65 years old.
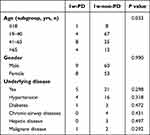 |
Table 1 Characteristics of the COVID-19 Subjects |
The history of underlying diseases was similar between the 1w-PD cohort and the 1w-non-PD cohort (29.41% vs 18.58%, P = 0.298). No significant difference in the history of hypertension, diabetes, chronic-airway diseases, hepatic disease, and malignant diseases between the two cohorts was observed (P > 0.05). No other self-reported diseases (including allergic rhinitis and autoimmune disease) were declared.
Clinical Manifestations in Omicron Infected-Patients
Upon admission, nearly half of the patients in both cohorts (52.94%, in 1w-PD cohort and 63.72% in 1w-non-PD cohort) were asymptomatic. In brief, there were no significant differences in fever, cough, dyspnea, myalgia, nasal congestion, and pharyngodynia between the two cohorts (35.29% vs 29.20%, 41.18% vs 33.63%, 0.00% vs 0.88%, 11.76% vs 11.50%, 0.00% vs 10.62%, and 23.53% vs 30.97%, P > 0.05 for each). Hence, we could not predict the disease progression based on the initial clinical manifestations.
Comparison of Biological Analyses in Both Cohorts
As shown in Table 2, the median levels of white blood cells, lymphocytes, and platelets in the 1w-PD cohort were similar to those of the 1w-non-PD cohort (P > 0.05 for each). In terms of organ function, the levels of alanine aminotransferase (ALT), total bilirubin (TBIL), blood urea nitrogen (BUN), creatinine (Cr), NT-pro-BNP and troponin I (TnI), and lactate dehydrogenase (LDH) were also comparable between the 1w-PD cohort and the 1w-non-PD cohort (P > 0.05, for each). Furthermore, comparable levels of PT, APTT, FDP, and AT-IIIA were observed in the 1w-PD cohort and the 1w-non-PD cohort (P > 0.05 for each). However, the serum hsCRP in the 1w-PD cohort was relatively higher than in the 1w-non-PD cohort (P = 0.010), suggesting that systematic inflammation was increased in the population of the 1w-PD cohort.
 |
Table 2 Laboratory Test Data |
Accordingly, as shown in Table 3, lymphopenia, thrombocytopenia, acute liver injury, acute cardiac injury, and coagulation were both observed in the 1w-PD cohort and the 1w-non-PD cohort, and they were similar to each other (P > 0.05 for each). Unexpectedly, more patients in the 1w-non-PD cohort suffered from acute kidney injury, compared to those in the 1w-PD cohort (P = 0.018).
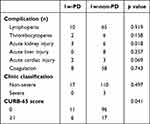 |
Table 3 Initial Disease Evaluations |
Distinct Disease Severity of Patients with Radiological Progression Within Admissive One Week
As shown in Table 3, at admission, 0 patient (0.00%) in the 1w-PD cohort and 3 patients (2.65%) in the 1w-non-PD cohort were categorized with severe pneumonia (P > 0.05). Although the CURB-65 score in the 1w-PD cohort was comparable to that of the 1w-non-PD cohort, more patients with admissive CURB-65 scores ≥1 were noticed in 1w-PD cohort (35.29% vs 15.04%, P = 0.041).
Vaccination, SARS-CoV-2 IgG, Viral Load in Omicron Infected-Patients Displaying Radiological Progression Within Admissive One Week
The SARS-CoV-2 IgG was analyzed among all of the Omicron-infected subjects (Table 4). In the 1w-PD cohort (n = 17), nine (52.94%) patients were not detected with serum SARS-CoV-2 IgG, and this was higher than that in 1w-non-PD cohort (28.32%, P = 0.042).
 |
Table 4 SARS-CoV-2 IgG and Viral Loads |
Table 4 also shows the SARS-CoV-2 viral load of all the Omicron-infected subjects in which two cohorts had comparable RT-PCR cycle threshold values (Ct value). This result suggested that the SARS-CoV-2 viral load could not be used to predict the disease progression of people who are hospitalized with Omicron. Consequently, radiological progression within admissive one week (1w-PD cohort) delayed viral shedding compared to that of 1w-non-PD cohort (13.06 d [7–19] vs 10.91 [2–22], P = 0.015).
Radiological Findings in Both Cohorts
As shown in Table 5, the lung opacities in the initial chest CTs were observed in 16 (16/17, 94.12%) and 13 (13/113, 11.50%) cases in the 1w-PD cohort and the 1w-non-PD cohort, respectively (P < 0.001). Furthermore, more patients in the 1w-PD cohort exhibited bilateral and multi-lobar distribution of lung opacities, which was significantly higher than that of the 1w-non-PD cohort (58.82% vs 7.96%, P < 0.001; 29.41% vs 3.54%, P = 0.002). Additionally, ground-glass opacity (GGO) was more common in the 1w-PD cohort than in the 1w-non-PD cohort (58.82% vs 7.96%, P < 0.001).
 |
Table 5 Initial Radiologic Findings and the One-Week Progression |
Risk Factors of Radiological Progression Within Admissive One Week in the Omicron Variant-Infected Patients
To explore the potential risk factors for radiological progression within admissive one week in all participants (Table 6), we first selected eight candidate variables based on the data above. We then placed them into the logistic regression model. According to the univariate analysis, Omicron-infected patients with ages ≥40 yrs (OR 3.627, 95% [CI] 1.196–10.996, P = 0.023), SARS-CoV-2 IgG ≤10 Au/mL (OR 0.351, 95% [CI] 0.125–0.990, P = 0.048), CURB-65 ≥1 scores (OR 3.080, 95% [CI] 1.005–9.445, P = 0.049), bilateral pneumonia (OR 16.508, 95% [CI] 5.063–53.821, P = 0.000), multi-lobar pneumonia (OR 11.354, 95% [CI] 2.680–48.096, P = 0.001), and GGO-pneumonia (OR 16.508, 95% [CI] 5.063–53.821, P = 0.000) were associated with a higher risk of radiological progression within admissive one week.
 |
Table 6 Univariate Analysis of the Risk Factors for Disease Progression in the Omicron Cohort |
Furthermore, according to the stepwise forward logistic regression model, two variables were identified as risk factors for radiological progression within admissive one week (Figure 2). In detail, by treating the GGO-pneumonia as a reference, it yielded an OR of 7.288 (95% CI, 1.677–31.670, P = 0.008). Furthermore, bilateral pneumonia was found to be the second most important risk factor, with an OR of 7.530 (95% CI, 1.402–40.426, P = 0.019).
Finally, the initial chest CT finding indicating progression within admissive one week was analyzed using the ROC curve. As shown in Figure 3, sensitivity and specificity for the “GGO-pneumonia” were found to be 92.0% and 58.8%, respectively, and the area under the curve (AUC) was 0.754. The sensitivity and specificity of the “bilateral pneumonia” were 92.9% and 58.8%, respectively, and the AUC was 0.759. Interestingly, this was indicated that the combination of the “GGO-pneumonia” and the “bilateral pneumonia” showed better performance for the clinical prediction of radiological progression within admissive one week, when the sensitivity and specificity were found to be 90.3% and 82.4%, and the AUC was 0.858.
Discussion
In previous studies, a different pattern of characteristics and outcomes in patients hospitalized with COVID-19 was observed in the recent Omicron wave compared to earlier waves, with a decrease in the severity and mortality.1 In this study, the total exacerbation rate for Omicron-infected patients was up to 13.08%. Therefore, it is still essential to ascertain the predictors that might help clinicians to identify the risky patients with Omicron quickly at an early stage. We systematically analyzed the clinical characteristics, disease progression and risk factors of Omicron-infected patients from a single center in China.
First, the middle-aged adults observed in Omicron-infected patients may have contributed to the progression of the disease. This risk factor has been identified in previous studies,4,5 while it has not been reported so far that the Omicron-infected patients aged 41–65 were prone to progress. Sometimes, it has also been suggested that patients with well-controlled comorbidities have a comparable risk of disease progression as those with health conditions.6
In addition, several previous studies have shown that patients with Omicron infection were often accompanied by little clinical presentation, and the proportion of asymptomatic cases in the Omicron wave was higher than that in the previous wave.1,7 Unexpectedly, our study demonstrated that a comparable proportion of asymptomatic cases was observed between the 1w-PD cohort and the 1w-non-PD cohort, and this did not elucidate the role of initial clinical manifestations in the prediction of the prognosis of Omicron-infected patients. In addition, the transmission from asymptomatic cases was also an important consideration for separating these patients from the others.
Similarly, although lymphopenia and the elevation of neutrophils were widespread manifestations in patients with COVID-19,4 we did not establish lymphopenia and thrombocytopenia as independent risk factors for exacerbation in Omicron-infected patients. We then introduced the hsCRP to a statistical analysis. As a biomarker, although the hsCRP might be associated with the exacerbation of Omicron infection in the Chi-squared test, the multivariate analysis indicated that it was not an independent early predictor for Omicron infection acceleration.
Several previous studies have shown that in addition to being capable of causing pneumonia, COVID-19 may also cause damage to other organs and systems such as the heart, liver, blood coagulation system, and kidneys.4,8,9 In the present study, we reported that similar extra-pulmonary complications were observed in the 1w-PD cohort and 1w-non-PD cohort, but acute kidney injury seemed to be associated with the exacerbation in Omicron-infected patients during hospitalization.
In particular, the correlation of the Ct value with the viral load has been established in prior studies.10,11 Liu et al reported that higher viral loads might be associated with severe clinical outcomes.12 There were observations that children with symptomatic disease had lower Ct values from respiratory specimens, but this result was in contrast to recently published data from Hurst et al.13–15 However, our data showed that no significant difference in the viral load was seen between the 1w-PD cohort and the 1w-non-PD cohort. An increased viral load in addition to higher Ct values may not account for Omicron infection acceleration. It is possible that while the Ct value was lower for a few patients, a robust immune response and decreased pathogenicity spared them from more progressive disease manifestations.
Alternatively, considering that the vaccination strategy might be of benefit for the recovery of patients with COVID-19 and reduce the transmission probability of SARS-CoV-2,16,17 we reviewed the COVID-19 vaccination histories in patient EHRs. It was noted that two cohorts displayed the diversity of vaccine and immunization. Due to the limitation of the single-center, a small-sample, and a retrospective study, our data could not update knowledge regarding the level of protection of the current vaccination for Omicron infection. Although it has been reported that the Omicron variant can escape immune surveillance,2,16 we found that more cases of the 1w-non-PD cohort had detectable SARS-CoV-2 IgG. Therefore, it was hypothesized that current COVID-19 vaccines would protect in reducing disease progression in vaccinated individuals. The potential impact of the COVID-19 vaccine is still being analyzed against this new variant to further investigate whether the antigen epitopes recognized by the vaccine-induced T cells are shifted.18
Finally, we hypothesized that the initial lung change may be the forerunner of later progression that would further degenerate into progressive pneumonia. This assumption was consistent with the previous theory that a gradual increase in the density of lesions represented the development of COVID-19 pneumonia from the early stage to the peak stage.19 As supported, the initial “bilateral pneumonia” and “GGO-pneumonia” were two independent early predictors for Omicron variant disease progression, and the combination of “GGO-pneumonia” and “bilateral pneumonia” showed the better performance for the clinical prediction of the one-week progression. This result suggested that the differences in outcomes between the 1w-PD cohort and the 1w-non-PD cohort were largely attributed to differences in the initial lung change. This may have important implications in response to new policy interventions.
Conclusions
Our study provided a preliminary understanding of the clinical course in patients with Omicron variant infection. Comparably, the impairment in respiratory function, coagulation dysfunction, and tissue injury were not associated with disease progression. Instead, increased age and initial lung change might have contributed to acquiring more risk factors for developing progressive pneumonia. These findings can help us assess the status of the disease in Omicron patients during hospitalization and provide direct advice for the strategies of clinical interventions. In the future, there is much work yet to be performed to understand these changes entirely. We also need to determine if the results are affected by preexisting acquired or natural immunity in the context of vaccination and the Omicron wave.
Abbreviations
ALT, alanine aminotransferase; AUC, area under the curve; BUN, blood urea nitrogen; CI, confidence interval; COVID-19, Coronavirus Disease 2019; Cr, creatinine; CT, computed tomography; Ct value, cycle threshold values; EHRs, electronic health records; GGOs, ground-glass opacities; hsCRP, high-sensitivity C-reactive protein; OR, odds ratio; RT-PCR, reverse transcription polymerase chain reaction; SOFA, sequential organ failure assessment; TBIL, total bilirubin; TnI, troponin I; VOC, variants of concern.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Consent for Publication
This study was approved by the Ethics Committee of The Fifth People’s Hospital of Suzhou (2022–009) and was conducted in compliance with ethical, legal, and regulatory norms. The consent was waived by the Ethics Committee of The Fifth People’s Hospital of Suzhou due to the retrospective nature of the review. Privacy of the participants was protected and the data was anonymized or maintained with confidentiality. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We thank the patients, the nurses, and the clinical staff who are providing care for the patients and staff at the local and state health departments. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Technology Research and Development Funding of Suzhou City SKY2021034 (to CC), the Science and Technology Project of Suzhou City Health Bureau LCZX201918 (to XHS), the Key Project of Health Care Commission of Jiangsu Province ZD2022041 (to XHS) and the Youth Science and Technology Project of Suzhou City KJXW2022049 (to BBG). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maslo C, Friedland R, Toubkin M, et al. Characteristics and outcomes of hospitalized patients in South Africa during the covid-19 omicron wave compared with previous waves. JAMA. 2022;327(6):583–584. doi:10.1001/jama.2021.24868
2. Araf Y, Akter F, Tang YD, et al. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022;94(5):1825–1832. doi:10.1002/jmv.27588
3. Kandeel M, Mohamed MEM, Abd El-Lateef HM, et al. Omicron variant genome evolution and phylogenetics. J Med Virol. 2022;94(4):1627–1632. doi:10.1002/jmv.27515
4. Hu H, Du H, Li J, et al. Early prediction and identification for severe patients during the pandemic of COVID-19: a severe COVID-19 risk model constructed by multivariate logistic regression analysis. J Glob Health. 2020;10(2):020510. doi:10.7189/jogh.10.020510
5. Fan T, Hao B, Yang S, et al. Nomogram for predicting COVID-19 disease progression based on single-center data: observational study and model development. JMIR Med Inform. 2020;8(9):e19588. doi:10.2196/19588
6. Liu W, Yang C, Liao YG, et al. Risk factors for COVID-19 progression and mortality in hospitalized patients without pre-existing comorbidities. J Infect Public Health. 2022;15(1):13–20. doi:10.1016/j.jiph.2021.11.012
7. Wang L, Berger NA, Kaelber DC, et al. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of Omicron. medRxiv. 2022. doi:10.1101/2021.12.30.21268495
8. Bhatt PJ, Shiau S, Brunetti L, et al. Risk factors and outcomes of hospitalized patients with severe coronavirus disease 2019 (COVID-19) and secondary bloodstream infections: a multicenter case-control study. Clin Infect Dis. 2021;72(12):e995–e1003. doi:10.1093/cid/ciaa1748
9. Tang G, Luo Y, Lu F, et al. Prediction of Sepsis in COVID-19 Using Laboratory Indicators. Front Cell Infect Microbiol. 2021;10:586054. doi:10.3389/fcimb.2020.586054
10. Ford L, Lee C, Pray IW, et al. Epidemiologic characteristics associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen-based test results, real-time reverse transcription polymerase chain reaction (rRT-PCR) cycle threshold values, subgenomic RNA, and viral culture results from university testing. Clin Infect Dis. 2021;73(6):e1348–e1355. doi:10.1093/cid/ciab303
11. Westblade LF, Brar G, Pinheiro LC, et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell. 2020;38(5):661–671. doi:10.1016/j.ccell.2020.09.007
12. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi:10.1016/S1473-3099(20)30232-2
13. Strutner J, Ramchandar N, Dubey S, et al. Comparison of RT-PCR cycle threshold values from respiratory specimens in symptomatic and asymptomatic children with SARS-CoV-2 infection. Clin Infect Dis. 2021:ciab403. doi:10.1093/cid/ciab403
14. Hurst JH, Heston SM, Chambers HN, et al. SARS-CoV-2 infections among children in the Biospecimens from Respiratory Virus-Exposed Kids (BRAVE Kids) study. medRxiv. 2020. doi:10.1101/2020.08.18.20166835
15. Kociolek LK, Muller WJ, Yee R, et al. Comparison of upper respiratory viral load distributions in asymptomatic and symptomatic children diagnosed with SARS-CoV-2 infection in pediatric hospital testing programs. J Clin Microbiol. 2020;59(1):e02593–20. doi:10.1128/JCM.02593-20
16. Mahase E. Covid-19: do vaccines work against omicron-and other questions answered. BMJ. 2021;375:n3062. doi:10.1136/bmj.n3062
17. Hippisley-Cox J, Coupland CA, Mehta N, et al. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021;374:n2244. doi:10.1136/bmj.n2244
18. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. medRxiv. 2021. doi:10.1101/2021.12.14.21267755
19. Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. 2020;295(3):715–721. doi:10.1148/radiol.2020200370
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


