Back to Journals » International Journal of General Medicine » Volume 16
Risk Factors for Left Ventricular Hypertrophy in Patients with Diabetic Kidney Disease: A Multi-Center Study
Authors Wang X, Zhu D, Peng L, Gao Y, Li X
Received 12 March 2023
Accepted for publication 1 May 2023
Published 8 May 2023 Volume 2023:16 Pages 1705—1712
DOI https://doi.org/10.2147/IJGM.S412230
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Xiangdong Wang,1,* Dongpo Zhu,2,* Leilei Peng,1 Yan Gao,3 Xiaodong Li4
1Department of Blood Purification, Baoding People’s Hospital, Baoding, 071030, People’s Republic of China; 2Department of Internal Medicine, Baoding People’s Hospital, Baoding, 071030, People’s Republic of China; 3Department of Nephrology, Affiliated Hospital of Hebei University, Baoding, 071030, People’s Republic of China; 4Department of Nephrology, Baoding No. 1 Central Hospital of Hebei Medical University, Baoding, 071000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaodong Li, Department of Nephrology, Baoding No. 1 Central Hospital of Hebei Medical University, Baoding, Hebei, 071000, People’s Republic of China, Email [email protected]
Objective: One of the earliest echocardiographic features of the left ventricle explored extensively was left ventricular hypertrophy (LVH). Numerous studies have identified a few risk factors for LVH, however, there are few for people with diabetic kidney disease (DKD). Therefore, we evaluated the risk factors in DKD patients with LVH by analyzing laboratory data and clinical traits.
Methods: In total, 500 DKD patients in the Baoding area from February 2016 and June 2020 were admitted and classified as an experimental group (240 cases, LVH group) and a control group (260 cases, non-LVH group). The clinical parameters and laboratory tests of the participants were collected and analyzed retrospectively.
Results: Compared with the control group, low-density lipoprotein (LDL), body mass index (BMI), intact parathyroid hormone (iPTH), systolic blood pressure, and 24-hour urine protein were higher in the experimental group (all P< 0.01). Multivariable logistic regression analysis results confirmed that high BMI (OR=1.332, 95% CI 1.016– 1.537, P=0.006), LDL (OR=1.279, 95% CI 1.008– 1.369, P=0.014) and 24-hour urine proteins (OR=1.446, 95% CI 1.104– 1.643, P=0.016) were statistically significant. The ROC analysis illustrated that the optimum cutoff value of BMI, LDL, and 24-hour urine proteins for diagnosis of LVH in patients with DKD was 27.36 kg/m2, 4.18 mmol/L, and 1.42 g respectively.
Conclusion: The increase in BMI, LDL, and 24-hour urine proteins quantification are independent risk factors for LVH in patients with DKD.
Keywords: diabetic kidney disease, chronic kidney disease, risk factors, left ventricular hypertrophy
Introduction
Chronic kidney disease (CKD), which is defined as chronic kidney structural dysfunction caused by various reasons such as increased urinary albumin excretion or abnormal glomerulus filtration rate, has become a serious threat to public health as a result of the prevalence of diabetes and hypertension. Diabetic kidney disease (DKD) is the most common type of CKD.1–3 Left ventricular hypertrophy (LVH) is characterized by myocyte hypertrophy and interstitial fibrosis and is a common target organ in cardiovascular diseases (CVDs).4–6 LVH is an adaptive change of the body to long-term increased hemodynamic load, which is manifested by thickening of the ventricular wall, remodeling of the myocardium, and myocardium weight gain, leading to severe heart failure and malignant ventricular arrhythmia. LVH is identified as an independent risk marker for many kinds of CVDs and is extremely frequent in CKD.7 However, the pathogenesis of LVH is complicated, since it can be caused by many factors including hemodynamic factors, neurohumoral factors, and family genetic factors. It is closely related to a variety of cardiac arrhythmias and early intervention can decrease the risk of cardiovascular events and fatalities.8–10 Hence, early diagnosis and prevention of LVH have important clinical value. Numerous research reports have revealed some risk factors for LVH, however, for patients with DKD, the data is lacking. Therefore, we assessed potential risk factors for DKD by analyzing clinical and laboratory data from patients with this disease.
This research aimed to identify potential risk factors for LVH in patients with DKD by collecting clinical and laboratory data from participants.
Methodology
Research Design and Subjects
This retrospective and multi-center study recruited patients with DKD in the Baoding area, between February 2016 and August 2020 from Baoding No. 1 Central Hospital, Baoding People’s Hospital, and Affiliated Hospital of Hebei University. This research was undertaken in compliance with the directions provided by the ethics committee of Baoding No. 1 Central Hospital (ethical approval number:2020079) to protect human subjects. Every procedure was carried out in conformity with the applicable policies, standards, and regulations.
Procedures
The clinical, laboratory, and cardiac color Doppler examination data of the patients were collected from electronic medical records by a group of medical researchers. The DKD patients were classified into the LVH group (experiment group) and the non-LVH group (control group).3,4 Five hundred patients with type 2 DKD from the Baoding area were included and confirmed by diagnostic standards published by American Diabetic Association′s standards for diagnosis and treatment of DKD in 2019. The inclusion criteria were as follows: a proteinuria was diagnosed with the urinary albumin to creatinine ratio (UACR) >30mg/g; b renal biopsy with clear pathological results without other CKD c estimated glomerular filtration rate (eGFR) >90mL/min/1.73m2. These patients all underwent renal biopsy, complete blood count, biochemistry, and infection indices tests. We recorded anthropometric parameters such as age, sex, weight, height, duration of diabetes, personal history, comorbidities, and therapy. In addition, blood pressure data were also obtained. The body mass index (BMI) was derived by dividing an individual’s total body weight by the square of their height (kg/m2).
Cardiac color Doppler examination included inter-ventricular septal thickness (IVST), left ventricular posterior diameter by echocardiography wall thickness (LVPWT), left ventricular end-systolic diameter (LVDs), and left ventricular end-diastolic diameter (LEVDD). Every echocardiographic examination was carried out because it was medically necessary, and cardiologists verified the measurement information. According to the echocardiographic results, the relative wall thickness (RWT), and the left ventricular mass index (LVMI), four patterns of left ventricular geometry have been identified: concentric remodeling (elevated RWT but normal LVMI), eccentric LVH (elevated LVMI but normal RWT), eccentric LVH (elevated LVMI but normal RWT), and normal (normal LVMI and RWT). LVMI > 95 g/m2 and > 115 g/m2 for women and men, correspondingly, was typically used to designate LVH.4
Exclusion criteria: ① Diabetic ketoacidosis, hyperosmolar nonketotic coma, urinary tract infection, heart, liver, and acute kidney insufficiency, rheumatic diseases, and other systemic diseases. ② Pregnant, lactating women or women of childbearing age who intend to become pregnant. ③ Renal artery stenosis. ④ History of kidney transplantation. ⑤ Decompensated stage of liver cirrhosis, or a history of hepatic encephalopathy. ⑥ Congenital heart disease, pulmonary heart disease, and myocardial infarction.
All of the patients who participated in the research submitted their written informed consent prior to participation.
Statistical Analysis
Mean±standard or median (inter-quartile range) were utilized to present continuous variables based on the normality of the data. Counts and proportions were used to present categorical variables, which were subjected to comparison with χ²-test or Fisher’s exact test between control and experiment groups. Univariate analysis was undertaken to examine patients’ clinical features, whereas binary logistic regression was employed to examine the multivariate differences (variations). Then, ROC curves of relevant potential markers were drafted. Two-tailed p value <0.05 denoted the significance level. The analyses of all statistical data were executed with SPSS, version 25.0 (IBM SPSS).
Results
Demographics and Characteristics
Five hundred participants were included in the research (Table 1). The mean age was 59.2 for the experimental group and 61.7 for the control group. Most patients had comorbid conditions, which include hypertension (n=176 [73.5%] for the experiment group and n=180 [69.1%] for the control group), CVDs (n=66 [27.5%] for the experiment group and n=75 [29.1%] for the control group). Most (n =242 [92.7%] for the experiment group and n=240 [91.5%] for the control group) patients need hypoglycemic agents. Moreover, around half of all need ACEI or ARB (n =123 [51.2%] for the experiment group and n=126 [48.6%] for the control group), lipid-lowering agents (n =153 [63.7%] for the experiment group and n=163 [62.5%] for the control group) and β blocking agents (n =160 [66.3%] for experiment group and n=156 [65.0%] for the control group). As opposed to the control patients, systolic blood pressure, BMI, low-density lipoprotein (LDL), intact parathyroid hormone (iPTH), and 24-hour urine proteins were elevated in the experimental group (all P<0.01).
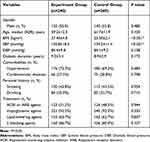 |
Table 1 Clinical Characteristics of Patients with Diabetic Kidney Disease |
Laboratory Examinations
Laboratory results at admission are inducted in Table 2. The LDL was elevated for patients of the experiment group (3.45±1.12), whereas that of the control group (2.85±0.76) remained at a normal level (p<0.001). The 24 h-Upros of the experiment group (1.85±0.62) was substantially higher than the control group (1.23±0.46) (p<0.001). The PTH of the experiment group (82.7[62.7, 96.3]) was also higher than that of the control group (73.6 [58.4, 82.3]) (p<0.001).
 |
Table 2 Laboratory Findings of Patients with Diabetic Kidney Disease |
Prognostic Value of Laboratory Findings
The statistically significant univariate variables above (p<0.05) were incorporated into the binary logistic regression analysis model. We analyzed the differences to find independent risk factors for predicting the disease outcome (Table 3). Multivariable analysis results illustrated that high BMI (OR=1.332, 95% CI 1.016–1.537, P=0.006), LDL (OR=1.279, 95% CI 1.008–1.369, P=0.014) and 24-hour urine proteins (OR=1.446, 95% CI 1.104–1.643, P=0.016) were statistically significant. Then, ROC curves of relevant potential markers were drafted (Figure 1), and we found that LDL, BMI, and 24 h-Upros all showed better performance. The ROC analysis confirmed that the optimum cut off value of BMI, LDL, and 24-hour urine proteins for diagnosis of LVH in patients with DKD was 27.36 kg/m2, 4.18 mmol/L and 1.42 g respectively (Table 4).
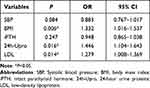 |
Table 3 Results of Multivariate Analysis for LVH in DKD Patients |
 |
Table 4 AUC of the Laboratory Findings for LVH in DKD Patients |
 |
Figure 1 ROC curves of various indicators for LVH in DKD patients. |
Discussion
LVH is an increase in the volume and the long axis of the heart during systolic or diastolic periods and can be divided into centripetal and centrifugal hypertrophy. Patients with CKD are predominantly affected by LVH, making it the most frequent structural heart lesion, and the prevalence of LVH in stage 1–3 CKD patients has exceeded 50%, which is significantly higher than the general population.11 LVH is a compensatory response of cardiomyocytes to strength training and aerobic exercise, as well as a pathological response to hypertension and cardiovascular disease. Cardiovascular events account for up to 43% of deaths in CKD patients, hence, the early intervention of LVH can effectively reverse LVH and improve the survival rate of CKD patients.12
The results of our research may have been influenced by numerous variables, which include BMI, LDL, and 24 h-Upros.
Urine Albumin is a sensitive detection marker for kidney and CVDs, indicating early renal damage in patients with hypertension and diabetes. Several research reports have illustrated that urine albumin is associated with LVH, however, most of them are single-center retrospective studies and need to be further confirmed by large-sample multi-center prospective studies.13 Jørgensen et al14 found that among 912 patients with type 2 diabetes who had no history of CVDs, both microalbuminuria and macroalbuminuria subjects had impaired diastolic function, however, the impaired systolic function was only found in the macroalbuminuria group. Another study showed that the LVMI increased with the increase of urinary albumin and subjects with microalbuminuria mainly exhibited impaired left ventricular diastolic function, while subjects with large albuminuria showed the impairment of both diastolic and systolic function.15 Chillawar et al discovered that LVH was associated with microalbuminuria or macroalbuminuria in patients with moderate-to-severe hypertension, following the adjustment for serum creatinine, age, diabetes, race, smoking, hypertension, and other confounding factors.16 In our study, we also confirmed that increased proteinuria served as a risk indicator for LVH in an independent manner in patients with DKD.
Low-density lipoprotein (LDL) is a type of lipoprotein that is involved in transporting cholesterol into the cells of peripheral tissue. Oxidation of LDL may result in oxidized LDL cholesterol (Ox-LDL-C), which is linked to an elevated risk of coronary heart disease, vascular disease mortality, myocardial infarction, and atherosclerosis. When there is an abundance of Ox-LDL, cholesterol will begin to accumulate on the artery wall, which will eventually contribute to the onset and progression of arteriosclerosis. Accumulation of small dense atherogenic LDL will stimulate xanthine oxidase, NAD(P)H oxidase, etc, causing accumulation of superoxide, which plays an integral function in hypertension, endothelial dysfunction, decreased bioavailability of nitric oxide, and vascular remodeling.17 Xiao FK et al18 found that among patients with high-normal blood pressure, the multivariate analyses showed a significant and independent association of LVMI with total cholesterol, triglyceride, andLDL.18 This is consistent with our findings. However, several research reports have demonstrated that LDL independently served as a risk indicator for LVH.19 This inconsistent result may be related to the study population, the type of disease, and the size of the sample., so it needs to be further clarified by large sample, multi-center and prospective studies.
LVH appears to occur frequently in obese patients, whether assessed by body mass index (BMI) or waist circumference. This is consistent with other observations, even in the absence of comorbidities such as hypertension.20 Nonetheless, it is unclear if obesity is a direct or indirect contributor to LVH risk. In our study, BMI is a risk indicator for LVH, suggesting that the elevated blood volume and long-term enhanced cardiac output caused by obesity-induced metabolic demand could be a significant factor for cardiac size. Nonetheless, the exact physiological reasons for this still need to be fully illustrated.
To the best of our knowledge, this study firstly showed that the increasing of LDL, BMI, and 24 h-Upros were independent risk markers for LVH in patients with DKD. NO study has examined the association between these markers and DKD patients with LVH. Hence, this result might be useful in early treating those patients with DKD and LVH. In contrast, as a retrospective study, our study has some limitations. First, the patients who participated in this research were all from the same geographic region, and the size of our sample was not very large. The clinical finding might not be applicable in other contexts. To additionally demonstrate the effectiveness of these biological markers, more independent validation datasets are required. Secondly, our research design failed to clarify the etiology and treatment of LVH in DKD patients, making it difficult to completely control the interference of other confounders. Lastly, some medications, such as sodium-dependent glucose transporters 2 inhibitor or glucagon like peptide-1 receptor agonist treatments may improve LVH in patients with DKD, but our study had not consider these drugs. As a result, these findings must be integrated with the actual clinical situation, and further prospective multi-center research is required to corroborate them.
Conclusion
In our study, the increase of LDL, BMI, and 24 h-Upros were risk markers for LVH in patients with DKD. For accurate assessments of patient severity and outcomes, more elaborate models incorporating more indicators are required.
Ethical Statement
The responsibility for investigating and resolving any questions and concerns raised about the accuracy or integrity of any part of the work is attributed to the authors of this work. The institutional review board at Baoding No. 1 Central Hospital approved this study, and the procedures in this study were conducted in compliance with the guidelines for protecting human subjects in research. There was strict adherence to all laws and regulations, and all procedures were undertaken accordingly.
Acknowledgments
The authors have completed the TRIPOD reporting checklist.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Baoding Science and Technology Plan Self-financing Project (1951ZF093).
Disclosure
The authors have no conflicts of interest to disclose. Reporting Checklist: The authors have completed the TRIPOD reporting checklist.
References
1. Sagoo MK, Gnudi L. Diabetic nephropathy: an overview. Methods Mol Biol. 2020;2067:3–7.
2. Oshima M, Shimizu M, Yamanouchi M, et al. Trajectories of kidney function in diabetes: a clinicopathological update. Nat Rev Nephrol. 2021;17:740–750. doi:10.1038/s41581-021-00462-y
3. Vasanth Rao VR, Tan SH, Candasamy M, et al. Diabetic nephropathy: an update on pathogenesis and drug development. Diabetes Metab Syndr. 2019;13(1):754–762. doi:10.1016/j.dsx.2018.11.054
4. Stewart MH, Lavie CJ, Shah S, et al. Prognostic implications of left ventricular hypertrophy. Prog Cardiovasc Dis. 2018;61:446–455. doi:10.1016/j.pcad.2018.11.002
5. Yildiz M, Oktay AA, Stewart MH, et al. Left ventricular hypertrophy and hypertension. Prog Cardiovasc Dis. 2020;63(1):10–21. doi:10.1016/j.pcad.2019.11.009
6. Bourdillon MT, Vasan RS. A contemporary approach to hypertensive cardiomyopathy: reversing left ventricular hypertrophy. Curr Hypertens Rep. 2020;22:85. doi:10.1007/s11906-020-01092-8
7. Nardi E, Mulè G, Giammanco A, et al. Left ventricular hypertrophy in chronic kidney disease: a diagnostic criteria comparison. Nutr Metab Cardiovasc Dis. 2021;31:137–144. doi:10.1016/j.numecd.2020.08.028
8. Brooks JE, Soliman EZ, Upadhya B. Is left ventricular hypertrophy a valid therapeutic target? Curr Hypertens Rep. 2019;21:47. doi:10.1007/s11906-019-0952-9
9. Iyengar SS, Ram CVS. Concentric vs eccentric left ventricular hypertrophy: does it matter? It is all “blood pressure centered”. Am J Hypertens. 2021;34:581–582. doi:10.1093/ajh/hpab037
10. Maron BJ, Rowin EJ, Maron MS. Is regression of left ventricular hypertrophy really a good thing for patients with hypertrophic cardiomyopathy? The emerging mavacamten story. Am J Cardiol. 2021;147:145–146. doi:10.1016/j.amjcard.2021.01.034
11. Xie X, Peng Z, Li H, et al. Association of urine albumin/creatinine ratio below 30 mg/g and left ventricular hypertrophy in patients with type 2 diabetes. Biomed Res Int. 2020;2020:5240153. doi:10.1155/2020/5240153
12. Wang J, Lv J, He K, et al. Association of left ventricular hypertrophy and functional impairment with cardiovascular outcomes and mortality among patients with chronic kidney disease, results from the C-STRIDE study. Nephrology. 2022;27:327–336. doi:10.1111/nep.14009
13. Al-Sharifi A, Mingher HM. Microalbuminuria and left ventricular hypertrophy in patients with essential hypertension. J Pak Med Assoc. 2019;69:S13–S16.
14. Jørgensen PG, Bieringsørensen T, Mogelvang R, et al. Presence of micro- and macroalbuminuria and the association with cardiac mechanics in patients with type 2 diabetes. Eur Heart J Cardiovasc Imaging. 2018;19:1034–1041. doi:10.1093/ehjci/jex231
15. Nagai H, Suzuki S, Ishii H, et al. Impact of low- grade albuminuria on left ventricular diastolic dysfunction. Ijc Metab Endocr. 2015;6:13–16. doi:10.1016/j.ijcme.2015.01.006
16. Chillawar S, Verna A, Acharya S, et al. Co-Relation of 24 Hours Proteinuria And Left Ventricular Hypertrophy in Hypertensive Patients.Iosr. J Dent Med Sci. 2017;16:1–8.
17. Xue-Wei H, Ke-Qiong D, Juan-Juan Q, et al. Association between lipid profiles and left ventricular hypertrophy: new evidence from a retrospective study. Chin Med Sci J. 2022;37:103–117. doi:10.24920/004066
18. Xiao FK, Li P, Han ZY, et al. Patients with dipper and nondipper high-normal blood pressure were associated with left ventricular mass. Int J Hypertens. 2021;2021:6946418. doi:10.1155/2021/6946418
19. Bjelakovic B, Stefanutti C, Vukovic V, et al. Lipid profile and left ventricular geometry pattern in obese children. Lipids Health Dis. 2020;19:109. doi:10.1186/s12944-020-01285-9
20. Zanib A, Anwar S, Saleem K, et al. Frequency of left ventricular hypertrophy among patients on maintenance hemodialysis by voltage criteria and its relationship with biophysical-chemical parameters. Cureus. 2020;12:e7426. doi:10.7759/cureus.7426
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
