Back to Journals » Infection and Drug Resistance » Volume 16
Risk Factors for Cefoperazone/Sulbactam-Induced Coagulation Disorder
Authors Miao W, Guo J , Cheng H , Zhao Q
Received 31 July 2023
Accepted for publication 20 September 2023
Published 21 September 2023 Volume 2023:16 Pages 6277—6284
DOI https://doi.org/10.2147/IDR.S429706
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Wan Miao, Jinlin Guo, Huifang Cheng, Qianqian Zhao
Department of Pharmacy, Shanxi Provincial People’s Hospital, Taiyuan, Shanxi Province, People’s Republic of China
Correspondence: Jinlin Guo, Department of Pharmacy, Shanxi Provincial People’s Hospital, Shuangtasi Street 59#, Taiyuan, Shanxi, 030001, People’s Republic of China, Fax +86-351-4960527, Email [email protected]
Purpose: Cefoperazone/sulbactam is a β-lactam/β-lactamase inhibitor combination effective against intra-abdominal, urinary tract, and respiratory infections. Although some studies have suggested that cefoperazone/sulbactam is associated with coagulation disorders, it remains debatable whether the combination of cefoperazone/sulbactam with tigecycline or valproic acid increases the risk of bleeding, as both drugs can lead to coagulation disorders. This study aimed to explore the risk factors of cefoperazone/sulbactam-induced coagulopathy.
Patients and Methods: This was a single-center, retrospective, nested case-control study. The sample groups were derived from individuals registered at the Department of Neurosurgery, Shanxi Provincial People’s Hospital. Propensity score matching (PSM) was used to adjust for demographic data. Conditional logistic regression was used to estimate the matched odds ratios representing the odds of cefoperazone/sulbactam-induced coagulopathy (CIC), and a receiver operating characteristic curve was used to determine the optimal cut-off conditions.
Results: After PSM, 155 and 56 patients were included in the control and case groups, respectively. Multivariate analysis revealed that advanced age, treatment duration, and total dose were independent risk factors of cefoperazone/sulbactam-induced coagulation disorders. Concomitant use of vitamin K was an independent protective factor against CIC. The optimal cut-off for the length of treatment was 5 d, and the cut-off for the total dose was 48 g.
Conclusion: Tigecycline and valproic acid were not associated with CIC. Advanced age and long treatment duration are risk factors for CIC. Supplementation with vitamin K during cefoperazone/sulbactam treatment was associated with a reduced risk.
Keywords: cefoperazone/sulbactam, coagulopathy, tigecycline, valproic acid
Introduction
Cefoperazone is a third-generation cephalosporin effective against intra-abdominal, urinary, and respiratory tract infections. Sulbactam is a β-lactamase inhibitor that enhances cefoperazone activity. Cefoperazone/sulbactam can be used to treat moderate-to-severe infections caused by ESBL-positive Escherichia coli, Klebsiella pneumoniae, and other multidrug-resistant bacteria.1,2 However, some studies have reported an association between cefoperazone/sulbactam and coagulation disorders.3,4 Case reports have shown that patients receiving cefoperazone/sulbactam treatment may experience bleeding events such as upper gastrointestinal bleeding, hematuria, and abdominal wall hematoma.5,6
A possible mechanism for the coagulation abnormalities associated with cefoperazone may be its N-methylthio-tetrazole (NMTT) side chain, which induces vitamin K deficiency and leads to hypoprothrombinemia and bleeding.6–9 A retrospective cohort study by Strom et al found that cefoperazone was associated with a higher risk of hypocoagulability than was antibiotics without NMTT.8 A nested case-control study conducted in China also found that cefoperazone may increase the risk of bleeding compared with other antibiotics.10
According to the China Antimicrobial Resistance Surveillance System, carbapenem-resistant Acinetobacter baumannii (CRAB) is a major causative pathogen of healthcare-associated infections in hospitals in China. Due to its ability to develop extensive resistance to antibiotics through complex mechanisms, treatment options are very limited, and patients often have a poor prognosis.11 Tigecycline and polymyxin are effective antibiotics against CRAB infections in China. However, given the high cost of polymyxins, tigecycline is the primary treatment option in most cases. Since tigecycline is generally not used as monotherapy because of its heterogeneous resistance,12,13 and sulbactam has antibacterial activity against CRAB, the combination of cefoperazone/sulbactam and tigecycline is the most commonly used treatment regimen for CRAB infections in China.14 According to the manufacturer’s indications, tigecycline may cause an increase in INR and prolong activated partial thromboplastin (APTT) and prothrombin times (PT).15 Both cefoperazone and tigecycline may cause coagulation disorders; however, it remains debatable whether the combination of cefoperazone/sulbactam and tigecycline increases the risk of bleeding.
Valproic acid (VPA) is a first-line, broad-spectrum antiepileptic drug used to prevent epilepsy in patients undergoing neurosurgery or brain trauma.16 These patients often develop secondary pulmonary infections due to coma and are unable to spontaneously cough sputum.17 Valproic acid and cefoperazone/sulbactam are simultaneously administered to patients at risk of epilepsy and pulmonary infection. However, valproic acid and cefoperazone/sulbactam can cause coagulation disorders.18,19 Further investigations are necessary to determine whether the combination of valproic acid or tigecycline with cefoperazone/sulbactam is associated with an increased risk of bleeding. Therefore, we conducted a retrospective nested case-control study to explore the risk factors for cefoperazone/sulbactam-induced coagulopathy (CIC).
Materials and Methods
This was a single-center, retrospective, nested case-control study. The sample groups were derived from individuals registered at the Department of Neurosurgery, Shanxi Provincial People’s Hospital. We enrolled 1056 adult patients who received cefoperazone/sulbactam between September 2019 and March 2023. This study was approved by the Ethics Committee of the Shanxi Provincial People’s Hospital (ID:201936).
Inclusion Definition for Selection of Cases and Controls
The inclusion criteria were patients who took cefoperazone/sulbactam (2:1, meaning each bottle of cefoperazone/sulbactam had 1 g cefoperazone and 0.5 gram sulbactam; the dosage in this article refers to cefoperazone/sulbactam combination), patients aged ≥18 years old, with normal coagulation before medication, and no liver disease or blood disorders history. The exclusion criteria included patients who were treated with cefoperazone/sulbactam for <3 d; those with missing data; patients aged <18 years old; those with abnormal coagulation before medication (patients who did not test coagulation function prior to medication were also excluded); those currently suffering from sepsis or hematological disease; and those prescribed the combined use of aspirin, low-molecular-weight heparin, warfarin, clopidogrel, ticagrelor, and rivaroxaban (Figure 1).
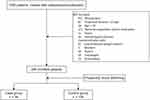 |
Figure 1 Cohort exclusions and nested case-control design. |
The normal range of coagulation function in our hospital is APTT 25.1–36.5 seconds (s) and PT 9.9–12.8 s. The coagulation disorder group (case group) was defined as PT or APTT that was prolonged by more than 30% compared to that before administration. Patients who met the inclusion criteria but did not show sufficient coagulation disorders served as controls. The time of the first cefoperazone/sulbactam administration was defined as the enrollment time, and the endpoint time was defined as the point at which the patient presented with drug withdrawal, death, or discharge. The period between enrollment and endpoint was defined as the follow-up period.
Data Collection
Clinical information was collected from the hospital information system and included body weight, height, age, sex, underlying diseases, site of infection, cefoperazone/sulbactam dosage, duration of cefoperazone/sulbactam treatment, concomitant drugs, ICU admission, Vitamin K supplementation, PT, APTT, white blood cell count, hemoglobin level, platelet count, transaminase level, total bilirubin level, albumin level, and creatinine level. All laboratory tests performed 48 h before cefoperazone/sulbactam administration until 48 h after discontinuation of cefoperazone/sulbactam were recorded.
Statistical Analysis
The design of the study was a retrospective, nested case-control study. The Shapiro–Wilk test was used to test normality. Statistical descriptions of quantitative variables are expressed as median and interquartile range (IQR) or mean and standard deviation. The propensity score matching (PSM) method was used to adjust for demographic data (body weight, sex, and site of infection). The data were modeled using a conditional logistic regression model, conditioning on the PSM-matched case-control sets (each set included one case and up to three controls). In the models, the dependent variable was coagulation disorders, and the main exposure variables included dosage, duration of treatment, concomitant drugs (tigecycline, carbapenems, and valproic acid), ICU admission, Vitamin K supplementation, white blood cell count, hemoglobin, platelet count, transaminase level, total bilirubin level, albumin level, and creatinine level to calculate the odds ratio (OR) with a 95% confidence interval (CI) of the association between variables and the risk of coagulation disorders. We used conditional logistic regression analysis to analyze the variables mentioned above. Vitamin K supplementation, concomitant drugs, and ICU admission were encoded with dummy variables, and P < 0.05 was considered statistically significant. Variables with P < 0.05 were selected; we then used the stepwise regression method to sequentially add them to the final model. A covariate was retained in the final model if it had a significant effect (P < 0.05).
Based on the conditional logistic regression data, an optimal cutoff point for the occurrence of coagulation disorders was determined using receiver operating characteristic (ROC) curves. The ROC curves were plotted, and the area under the curves (AUC) were calculated with a 95% CI.
Descriptive statistics and PSM were calculated using R version 3.6.3 and Python version 3.7. Conditional logistic regression, Student’s t-test, and ROC were calculated using SPSS AU software (Version 21.0 [Online Application Software]) retrieved from https://www.spssau.com.
Results
The study cohort comprised 249 patients who met the inclusion criteria. PSM was used to match the two groups to eliminate baseline differences. After PSM, 155 and 56 patients were included in the control and case groups, respectively. The baseline clinical characteristics of the patients are presented in Table 1. The lungs were the most common site of infection in both groups.
 |
Table 1 Baseline Information |
Univariate analysis showed that advanced age, treatment duration, total dose, and concomitant use of tigecycline and Vitamin K were significantly different between the two cohorts (P < 0.05). Multivariate analysis (Table 2) revealed that advanced age (OR: 1.04, 95% CI: 1.01~1.08, P=0.026), treatment duration (OR: 1.25, 95% CI: 1.04~1.50, P=0.019), and total dose (OR: 1.02, 95% CI: 1.01~1.05, P=0.047) were the independent risk factors for cefoperazone/sulbactam-induced coagulation disorders. Cefoperazone/sulbactam in combination with tigecycline (OR: 2.31, 95% CI: 0.81~6.57, P=0.116) or valproic acid (OR: 1.20, 95% CI: 0.42~3.44, P=0.737) was not associated with CIC. Although the case group had more patients admitted to the ICU, ICU admission was not an independent risk factor for CIC. Liver dysfunction, kidney dysfunction, and fibrinogen levels were not associated with CIC. Concomitant use of Vitamin K was an independent protective factor against CIC (OR: 0.30, 95% CI: 0.14~0.66, P=0.002).
 |
Table 2 Risk Factors for Cefoperazone/Sulbactam-Induced Coagulation Disorders |
Table 3 shows the ROC curves for the incidence of coagulation disorders. The AUC of the ROC curve was 0.73 (95% CI: 0.66–0.81, sensitivity: 0.82, specificity: 0.59) for treatment duration, and 0.71 (95% CI: 0.63–0.78, sensitivity: 0.61, specificity: 0.73) for total dose (Figure 2). The optimal cutoff for the length of treatment was 5 d, and the cutoff for the total dose was 48 g.
 |
Table 3 Results of ROC |
 |
Figure 2 Receiver operating characteristic (ROC) curve. |
Discussion
Many previous studies have reported risk factors for CIC, such as advanced age, treatment duration, and dosage. However, these studies failed to establish strict inclusion criteria.10,20–22 The strength of this study was the establishment of strict inclusion criteria that excluded the influence of antiplatelet drugs, anticoagulants, and underlying diseases on coagulation function. Additionally, we explored whether the concomitant use of cefoperazone/sulbactam with tigecycline or valproic acid increased the risk of bleeding.
However, the mechanisms underlying CIC remain unclear. Possible mechanisms include the following: 1.) Cefoperazone is mainly excreted through bile, which can eliminate Escherichia coli, Bacteroides, and other bacteria that produce vitamin K in the intestine. This leads to a reduction in vitamin K synthesis. When the body lacks sufficient vitamin K, coagulation factors II, VII, IX, and X cannot undergo carboxylation, resulting in a loss of coagulation activity. This can cause prolonged PT and APTT, abnormal international normalized ratio (INR), or bleeding. 2.) Cefoperazone contains an NMTT side chain that can competitively bind to gamma-glutamyl carboxylase, an enzyme responsible for the carboxylation of vitamin K-dependent coagulation factors and their conversion into active forms. The inhibition of this enzyme by cefoperazone can lead to impaired carboxylation of coagulation factors, resulting in reduced clotting activity and an increased risk of bleeding.6,21
Our results indicate that supplementation with vitamin K during the use of cefoperazone was an independent protective factor against cefoperazone-induced CIC. Humans cannot synthesize vitamin K on their own, but they can maintain normal function by consuming vegetables rich in vitamin K. Additionally, vitamin K synthesized by the intestinal microbiota can provide additional supplementation. In this study, 58.29% (123/211) of patients were admitted to the ICU. Most of these patients were unable to intake vegetables to obtain vitamin K. We suggest that cefoperazone further hindered the acquisition of vitamin K, leading to CIC. Although supplementing vitamin K seems to prevent CIC, we believe that the prophylactic use of vitamin K for all patients using cefoperazone is debatable. Not all patients receiving cefoperazone experience coagulation abnormalities. The potential risk of venous thrombosis associated with vitamin K supplementation in low-risk patients receiving cefoperazone remains unknown. Some studies also suggest that routine vitamin K supplementation in patients with short-term cefoperazone use and no bleeding risk is not supported by evidence and carries the potential risk of severe allergic reactions.20,23
High doses and prolonged cefoperazone treatment can exacerbate the inhibition of vitamin K synthesis.20,23 Case-control studies have shown that the greater the cumulative exposure to cefoperazone, which can induce coagulation abnormalities, the higher the risk of bleeding.10 Our study indicated that the treatment duration and total dose were risk factors for CIC. The optimal cutoff for the length of treatment was five days, and the cutoff for the total dose was 48 g. Since cumulative exposure is equal to the daily dosage multiplied by the treatment duration, we considered CIC to be dose-dependent.
Our study found that advanced age was an independent risk factor for CIC, which is consistent with previous research.20,23 Coagulation factors are primarily synthesized in the liver. We speculate that the higher occurrence of CIC in elderly patients is due to an age-related decline in liver function compared with younger patients.
Numerous studies have reported coagulation disorders caused by tigecycline,24–26 but none have specifically addressed whether the combination of cefoperazone/sulbactam and tigecycline increases the risk of bleeding. Our results suggest that the combination of tigecycline and cefoperazone/sulbactam is not an independent risk factor for CIC. Tigecycline decreases fibrinogen levels, particularly at high doses.24,27 CIC is primarily associated with vitamin K levels. We speculate that the mechanisms underlying coagulation disorders induced by cefoperazone/sulbactam and tigecycline are different; therefore, the concomitant use of both drugs does not exacerbate side effects.
In neurosurgical cases, cefoperazone/sulbactam is sometimes combined with VPA to treat pneumonia and prevent seizures. VPA can lead to coagulation disorders in both pediatric and adult patients due to excessive fibrinogen consumption or deficient production.28,29 Our study suggests that cefoperazone/sulbactam combined with VPA is not associated with CIC. However, coagulation disorders induced by valproic acid typically require several weeks or longer, whereas the treatment duration for cefoperazone/sulbactam is usually only one or two weeks. Therefore, it is challenging to observe the interactions between these two drugs. Further research is required to understand the relationship between valproic acid and cefoperazone/sulbactam interactions.
This study had several limitations. First, this was a retrospective cohort study; therefore, there may have been a bias that was not controlled for. Second, the sample size was relatively small, and limited parameters were collected. Despite these limitations, we investigated the effects of tigecycline combined with cefoperazone/sulbactam on coagulation function to facilitate clinical decision making.
Conclusion
In conclusion, advanced age and prolonged treatment duration are risk factors for cefoperazone/sulbactam-induced coagulopathy. Supplementation with vitamin K during cefoperazone/sulbactam treatment was associated with a reduced risk. Tigecycline and valproic acid were not associated with CIC.
Abbreviations
APTT, activated partial thromboplastin time; AUC, area under the curve; CI, confidence interval; CIC, cefoperazone/sulbactam-induced coagulopathy; CRAB, carbapenem-resistant Acinetobacter baumannii; g, gram; ICU, intensive care unit; IQR, interquartile range; INR, international normalized ratio; PSM, propensity score matching; PT, prothrombin time; ROC, receiver operating characteristic; VPA, valproic acid.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author (Professor Jinlin Guo) upon reasonable request.
Ethical Approval Statement
The study protocol was approved by the Ethics Committee of Shanxi Provincial People’s Hospital (201936) and was conducted following the legal requirements and tenets of the Declaration of Helsinki and its subsequent amendments. Written informed consent was waived by the Ethics Committee of Shanxi Provincial People’s Hospital because this was a retrospective study, and the clinical data collected were only for the purpose of scientific research, with no additional burden on patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev. 2010;23(1):160–201. doi:10.1128/CMR.00037-09
2. Su J, Guo Q, Li Y, et al. Comparison of empirical therapy with cefoperazone/sulbactam or a carbapenem for bloodstream infections due to ESBL-producing Enterobacteriaceae. J Antimicrob Chemother. 2018;73(11):3176–3180. doi:10.1093/jac/dky323
3. Xin X, Jian L, Xia X, et al. A multicentre clinical study on the injection of ceftriaxone/sulbactam compared with cefoperazone/sulbactam in the treatment of respiratory and urinary tract infections. Ann Clin Microbiol Antimicrob. 2013;12(1):38. doi:10.1186/1476-0711-12-38
4. Greenberg RN, Reilly PM, Weinandt WJ, Bollinger M, Kennedy DJ. Cefoperazone-sulbactam combination in the treatment of urinary tract infections: efficacy, safety, and effects on coagulation. Clin Ther. 1987;10(1):52–56.
5. Wong RS, Cheng G, Chan NP, Wong WS, Ng MH. Use of cefoperazone still needs a caution for bleeding from induced vitamin K deficiency. Am J Hematol. 2006;81(1):76. doi:10.1002/ajh.20449
6. Cai Z, Yang W, He Y, et al. Cefoperazone/sulbactam-induced abdominal wall hematoma and upper gastrointestinal bleeding: a case report and review of the literature. Drug Saf Case Rep. 2016;3(1):2. doi:10.1007/s40800-016-0025-9
7. Lipsky JJ. N-methyl-thio-tetrazole inhibition of the gamma carboxylation of glutamic acid: possible mechanism for antibiotic-associated hypoprothrombinaemia. Lancet. 1983;2(8343):192–193. doi:10.1016/s0140-6736(83)90174-5
8. Strom BL, Schinnar R, Gibson GA, Brennan PJ, Berlin JA. Risk of bleeding and hypoprothrombinaemia associated with NMTT side chain antibiotics: using cefoperazone as a test case. Pharmacoepidemiol Drug Saf. 1999;8(2):81–94. doi:10.1002/(SICI)1099-1557(199903/04)8:2<81::AID-PDS411>3.0.CO;2-G
9. Mueller RJ, Green D, Phair JP. Hypoprothrombinemia associated with cefoperazone therapy. South Med J. 1987;80(11):1360–1362. doi:10.1097/00007611-198711000-00007
10. Chen LJ, Hsiao FY, Shen LJ, et al. Use of hypoprothrombinemia-inducing cephalosporins and the risk of hemorrhagic events: a nationwide nested case-control study. PLoS One. 2016;11(7):e0158407. doi:10.1371/journal.pone.0158407
11. Group of Infectious Diseases RDBoCMA. Guidelines for the diagnosis and treatment of hospital-acquired pneumonia and ventilator-associated pneumonia of adults in China. Chin J Tuberculosis Respir Dis. 2018;41(4):255–280.
12. Liu H, Jia X, Zou H, et al. Detection and characterization of tigecycline heteroresistance in E. cloacae: clinical and microbiological findings. Emerg Microbes Infect. 2019;8(1):564–574. doi:10.1080/22221751.2019.1601031
13. Paul M, Carrara E, Retamar P, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. 2022;28(4):521–547. doi:10.1016/j.cmi.2021.11.025
14. Garnacho-Montero J, Dimopoulos G, Poulakou G, et al. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med. 2015;41(12):2057–2075. doi:10.1007/s00134-015-4079-4
15. Pfizer. Tygacil (tigecycline) for injection [prescribing information]. PA; 2013.
16. Guo J, Ma J, Wang S, et al. Valproic acid after neurosurgery induces elevated risk of liver injury: a prospective nested case-control study. Ann Pharmacother. 2022;56(8):888–897. doi:10.1177/10600280211055508
17. Rafa E, Kolpa M, Walaszek MZ, et al. Healthcare-acquired infection surveillance in neurosurgery patients, incidence and microbiology, five years of experience in two polish units. Int J Environ Res Public Health. 2022;19(12):7544. doi:10.3390/ijerph19127544
18. Abdallah C. Considerations in perioperative assessment of valproic acid coagulopathy. J Anaesthesiol Clin Pharmacol. 2014;30(1):7–9. doi:10.4103/0970-9185.125685
19. Sharma A, Sanchez J, Dew M, Shergill G. Valproate-associated transaminitis and rhabdomyolysis. Cureus. 2023;15(4):e38348. doi:10.7759/cureus.38348
20. Yuanchao Zhu LL, Shen J. Clinical features and risk factors of coagulation disorders caused by cefoperazone sodium and sulbactam sodium. Chin Pharm J. 2022;57(9):741–746.
21. Shao X, Ren Y, Xie N, et al. Effect of cefoperazone/sulbactam on blood coagulation function in infected emergency department patients and the necessity of Vitamin K1 (VK1) preventive intervention: a single-center, retrospective analysis. Med Sci Monit. 2023;29:e939203. doi:10.12659/MSM.939203
22. Wu S, Wu G, Wu H. A comparison of coagulation function in patients receiving aspirin and cefoperazone-sulbactam with and without vitamin K(1): a retrospective, observational study. Clin Ther. 2021;43(12):e335–e345. doi:10.1016/j.clinthera.2021.10.005
23. Yuying Wang JY, Duan J. Analysis of influencing factors of coagulation dysfunction caused by cefoperazone sulbactam. Chin General Pract. 2020;23(S1):138–140.
24. Liu J, Yan Y, Zhang F. Risk factors for tigecycline-associated hypofibrinogenemia. Ther Clin Risk Manag. 2021;17:325–332. doi:10.2147/TCRM.S302850
25. McMahan J, Moenster RP. Tigecycline-induced coagulopathy. Am J Health Syst Pharm. 2017;74(3):130–134. doi:10.2146/ajhp150894
26. Zhang L, Cai X, Peng F, et al. Comparison of bleeding risk and hypofibrinogenemia-associated risk factors between tigecycline with cefoperazone/sulbactam therapy and other tigecycline-based combination therapies. Front Pharmacol. 2023;14:1182644. doi:10.3389/fphar.2023.1182644
27. Treml B, Rajsic S, Hell T, Fries D, Bachler M. Progression of fibrinogen decrease during high dose tigecycline therapy in critically ill patients: a retrospective analysis. J Clin Med. 2021;10(20):4702. doi:10.3390/jcm10204702
28. Chen HF, Xu LP, Luo ZY, et al. Valproic acid-associated low fibrinogen and delayed intracranial hemorrhage: case report and mini literature review. Drug Des Devel Ther. 2013;7:767–770. doi:10.2147/DDDT.S47718
29. Meng QGH. Literature Analysis on 322 Cases of Fibrinogen Reduction Induced by Valproate. China Pharmacist. 2019;22(2):144–147.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
