Back to Journals » International Journal of General Medicine » Volume 16
Risk Factors Analysis for the Development of Hypocomplementemia in Rheumatoid Arthritis Patients: A Single-Center Retrospective Study
Authors Chen Y, Xiao C, Liao Y, Tan L
Received 30 May 2023
Accepted for publication 8 August 2023
Published 18 August 2023 Volume 2023:16 Pages 3583—3592
DOI https://doi.org/10.2147/IJGM.S422547
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yinyi Chen,1 Chunlan Xiao,2 Yubin Liao,2 Liming Tan1
1Department of Clinical Laboratory, Jiangxi Province Key Laboratory of Laboratory Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, People’s Republic of China; 2Department of Clinical Laboratory, The First Affiliated Hospital of University of South China, Hengyang, Hunan, People’s Republic of China
Correspondence: Liming Tan, Email [email protected]
Objective: The purpose of the research was to explore the possible risk factors for the development of hypocomplementemia (HC) in rheumatoid arthritis (RA) patients by analyzing their clinical and laboratory features.
Methods: This retrospective research contained 501 RA patients, divided into RA patients with HC (n=78) and RA patients without HC (n=423). Demographic characteristics and laboratory test results of RA patients were collected and analyzed, such as age, sex, anti-mutated citrullinated vimentin antibody (Anti-MCV), serum complements (C3, C4), immunoglobulins (IgA, IgG, IgM), hemoglobin (Hb), platelets (PLT) and erythrocyte sedimentation rate (ESR), etc. Spearman correlation was served as assessing the correlations of the levels of serum C3 and C4 with each index. Receiver operating characteristic (ROC) curves were served as assessing the diagnostic efficacy of each index for RA patients with HC. Furthermore, risk factors for the occurrence of HC in RA patients were analyzed by employing binary logistic regression of single and multiple factors.
Results: Compared RA patients with HC to without HC, the former were older and had a longer disease duration with increased levels of Anti-MCV, IgM and DAS28 and lower levels of Hb, PLT and ESR; Spearman correlation analysis verified the level of serum Anti-MCV was a negative correlation with C3 (r=− 0.156); the area under the ROC curve (AUC) of PLT in diagnosing RA patients with HC was the largest at 0.65 (95% CI: 0.60– 0.69); binary logistic regression analysis indicated that advanced age (> 66 years), long disease duration (> 62 months), high DAS28 value (> 6.13), the levels of Anti-MCV> 107.68IU/mL, IgM> 1.54g/L, ESR≤ 69.00mm/h, Hb≤ 99.00g/L and PLT≤ 305.00× 109/L were probable risk factors for the occurrence of HC in RA patients.
Conclusion: Age and disease duration, DAS28, Anti-MCV, IgM, ESR, Hb, and PLT are closely related to the development of HC in RA patients. Timely monitoring of these indicators can help to evaluate disease activity of RA patients and further improve their prognosis.
Keywords: rheumatoid arthritis, hypocomplementemia, risk factors, disease activity
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation and systematicness, which causes joint pain and structural damage and involves extra-articular organs.1,2 RA affects approximately 1% of the adult population, and the incidence rate among females is 3–5 times higher than of males.3 Due to the complexity of RA, its etiology and pathophysiological mechanisms have not been fully elucidated.
The complement system is a main constituent within human immune system, and inadequate control of complement activation constitutes the formation of the pathogenesis of inflammatory and autoimmune diseases, containing RA.4,5 The classical and lectin pathways of the complement system may involve in vasculitic neuropathy related to RA and systemic lupus erythematosus (SLE).6 The complement system involved in the pathogenesis of RA acts only on localized mucous in the preclinical stage, whereas it acts on joints and system in RA patients with clinically significant arthritis.7 The activation of the complement system in RA appears to not only facilitate direct tissue damage but also contribute to the initiation of RA pathogenesis by interacting with citrullination protein.8
C3 is the central molecule activated by the three complement pathways (classic, lectin, and alternative), which supports all important functions performed by it.9 Hypocomplementemia (HC) is not uncommon in patients with autoimmune diseases. For example, low levels of C3 and C4 are correlated with extraglandular manifestations, and they have clinical and prognostic value in patients with primary Sjögren’s syndrome (pSS).10 RA patients with HC exhibit more severe joint involvement and obvious extra-articular manifestations than RA patients without HC, such as subcutaneous nodules.11,12 However, there is scarce research about risk factors analysis for the development of HC in RA patients. Hence, it is necessary to understand them, which will help to monitor the disease condition and improve the prognosis of RA patients.
Materials and Methods
Patients and Study Design
This retrospective single-center cohort was conducted at a 2400-bed tertiary, comprehensive, university-affiliated hospital in Nanchang, China, with a population of over 6 million. The study population was hospitalized patients with established RA at the Second Affiliated Hospital of Nanchang University from January 2021 to July 2022. And this is one of the largest tertiary hospitals in Jiangxi Province. All the patients were eligible for the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for RA.13 The diagnoses of these patients were conducted by physicians with senior qualifications in the rheumatology department, which had 45 hospital beds. Each patient was included only once. The exclusion criteria for this research were as follows: 1) less than 18 years of age; 2) combination of other autoimmune diseases; 3) acute inflammatory diseases, infectious diseases, malignant neoplasms and severe blood diseases; 4) bacteraemia; 5) with underlying diseases that affected serum complement levels such as acute and chronic hepatitis, chronic liver disease, cirrhosis, nephrotic syndrome and glomerulonephritis; 6) missing key clinical information. Using RA as the search term, a total of 614 patients diagnosed with RA were initially exported from the hospital information system (HIS), and after applying the aforementioned exclusion criteria, a subset of 501 RA patients were contained in the subsequent research, which were divided into RA patients with HC (n=78) and without HC (n=423) according to the presence or absence of HC. Demographic characteristics and laboratory test results of RA patients were collected and analyzed.
Data Collection
Clinical data of patients were obtained from the HIS, including sex, age, age at onset, disease duration and DAS28. Laboratory results of patients were collected from the Laboratory Information System (LIS), containing the values of serum anti-cyclic citrullinated peptide antibody (Anti-CCP), anti-mutated citrullinated vimentin antibody (Anti-MCV), rheumatoid factor (RF) subtypes (RF-IgA, RF-IgG, RF-IgM), complements (C3,C4), immunoglobulins (IgA, IgG, IgM) and C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR), white blood cell (WBC), neutrophils (N), lymphocytes (L), hemoglobin (Hb) and platelets (PLT) values.
Sample Measurement Methods
Anti-CCP, Anti-MCV and RF subtypes (RF-IgA, RF-IgG, RF-IgM) were tested by ELISA, and the former reagents were purchased from ORGENTEC Diagnostika GmbH and the others were purchased from Aesku. Diagnostics GmbH&Co.KG. The levels of serum C3, C4, IgA, IgG, IgM and CRP were detected by immunoturbidimetric assay, using A25 automatic specific protein analyzer and supporting reagents (BIOSTEC Biotechnology Co.Ltd, China). The level of ESR was detected through using Dynamic hematocrit tester SD-1000 (SUCCEEDER Technology Co.Ltd, China). The levels of WBC, N, L, Hb and PLT were measured through using Sysmex XN-9000 automatic blood cell analyzer (Sysmex Corporation, Japan) and supporting reagents.
Statistical Analysis
SPSS software, MedCalc software and the OmicStudio tools at https://www.omicstudio.cn were used to statistics and charting. The chi-squared test was applied for comparisons for the count data. Continuous variables were represented as M (Q1, Q3), and the Mann–Whitney U-test was applied for comparing measurement data. Spearman correlation was served as assessing the correlations of the levels of serum C3 and C4 with each index. Receiver operating characteristic (ROC) curves were served as assessing the diagnostic efficacy of each index for RA patients with HC. The optimum cut-off value of each index for RA patients with HC was selected according to Youden index J. And risk factors for the occurrence of HC in RA patients were analyzed by employing binary logistic regression of single and multiple factors with the cut-off values as the boundary, and the results were expressed as odds ratio (OR) and 95% confidence interval (CI). P<0.05 was of statistical significance.
Results
Demographic Characteristics and Laboratory Test results of RA Patients
The clinical characteristics of RA patients were shown in Table 1. All together 501 RA patients were included, and most of them were females (n=343, 68.5%). The mean age was 61.54±10.68 years (range: 55–69 years). They were divided into RA with HC group (n=78) and RA without HC group (n=423) on the basis of the serum C3 and C4 levels, and the incidence of HC in RA patients was 15.57%. There were no statistically significant differences in terms of gender (P=0.090) and age at onset (P=0.721) between the above groups. Compared RA patients with HC to RA patients without HC, the former were older and had a longer disease duration with increased levels of Anti-MCV, RF (RF-IgA, RF-IgG, RF-IgM), IgM and DAS28 and lower levels of WBC, N, L, Hb, PLT and ESR (all P<0.05). And there were no significant differences (P>0.05) in other indicators (Table 1).
 |
Table 1 Demographic and Serological Descriptors of RA Patients with HC and RA Patients Without HC |
Correlation Analysis Among Each Index of RA
Correlation Cluster Analysis
The outcomes of Spearman correlation analysis indicated the level of N was a significant positively related to WBC (r>0.80), and the level of serum C3 was positively related to C4 (r>0.40), while the level of ESR was negatively related to Hb (r<-0.4). And all P values were less than 0.01 (Figure 1).
Correlation Network Analysis
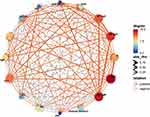
The outcomes of further correlation network analysis suggested WBC level had the strongest correlation degree of each indicator, whereas the correlation degree of age was the weakest. Among the levels of C3, C4 and other indexes of patients with RA, the results of correlation analysis with statistical significance implied the level of serum Anti-MCV was negatively related to C3 (r=−0.156), the level of PLT was positively related to C3 (r=0.265). Similarly, serum RF-IgG level was negatively related to C4 (r=−0.309), serum C3 level was positively related to C4 (r=0.428), and all P values were less than 0.05 (Figure 2, Table 2).
 |
Table 2 Correlation Analysis of C3, C4 and Other Indexes in Patients with RA |
Assessment of the Diagnostic Efficacy of Each Index in RA Patients with HC
The area under the ROC curve (AUC) of PLT in diagnosing RA patients with HC was the largest at 0.65 (95% CI: 0.60–0.69) and its Youden’s index was 0.24; whereas the AUC of Hb was the smallest at 0.58 (95% CI: 0.53–0.62). The best cut-off for Anti-MCV had the highest diagnostic sensitivity of 87.18% and negative predictive value (-PV) of 93.50 for RA patients with HC. The best cut-off for RF-IgM had the highest diagnostic specificity of 75.18%, which its positive likelihood ratio (+LR) and positive predictive value (+PV) were the biggest at 1.81 and 25.00, respectively (Figure 3 and Table 3).
 |
Table 3 Diagnostic Value of Anti-MCV, RF-IgM, PLT and Other Indexes in RA Patients with HC |
Binary Logistic Regression Analysis of RA Patients with HC and Indexes
Univariate binary logistic regression analysis indicated the levels of Anti-MCV>107.68IU/mL, RF-IgM>316.57IU/mL, ESR≤69mm/h and the other results were related to higher risk for the occurrence of HC in RA patients. With this as a basis, multivariate analysis was performed, and the consequences confirmed that the levels of Anti-MCV>107.68IU/mL, ESR≤69.00mm/h, Hb≤99.00g/L and the levels of other indicators were risk factors (Table 4).
 |
Table 4 Binary Logistic Regression Analysis of RA Patients with HC and Influencing Factors |
Discussion
RA with HC is often characterized by a decrease in serum complement, especially in C3 and/or C4, due to excessive consumption of complement in the process of complement activation, and complement deficiency may precipitate RA.14 Consequently, monitoring complement (C3, C4) levels plays a part in assessing the disease activity and prognosis of RA,15 which is in line with our study. These results provided evidence supporting our findings: (1) Compared RA with HC group to RA without HC group, the former were older and had a longer disease duration with increased levels of Anti-MCV, RF (RF-IgA, RF-IgG, RF-IgM), IgM and DAS28 and lower levels of WBC, N, L, Hb, PLT and ESR (all P<0.05). (2) Spearman correlation coefficient analysis suggested that the levels of Anti-MCV, RF (RF-IgA, RF-IgG, RF-IgM), PLT and other indicators were correlated with complement (C3, C4). (3) The results of AUC revealed that PLT in diagnosing RA patients with HC was the largest at 0.65 (95% CI:0.60–0.69). (4) Finally, binary logistic regression analysis of multiple factors declared that advanced age (>66 years), long disease duration (>62 months), high DAS28 value (>6.13), the levels of Anti-MCV>107.68IU/mL, IgM>1.54g/L, ESR≤69.00mm/h, Hb≤99.00g/L and PLT≤305.00×109/L were possible risk factors for the occurrence of HC in RA patients.
The complement system is associated with inflammation and immunity, and its activation plays a major part in the pathogenesis of vasculitis, dermatomyositis, SLE, RA, etc.4,16 Episodes of HC usually occurs in RA patients with multiple joints, vasculitis, rheumatoid pleurisy or other obvious destructive changes.11,17 And Some of these diseases are related to complement consumption and reduced serum complement levels. Several autoantibodies could be tested in RA patients, including RF subtypes, Anti-CCP and Anti-MCV, etc.18 In the research, RA patients with HC were older and had a longer disease duration than RA patients without HC, which was consistent with AE Franco’s findings: the average age and disease duration in RA patients with HC were 59 years and 13.6 years, while they were 57 years and 10 years in RA patients without HC, respectively (all P<0.05).11 These could lead to more severe clinical symptoms and poor prognosis in RA patients with HC. Consequently, it was easy to understand that advanced age (>66 years) and long disease duration (>62 months) were possible risk factors for the occurrence of HC in RA patients. Anti-MCV was an early diagnostic indicator of RA, and the serum Anti-MCV levels had no significant correlation with C3 and C4 levels.19 However, the serum Anti-MCV levels were negatively correlated with C3 and C4 levels (all P<0.001) in this study, and the level of Anti-MCV>107.68IU/mL was also a risk factor, which might be due to different demographic composition and geographical differences. Consistent with the findings of Hunder GG’s research, RA patients with HC exhibited more severe joint involvement and had increased IgM values than RA patients without HC,12 and the level of IgM>1.54g/L was a possible risk factor. Moreover, for pSS patients, IgG elevation is an important risk factor for the occurrence of HC, Lin et al also assess HC not only correlates with disease activity at diagnosis, but also plays an important role as a prognostic marker in predicting outcome.20 Therefore, HC may be closely correlated with more severe joint involvement and poor prognosis of RA patients.
ESR is a common inflammatory indicator, and the detection of ESR level can accurately reflect the systemic inflammatory status of RA patients; in addition, combined with clinical manifestations such as swelling and tenderness of 28 joints, DAS28 is often served as evaluating the disease activity of RA patients.21,22 The results of this research showed that ESR levels were significantly above its reference value in RA with HC group or without HC group, and comparing to RA without HC group, RA with HC group had lower ESR levels (P=0.024), considering reduced ESR level caused by a decrease of globulin level. Further more, DAS28 values were lower among RA patients without HC than those with HC (P=0.006), suggesting that patients in RA with HC had more severe disease activity. In this research, the level of ESR≤69.00mm/h and high DAS28 value (>6.13) were probable risk factors for the occurrence of HC in RA patients, which indicated that HC might be closely correlated with the strengthened disease activity of RA patients. Anemia is a relatively common clinical expression of RA, influencing 30–70% of patients.23 Anemia is closely related to the disease activity of RA and can be served as predicting incipient joint damage of RA.24 The level of Hb104.50 (89.75–119.50)g/L in RA with HC group was lower than the level of Hb110.00 (98.00–121.00)g/L in RA without HC group, and anemia was more serious, which suggested that HC might correlate with disease activity of RA. Furthermore, this research also revealed that the level of Hb≤99.00g/L and PLT≤305.00×109/L were potential risk factors for the development of HC in RA patients. The results of this research were similar to those of Zhou et al: pSS patients with HC had lower Hb and PLT values than pSS patients without HC with statistically significant difference.25 Hence, HC can be related to more severe disease activity of RA patients.
Limitations
Despite the above findings, this study still had some limitations. Firstly, this study was a single-center retrospective study, which could not avoid the existence of selection bias. And due to the sample size was relatively insufficient, more multi-center studies were expected to further confirm our findings. Secondly, although the above differential indicators had been analyzed and their impact on disease activity and prognosis of RA had been confirmed, the dynamic changes of these indicators were still unclear. Finally, even though this study verified that RF subtypes (RF-IgA, RF-IgG, RF-IgM) were possible risk factors for HC in RA patients through univariate analysis, multivariate analysis indicated they were not risk factors. We look forward to further confirming this finding in future studies.
Conclusion
In conclusion, age and disease duration, DAS28, Anti-MCV, IgM, ESR, Hb, and PLT are closely related to the occurrence of HC in RA patients. Timely monitoring of these indicators can help to evaluate disease activity of RA patients and further improve their prognosis.
Ethics Approval
The procedures used in this study involving human participants were in accordance with the tenets of the Declaration of Helsinki and obtained approval from the Ethics Committee of the Second Affiliated Hospital of Nanchang University. Because this study was a retrospective study with exclusion of patient privacy concerns, and we were committed to maintaining absolute confidentiality of patients’ data. Meanwhile, the utilization of this data for any purpose beyond the scope of this study was prohibited. Consequently, the Ethics Committee has granted an exemption from obtaining informed consent.
Funding
This work was kindly supported by the Key Research and Development Planning Project of Jiangxi Province (20203BBGL73149), the Science and Technology Plan of Jiangxi Provincial Health and Family Planning Commission (202210628) and the Research Project of Nanchang University Degree and Postgraduate Education &Teaching Reform (NCUYJSJG-2022-064).
Disclosure
The authors have no conflict of interest to declare.
References
1. Arias de la Rosa I, Escudero-Contreras A, Ruiz-Ponce M, et al. Molecular changes in the adipose tissue induced by rheumatoid arthritis: effects of disease-modifying anti-rheumatic drugs. Front Immunol. 2021;12:744022. doi:10.3389/fimmu.2021.744022
2. Conforti A, Di Cola I, Pavlych V, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. 2021;20(2):102735. doi:10.1016/j.autrev.2020.102735
3. Radu AF, Bungau SG. Management of rheumatoid arthritis: an overview. Cells. 2021;10(11):2857. doi:10.3390/cells10112857
4. Holers VM, Banda NK. Complement in the initiation and evolution of rheumatoid arthritis. Front Immunol. 2018;9:1057. doi:10.3389/fimmu.2018.01057
5. Nass FR, Skare TL, Goeldner I, Nisihara R, Messias-Reason IJ, Utiyama SR. Association of complement factor B allotypes and serum biomarkers in rheumatoid arthritis patients and their relatives. Int J Immunogenet. 2015;42(6):439–444. doi:10.1111/iji.12232
6. Fukami Y, Koike H, Iijima M, Mouri N, Nishi R, Katsuno M. Role of complement components in vasculitic neuropathy associated with systemic lupus erythematosus and rheumatoid arthritis. Muscle Nerve. 2022;66(2):175–182. doi:10.1002/mus.27636
7. Bemis EA, Norris JM, Seifert J, et al. Complement and its environmental determinants in the progression of human rheumatoid arthritis. Mol Immunol. 2019;112:256–265. doi:10.1016/j.molimm.2019.05.012
8. Triggianese P, Conigliaro P, De Martino E, Monosi B, Chimenti MS. Overview on the link between the complement system and auto-immune articular and pulmonary disease. Open Access Rheumatol. 2023;15:65–79. doi:10.2147/OARRR.S318826
9. Ricklin D, Reis ES, Mastellos DC, Gros P, Lambris JD. Complement component C3 - The “Swiss Army Knife” of innate immunity and host defense. Immunol Rev. 2016;274(1):33–58. doi:10.1111/imr.12500
10. Jordán-González P, Gago-Piñero R, Varela-Rosario N, Pérez-Ríos N, Vilá LM. Characterization of a subset of patients with primary Sjögren’s syndrome initially presenting with C3 or C4 hypocomplementemia. Eur J Rheumatol. 2020;7(3):112–117. doi:10.5152/eurjrheum.2020.19132
11. Franco AE, Schur PH. Hypocomplementemia in rheumatoid arthritis. Arthritis Rheum. 1971;14(2):231–238. doi:10.1002/art.1780140206
12. Hunder GG, McDuffie FC. Hypocomplementemia in rheumatoid arthritis. Am J Med. 1973;54(4):461–472. doi:10.1016/0002-9343(73)90042-9
13. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/art.27584
14. Ballanti E, Perricone C, Di Muzio G, et al. Role of the complement system in rheumatoid arthritis and psoriatic arthritis: relationship with anti-TNF inhibitors. Autoimmun Rev. 2011;10(10):617–623. doi:10.1016/j.autrev.2011.04.012
15. Bieber A, Markovits D, Toledano K, et al. Hypocomplementemia during tocilizumab treatment: long-term follow-up results. Medicine. 2022;101(24):e29528. doi:10.1097/MD.0000000000029528
16. Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34(3):J276–86. doi:10.1016/j.jaut.2009.11.014
17. Saraux A, Bourdon V, Devauchelle V, Le Goff P. Is hypocomplementemia useful for diagnosing or predicting extra-articular manifestations in patients with rheumatoid arthritis? Joint Bone Spine. 2001;68(6):487–492. doi:10.1016/S1297-319X(01)00312-8
18. Rönnelid J, Turesson C, Kastbom A. Autoantibodies in rheumatoid arthritis - laboratory and clinical perspectives. Front Immunol. 2021;12:685312. doi:10.3389/fimmu.2021.685312
19. Liu X, Jia R, Zhao J, Li Z. The role of anti-mutated citrullinated vimentin antibodies in the diagnosis of early rheumatoid arthritis. J Rheumatol. 2009;36(6):1136–1142. doi:10.3899/jrheum.080796
20. Lin W, Xin Z, Wang J, et al. Hypocomplementemia in primary Sjogren’s syndrome: association with serological, clinical features, and outcome. Clin Rheumatol. 2022;41(7):2091–2102. doi:10.1007/s10067-022-06135-w
21. Barczyńska TA, Dura M, Blumfield E, et al. DAS28 score vs. ultrasound examination for assessment of rheumatoid arthritis disease activity: comparison and discussion of pros and cons. Reumatologia. 2015;53(4):213–218. doi:10.5114/reum.2015.53999
22. van Riel PL, Renskers L. The Disease Activity Score (DAS) and the disease activity score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(5 Suppl 101):S40–s4.
23. Sun Y, Liu J, Xin L, et al. Factors influencing the Sharp score of 1057 patients with rheumatoid arthritis and anemia: a retrospective study. J Int Med Res. 2022;50(3):3000605221088560. doi:10.1177/03000605221088560
24. Möller B, Everts-Graber J, Florentinus S, Li Y, Kupper H, Finckh A. Low Hemoglobin and Radiographic Damage Progression in Early Rheumatoid Arthritis: secondary Analysis From a Phase III Trial. Arthritis Care Res. 2018;70(6):861–868. doi:10.1002/acr.23427
25. Zhou M, Yuan F. Hypocomplementemia in primary sjogren’s syndrome: a retrospective study of 120 treatment-naive Chinese patients. Int J Gen Med. 2022;15:359–366. doi:10.2147/IJGM.S346188
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.