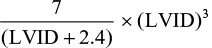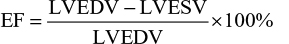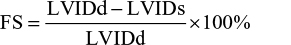Back to Journals » Vascular Health and Risk Management » Volume 13
Right ventricular systolic function in hypertensive heart failure
Authors Oketona OA , Balogun MO , Akintomide AO, Ajayi OE, Adebayo RA , Mene-Afejuku TO , Oketona OT, Bamikole OJ
Received 23 May 2017
Accepted for publication 26 July 2017
Published 27 September 2017 Volume 2017:13 Pages 353—360
DOI https://doi.org/10.2147/VHRM.S142429
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Naga Venkata Amarnath Kommuri
OA Oketona,1 MO Balogun,2 AO Akintomide,2 OE Ajayi,2 RA Adebayo,2 TO Mene-Afejuku,3 OT Oketona,1 OJ Bamikole2
1Fort Nelson General Hospital, Fort Nelson, BC, Canada; 2Cardiology Unit, Department of Medicine, Obafemi Awolowo University Teaching Hospitals Complex, Ife, Osun State, Nigeria; 3Department of Medicine, Metropolitan Hospital Center, New York, NY, USA
Background: Heart failure (HF) is a major cause of cardiovascular admissions and hypertensive heart failure (HHF) is the most common cause of HF admissions in sub-Saharan Africa, Nigeria inclusive. Right ventricular (RV) dysfunction is being increasingly recognized in HF and found to be an independent predictor of adverse outcomes in HF. This study aimed to determine the prevalence of RV systolic dysfunction in HHF by several echocardiographic parameters.
Methodology: One hundred subjects with HHF were recruited consecutively into the study along with 50 age and sex-matched controls. All study participants gave written informed consent, and had a full physical examination, blood investigations, 12-lead electrocardiogram, and transthoracic echocardiography. RV systolic function was assessed in all subjects using different methods based on the American Society of Echocardiography guidelines for echocardiographic assessment of the right heart in adults. This included tricuspid annular plane systolic excursion (TAPSE), RV myocardial performance index (MPI), and RV systolic excursion velocity by tissue Doppler (S′).
Results: RV systolic dysfunction was found in 53% of subjects with HHF by TAPSE, 56% by RV MPI, and 48% by tissue Doppler systolic excursion S′. RV systolic dysfunction increased with reducing left ventricular ejection fraction (LVEF) in subjects with HHF.
Conclusion: A high proportion of subjects with HHF were found to have RV systolic functional abnormalities using TAPSE, RV MPI, and RV S′. Prevalence of RV systolic dysfunction increased with reducing LVEF.
Keywords: right ventricle, systolic function, TAPSE, RV MPI, RV S′, hypertensive heart failure
Introduction
Heart failure (HF) is a major public health problem with increasing worldwide prevalence and is singled out as an emerging epidemic.1 The aging of the population and increasing survival of subjects with cardiac disease due to availability of new treatment methods may account for some of the increase in the prevalence of HF. HF accounts for 3%–7% of hospital admissions in Africa, including Nigeria.2
Hypertensive heart disease is the commonest echocardiographic diagnosis and the commonest cause of HF in Nigeria3 compared to ischemic heart disease in the western world.
The function of the right ventricle is being increasingly recognized as a major determinant of adverse outcomes in subjects with cardiac diseases. Studies have shown that right ventricular (RV) function correlates more closely to exercise capacity in HF than the left ventricular (LV) function.4
The advent of reliable and reproducible echocardiographic measures of RV function such as tricuspid annular plane systolic excursion (TAPSE), RV myocardial performance index (MPI), and tissue Doppler-derived tricuspid lateral annular velocities (S′, E′, A′, E′/A′)5 has made the assessment of RV function in cardiac diseases easier and helped identify the important role of RV in HF subjects. We undertook this study to determine the prevalence of RV systolic dysfunction in subjects with hypertensive heart failure (HHF) using several echocardiographic parameters and to determine if RV systolic dysfunction was associated with other markers of prognosis in HF.
Materials and methods
This study was a cross-sectional study carried out at the Cardiac Care Unit of the Department of Medicine, Obafemi Awolowo University Teaching Hospitals’ Complex, Ife, Osun State. Ethical clearance for the study was obtained from the Ethics and Research Committee of the Obafemi Awolowo University Teaching Hospital. One hundred newly and previously diagnosed subjects with hypertensive HF attending the Cardiology Outpatient Clinics were studied. Fifty apparently healthy subjects were recruited to serve as controls. All subjects with HF were recruited while on HF medications. Age criterion was ≥18 years with no upper age limit. All participants included in the study provided written informed consent prior to enrollment in the study.
The inclusion criterion was subjects who had a clinical diagnosis of hypertensive HF.6 Hypertension was defined as average of three blood pressure measurements ≥140/90 mmHg, according to the Joint National Committee VII guidelines7 and subjects had features of longstanding hypertension including thickened arterial wall, locomotor brachialis, and at least grade 2 hypertensive retinopathy. All subjects met the Framingham criteria8 for congestive HF and were classified based on the New York Heart Association (NYHA) functional classification.9
Subjects with suspected HF from causes other than hypertensive HF based on history, physical examination, and echocardiographic features were excluded from the study. Subjects with chest pain, electrocardiogram (ECG) and echocardiographic features suggestive of ischemic heart disease, elevated troponins, and previous cardiac catheterizations diagnostic of ischemic heart disease were excluded from the study. Cardiac catheterization was not done in all subjects. Other subjects with comorbid diseases such as uncontrolled asthma, chronic obstructive pulmonary disease, stages 3–5 chronic kidney disease, and diabetes mellitus were also excluded from the study. Subjects who had suboptimal echocardiographic windows were also excluded from the study.
Procedure
Data were obtained using a questionnaire that included demographic data and other relevant history details. Complete physical examination was carried out on each study participant. The blood pressure was measured on the right arm in supine and standing positions with an Accoson’s mercury sphygmomanometer. Korotkoff sounds I and V were used for systolic and diastolic blood pressure, respectively. Three consecutive measurements were taken at 5 minute intervals and the average values were recorded.
Investigations
Routine laboratory investigations were done to further assess clinical state of the subjects and rule out comorbidities. These included full blood count (anemia defined as packed cell volume <33%), electrolytes, urea and creatinine, and random blood glucose tests.
Resting 12-lead ECG
The resting 12-lead ECG was obtained using a 3-channel electrocardiograph (Cardiofax YD-907D; Nihon Kohden, Tokyo, Japan). The machine was set at a paper speed of 25 mm/s and amplitude 10 mm/mv. Lead II was used for recording a long rhythm strip. The ECG was specifically analyzed for heart rate and rhythm, QRS axis, PR interval, QRS duration, QT intervals, QTc duration, P wave morphology, pathological Q waves, ST segment and T wave changes, presence of LV or RV hypertrophy, and arrhythmias according to the recommendation of the American Heart Association.10
Transthoracic echocardiography
Two-dimensional (2D), M-mode, and Doppler studies were performed on all subjects using vivid 7 dimension ultrasound imaging system (GE Healthcare Bio-sciences Corp, Piscataway, NJ, USA) with P5s MHz transducer with subjects in the left lateral position. Measurements were taken in accordance with the recommendations of the American Society of Echocardiography by noting the leading edge to leading edge recordings.11 Echocardiographic studies were analyzed for pericardial abnormalities, wall dimensions, chamber size, wall motion abnormalities, intramural thrombi, valve morphology, LV systolic and diastolic function, septal defects, and other congenital heart defects, and estimated intracardiac and vascular pressures.
LV wall and chamber dimensions were measured using 2D-guided M-mode echocardiography with simultaneous electrocardiographic monitoring of the cardiac cycle. The M-mode cursor cut perpendicularly across the interventricular septum, LV chamber, and posterior wall at the level of the mitral valve tips as the cardiac anatomy was visualized with the 2D echo from the parasternal long axis view. Measurements were made with leading edge to leading edge recordings taken at end-diastole and end-systole. End-diastole was recognized as the beginning of the electrocardiographic qRs complex while end-systole was recognized as the peak downward deflection of the interventricular septum and the peak upward deflection of the LV posterior wall.11
LV systolic function
This was assessed using M-mode echocardiography taken from the parasternal long axis view to determine LV wall dimensions, ejection fraction (EF), and fractional shortening (FS). The Teichholz formula12 programmed by the automated facilities in the echocardiography machine was used for determining the LV volumes in end-diastole and end-systole:
|
where LVID is the LV internal dimension.
EF was then determined by the formula
|
where LVEDV is the LV end-diastolic volume and LVESV is the LV end-systolic volume.
FS was determined by the formula
|
where LVIDd and LVIDs are the LV internal diameters in the diastole and systole, respectively.
The above formulae were programmed by the automated facilities in the echocardiography machine. A similar study13 employed the use of the Teichholz formula in determining LV systolic function.
The RV study included RV dimension (RVD), systolic function using TAPSE, and tissue Doppler MPI and RV S′ and diastolic function using pulsed and tissue Doppler velocities of the tricuspid annulus (E, A, E/A, E′, A′ E/E′) according to the American Society of Echocardiography guidelines for the echocardiographic assessments of the right heart in adults.5
RVD
RVD was measured in the apical four-chamber view with focus on the RV. It was recorded as the RV basal diameter taken as the maximal short axis dimension in the basal one third of the RV cavity at end-diastole. Values >4.2 cm were considered abnormal indicating RV dilatation. The relative size of the RV was also compared visually with that of the LV to help determine RV dilatation.
TAPSE
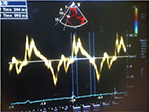
This was acquired in the apical four-chamber RV-focused view by placing the M-mode cursor through the tricuspid annulus and measuring the amount of longitudinal motion of the annulus at peak systole. Values ≤1.6 cm indicated impaired RV systolic function (Figure 1).
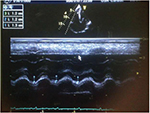  | Figure 1 Abnormal TAPSE, 1.2cm (average value of 1,2 and 3 in Figure 1) in subjects with HF. Abbreviations: HHF, hypertensive heart failure; TAPSE, tricuspid annular plane systolic excursion. |
RV tissue Doppler MPI
This is an index of global RV function and was obtained in the apical four-chamber view and in tissue Doppler mode. Pulsed Doppler sample volume was then placed on the tricuspid annulus (Figure 2). The RV tissue Doppler MPI was calculated as follows
|
The tricuspid valve closure–opening time (TCO) encompasses isovolumic contraction time, ejection time (RVET), and isovolumic relaxation time. RV MPI values >0.55 implied RV dysfunction (Figure 2).
Tissue Doppler systolic velocity of the tricuspid annulus, S′
This was obtained using the same pulsed tissue Doppler image with sample volume on the tricuspid annulus as for RV MPI. RV S′ was recorded as the highest systolic velocity and values ≤10 cm/s indicated impaired RV systolic function (Figure 3).
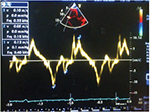  | Figure 3 Abnormal RV S’, 0.10m/s in subjects with HF. Abbreviations: HF, heart failure; RV, right ventricular; S’, systolic excursion velocity by tissue Doppler. |
Data analysis
Data were analyzed using the statistical package for social sciences (IBM Corporation, Armonk, NY, USA), version 20.0 software. Data summary and presentation techniques such as arithmetic means, standard deviation, and frequency distribution tables and charts were used where appropriate. Categorical variables were represented in numbers and percentages and charted where appropriate. Chi-square analysis was used to describe differences between categorical variables. Statistical 2 sample t-test and t-test in regression output were used to assess significant differences between group means of quantitative variables. Analysis of variance was used to compare means of variables with more than two categories. Pearson’s correlation analysis was used where necessary. Statistical significance was defined as p-values <0.05.
Results
One hundred subjects with HF and 50 apparently healthy subjects were studied.
Clinical parameters of study subjects
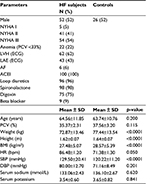
Table 1 shows the clinical parameters of the study subjects. The HF subjects were matched for age and sex with healthy controls in a ratio of 2:1. The mean ages of both groups were similar (64.56±11.85 vs 63.74±10.76 years; p=0.200). Body mass index was significantly lower in HF subjects compared to controls. HF subjects had significantly higher baseline systolic blood pressures than controls (129.50±20.41 vs 120.22±11.20 mmHg, p<0.001).
Fifty-two percent of subjects satisfied Framingham’s major criteria for HF. All subjects with HF were in NYHA stages I–III, with subjects in NYHA II and III accounting for 41% and 54% of HF subjects, respectively.
Echocardiographic parameters of study subjects
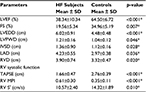
Table 2 shows the echocardiographic parameters in study subjects. All echocardiographic variables were significantly different between subjects and controls. LV ejection fraction (LVEF) and FS mean values were significantly lower in subjects with HF than controls, while LV end-diastolic diameter (LVEDD) and left atrial diameter were significantly higher in HF subjects than controls. TAPSE and RV S′ were significantly lower in HF subjects than in controls while RV MPI was significantly higher in HF subjects than in controls. Eighty-four percent of HF subjects had reduced LVEF (LVEF <50%).
RV systolic dysfunction in HF subjects
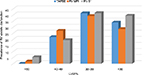
Figure 4 shows the prevalence of RV systolic dysfunction in HF subjects by TAPSE, RV MPI, and S′. Prevalence of RV systolic dysfunction in HF subjects with preserved EF (LVEF >50%) was 1.89% by TAPSE and 5.36% by RV MPI. The prevalence of RV systolic dysfunction was highest in HF subjects with LVEF between 30% and 39% (41.51% by TAPSE, 39.29% by RV MPI, and 41.67% by RV S′) and subjects with LVEF <30% (33.96% by TAPSE, 28.57% by RV MPI, and 39.58% by RV S′).
Correlation of RV echocardiographic variables with clinical and other echocardiographic variables in HF subjects
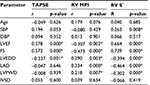
Bivariate correlation analysis showed that TAPSE and RV S′ correlated positively and significantly with LVEF (r=0.578, p<0.001; r=0.664, p=0.002) while RV MPI showed a significant negative correlation with LVEF (r=−0.307, p<0.001). TAPSE, RV MPI, and RV S′ also significantly correlated with FS (p<0.001) and LVEDD (p<0.05). RV S′ had a significant positive correlation with SBP (r=0.263, p=0.008) as shown in Table 3.
Discussion
This study shows that RV systolic dysfunction is common in subjects with HF and goes further to investigate RV systolic function using alternative echocardiographic parameters other than TAPSE which is mostly routinely used to assess RV function in echocardiographic studies. It also shows that RV MPI and RV S′ can also be reliably used to assess RV systolic function in HF subjects.
We found statistically significant differences in the prevalence of RV systolic dysfunction between subjects with HHF and control subjects using all three echocardiographic parameters. A high prevalence of RV systolic dysfunction was found using all three echocardiographic parameters – 53% by TAPSE, 56% by RV MPI, and 48% by RV S′. Ojji et al13 also found a high prevalence of RV systolic dysfunction in subjects with HHF in Nigeria using TAPSE (44.5%). TAPSE is simple and easily reproducible and has been found to correlate with the radionuclide angiographic estimate of RV global systolic function.14 However, TAPSE assumes that the longitudinal displacement of a single segment represents the function of a three-dimensional (3D) structure and is found to be angle and load dependent.5 Kjaergaard et al15 studied RV function in HF subjects using TAPSE and found that although TAPSE is reduced with LV dysfunction in HF the absolute reduction is small and seemed to be of minor importance in the clinical utilization of TAPSE as a measure of RV systolic function or as a prognostic factor. The fact that there are limitations to the use of a single method of assessing function necessitates the validation of other methods to serve as alternatives or further validate results obtained using one method.
Right sided MPI
The right sided MPI also known as the Tei index is a global estimate of both systolic and diastolic function of the right ventricle. We found the RV systolic dysfunction in HHF subjects in this study to be 56% using the RV MPI.
MPI was shown by Karnati et al16 to correlate with radionuclide-derived RV EF. Tei et al17 found that RV MPI was a strong predictor of clinical status and survival in subjects with pulmonary hypertension. The LV Tei index has been well studied in subjects with HF18,19 however, there are less studies on the clinical value of the RV Tei index. Thus, we decided to also assess RV function in subjects with HHF using MPI.
Tissue Doppler-derived tricuspid lateral annular systolic velocity (RV S′)
This was also used to assess RV systolic function in this study. RV S′ velocity <10 cm/s indicates RV systolic dysfunction5 and was found in up to 48% of subjects with HHF in this study. Damy et al20 compared four RV systolic echocardiographic parameters (TAPSE, RV S′ aka peak systolic velocity [PSV] tdi, RV fractional area, and 3-integral of the systolic wave) to predict adverse outcomes in chronic HF and concluded that RV S′ (PSV tdi) <9.5 cm/s was the best predictor of the combined endpoint (death, urgent heart transplantation, urgent ventricle assist device implantation, or acute HF episode). RV S′ has population-based validation studies and mean values for normal populations are available21 which makes this echocardiographic parameter even more useful.
Clinical and echocardiographic correlates of RV systolic dysfunction
Echocardiographic measures of RV systolic function have been previously found to be independent of age and gender in the healthy population.15,22 This study also found the same to be true in subjects with HHF. Kjaergaard22 et al reported that TAPSE does not correlate with age or gender in HF subjects. Ojji in Nigeria23 also reported that TAPSE did not correlate with gender in HHF. It is not known why echocardiographic measures of RV function do not correlate with age or gender; however, some studies showed that RV end-diastolic volume and 3D echocardiographic imaging of the RV demonstrate significant differences with gender and age.15 The lack of a relation of TAPSE with gender makes it possible for the same reference values to be used for both sexes.
This study found significant correlation between echocardiographic variables of RV systolic function and several indices of LV function. TAPSE and RV S′ correlated positively and significantly with LVEF (r=0.578, p<0.001; r=0.664, p=0.002) while RV MPI showed a significant negative correlation with LVEF (r=−0.307, p<0.001). TAPSE, RV MPI, and RV S′ also significantly correlated with FS (p<0.001) and LVEDD (p<0.05). RV S′ had a positive correlation with SBP. Prevalence of RV dysfunction increased with decreasing LVEF in this study. Several studies24,25 have reported positive correlations between echocardiographic variables of RV systolic function and LVEF and LV chamber dimensions. Guglin et al25 noted that RV systolic dysfunction correlated positively with LVEDD in HF subjects. Karaye et al26 also reported that LVEF was the best correlate of TAPSE and RV S′; however, this was in a cohort of hypertensive subjects without features of HF. In this study, subjects with reduced EF had the highest prevalence of RV systolic dysfunction (61.9% and 63% by TAPSE and RV MPI, respectively). This observation was also reported in studies by de Groote et al4 (52%) and by Puwanant et al27 (63%–76%).
RV function worsens with increasing severity of LV dysfunction and this may result from the concept of ventricular interdependence.28 HF subjects with reduced EF but normal RV function have better prognosis compared to those with impaired RV systolic function.29 Subjects with HF with preserved EF may also have RV dysfunction and this has been found to be associated with clinical and echocardiographic evidence of more advanced HF and is predictive of poorer outcomes.29
Limitations of the study
Ischemic heart disease was excluded in subjects by history, physical eaxmination, electrocardiography, and wall motion abnormalities on echocardiography. Cardiac catheterization or myocardial perfusion imaging was not performed; so subjects with subclinical features of ischemic heart disease may have been missed.
Conclusion
RV systolic dysfunction is common in subjects with hypertensive HF and was found in >50% of such subjects using TAPSE and RV MPI and in 48% with RV S′. All three echocardiographic parameters of RV systolic function correlated with LVEF and FS.
Disclosure
The authors report no conflicts of interest in this work. OA Oketona and OT Oketona are a married couple.
References
Braunwald E. Shattuck lecture – cardiovascular medicine at the turn of the millenium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. | ||
Oyoo GO, Ogola EN. Clinical and socio demographic aspects of congestive heart failure patients at Kenyatta National Hospital, Nairobi. East Afr Med J. 1999;76:23–27. | ||
Adebayo RA, Akinwusi PO, Balogun MO, et al. Two-dimensional and Doppler echocardiographic evaluation of patients presenting at Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria: a prospective study of 2501 subjects. Int J Gen Med. 2013;6:541–544. | ||
deGroote P, Millaire A, Foucher-Hossein C, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. | ||
Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. | ||
Araoye MA, Olowoyeye O. The clinical spectrum of hypertensive heart failure: a point score system for solving an old problem. E Afr Med J. 1984;61:306–314. | ||
US Department of Health and Human Sciences. The Seventh Report of the Joint National Committee on Prevention, Evaluation and Treatment of High Blood Pressure. NIH publication 2004;04:5230. | ||
McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1442. | ||
The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Little, Brown & Co; 1994: 253–256. | ||
Mason JW, Hancock EW, Gettes LS, et al. Recommendations for the standardization and interpretation of the electrocardiogram. Part 11: electrocardiography diagnostic statement list. A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology Foundation; and the Heart Rhythm Society endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2007;49:1128–1135. | ||
Sahn DJ, DeMarria A, Kisslo J, Weyman. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58(6):1072–1083. | ||
Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic–angiographic correlations in the presence or absence of synergy. Am J Cardiol. 1976;37:7. | ||
Ojji DB, Lecour S, Atherton JJ, Blauwet LA, Alfa J, Sliwa K. Right ventricular systolic dysfunction is common in hypertensive heart failure: a prospective study in sub-Saharan Africa. PLoS One. 2016;11(4):e0153479. | ||
Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–531. | ||
Kjaergaard J, Sogaard P, Hassager C. Quantitative echocardiographic analysis of the right ventricle in healthy individuals. J Am Soc Echocardiogr. 2006;19:1365–1372. | ||
Karnati PK, El-Hajjar M, Torosoff M, Fein SA. Myocardial performance index correlates with right ventricular ejection fraction measured by nuclear ventriculography. Echocardiography. 2008;25:381–385. | ||
Tei C, Dujardin KS, Hodge DO, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838–847. | ||
Akintunde AA. The clinical value of Tei index among Nigerians with hypertensive heart failure: correlation with other conventional indices. Cardiovasc J Afr. 2012;23:40–43. | ||
Ogunmola OJ, Akintomide AO, Olamoyegun AM. Relationship between clinically assessed heart failure severity and the Tei index in Nigerian patients. BMC Res Notes. 2013;6:488. | ||
Damy T, Viallet C, Lairez O, et al. Comparison of four right ventricular systolic echocardiographic parameters to predict adverse outcomes in chronic heart failure. Eur J Heart Fail. 2009;11:818–824. | ||
Lindqvist P, Waldenstrom A, Henein M, Morner S, Kazzam E. Regional and global right ventricular function in healthy individuals aged 20–90 years: pulsed Doppler tissue imaging study: Umea General Population Heart Study. Echocardiography. 2005;22:305–314. | ||
Kjaergaard J, Iversen KK, Akkan D, et al. Predictors of right ventricular function as measured by tricuspid annular plane systolic excursion in heart failure. Cardiovasc Ultrasound. 2009;7:51–57. | ||
Ojji D. Biomarkers of Ventricular Remodelling in African Hypertensives [dissertation]. Cape Town: Faculty of Medicine, University of Cape Town; 2013. | ||
Lopez-Candales A, Dohi K, Rajagopalan N, Edelman K, Gulyasy B, Bazaz R. Defining normal variables of right ventricular size and function in pulmonary hypertension: an echocardiographic study. Postgrad Med J. 2008;84:40–45. | ||
Guglin M, Win CM, Darbinyan N, Wu Y. Predictors of right ventricular systolic dysfunction in compensated and decompensated heart failure. Congest Heart Fail. 2012;18:278–283. | ||
Karaye KM, Sai´du H, Shehu MN. Right ventricular dysfunction in a hypertensive population stratified by patterns of left ventricular geometry. Cardiovasc J Afr. 2012;23:478–482. | ||
Puwanant S, Priester TC, Mookadam F, Bruce CJ, Redfield MM, Chandrasekaran K. Right ventricular function in patients with preserved and reduced ejection fraction heart failure. Eur J Echocardiogr. 2009;10:733–737. | ||
Santamore WP, Dell Italia LJ. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis. 1998;40:289–308. | ||
Mohammed SF, Roger VL, Abou Ezzeddine OF, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction. Circulation. 2014;130:2310–2320. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.