Back to Journals » Infection and Drug Resistance » Volume 13
Rifampicin-Resistant Mycobacterium tuberculosis Among Patients with Presumptive Tuberculosis in Addis Ababa, Ethiopia
Authors Araya S , Negesso AE , Tamir Z
Received 21 May 2020
Accepted for publication 25 August 2020
Published 6 October 2020 Volume 2020:13 Pages 3451—3459
DOI https://doi.org/10.2147/IDR.S263023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Shambel Araya, Abebe Edao Negesso, Zemenu Tamir
Department of Medical Laboratory Sciences, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Zemenu Tamir
Department of Medical Laboratory Sciences, College of Health Sciences, Addis Ababa University, P.O. Box: 1176, Addis Ababa, Ethiopia
Tel +251 915 992 362
Email [email protected]
Background: Drug-resistant tuberculosis remains a major public health threat complicating tuberculosis control programs globally. Data on rifampicin resistance (RR), which is a surrogate marker for multidrug resistance, are limited among Ethiopian tuberculosis patients. This study aimed to determine the magnitude of rifampicin-resistant Mycobacterium tuberculosis (RR-MTB) among presumptive tuberculosis patients attending St. Peter Tuberculosis Specialized Hospital, Addis Ababa, Ethiopia.
Patients and Methods: A retrospective cross-sectional study was conducted at St. Peter Tuberculosis Specialized Hospital from January 2016 to December 2018. After checking completeness of the necessary information, data of tuberculosis-presumptive cases who underwent Gene Xpert® testing were collected from medical records using a data-extraction format prepared for this study purpose. Data were double entered and analyzed using SPSS version 20 statistical software.
Results: A total of 12,685 presumptive tuberculosis patients were included; of whom 54.5% were males and the mean age of the study participants was 40.3± 18.7 years. Mycobacterium tuberculosis (MTB) was detected in 1714 participants (13.5%). Of these MTB cases, 169 cases (9.8%) were confirmed to have RR-MTB. Prevalence of MTB was relatively higher among males (15.1%, P=0.78); whereas RR-MTB was higher among females (10.3%, P=0.81). The incidence of MTB and RR-MTB was significantly associated with treatment history (P=0.042 and P=0.025), respectively. HIV infection has significantly associated with incidence of RR-MTB (P=0.032), but not with MTB (P˃0.05). Prevalence of MTB and RR-MTB had a declining trend through time, being 16.7% and 12.9%, 12.8% and 9.1%, and 12.2% and 7.9% in 2016, 2017 and 2018, respectively.
Conclusion: This study showed a decreasing trend of both MTB and RR-MTB from 2016 to 2018 in an MTB, MDR-MTB, and TB/HIV co-infection high-burden setting, Addis Ababa, Ethiopia. Occurrence of MTB and RR-MTB was associated with treatment history. Therefore, improvement in treatment adherence of identified cases would be helpful to prevent emergence or re-emergence of MTB and RR-MTB cases.
Keywords: Mycobacterium tuberculosis, rifampicin resistance, Ethiopia, St. Peter Tuberculosis Specialized Hospital
Introduction
Despite the recent progress of global control efforts, tuberculosis (TB) remains a major public health threat globally.1,2 It remains the top infectious killer worldwide with 10 million people falling ill in 2018.3 According to the World Health Organization (WHO) 2019 report, there were an estimated 1.2 million TB deaths among HIV-negative people in 2018, and an additional 251,000 deaths among HIV-positive people. Since 2007, TB has been the leading cause of death from a single infectious agent, ranking above HIV/AIDS.3 Globally, the TB mortality rate is falling at about 3% per year. TB incidence is falling at about 2% per year; this needs to improve to 4–5% per year by 2020 to reach the first milestones of the End TB Strategy.1,4
Multidrug-resistant tuberculosis (MDR-TB) is said to occur if the strain of Mycobacterium tuberculosis is resistant to at least rifampicin and isoniazid is a threat, which complicates diagnosis, treatment and control of the disease.4,5 In 2017, the WHO estimated 558,000 new cases of rifampicin-resistant TB (RR-TB) globally, and among these cases an estimated 82% had multidrug-resistant TB (MDR-TB); on average 3.5% of new TB cases and 18% of previously treated cases had MDR/RR-TB.6 The WHO-recommended rapid diagnostic test for detection of TB and RR is the Gene Xpert® MTB/RIF assay (Cepheid, USA). The assay is performed using the Gene Xpert® platform and is a fully automated nested real-time polymerase chain reaction (PCR) system, which simultaneously detects MTB complex DNA in sputum and identifies mutations in the gene that are associated with RR.7,8
Ethiopia ranks 10th among the high TB-pandemic countries and 15th among the 27 high-MDR-TB countries with more than 5000 estimated MDR-TB patients each year.5,9 A recently published meta-analysis report indicated that in Ethiopia 2.18% of newly diagnosed and 21.07% of previously treated patients have MDR-TB with overall prevalence of 7.24%.10 Similarly, a meta-analysis and systematic review by Eshetie et al has revealed that the burden of MDR-TB remains high in Ethiopian settings, and previous TB treatment was the most powerful predictor for MDR-MTB infection.11
In Ethiopia, sputum-smear microscopy using an acid fast bacilli staining technique has been the backbone of TB-case detection in the past few decades. Mycobacterium tuberculosis culture, the gold standard test, and drug resistance testing is limited only to regional laboratories and primarily used for research purposes due to a scarcity of resources and equipped laboratory facilities. Determining the magnitude of RR-MTB with advanced technology is critical to establish the preventive strategies of drug-resistant tuberculosis. As long as the published literatures are concerned, the magnitude of RR-MTB using Xpert® MTB/RIF in Ethiopia is limited. Therefore, this study aimed to retrospectively determine the magnitude of MTB and RR-MTB among presumptive TB patients at St. Peter Tuberculosis Specialized Hospital, Addis Ababa, Ethiopia.
Patients and Methods
This retrospective cross-sectional study was conducted at St. Peter Tuberculosis Specialized Hospital, Addis Ababa, Ethiopia. The hospital is under the Ethiopian Federal Health Ministry which has been providing MDR‑TB treatment for patients referred from all regional states and city administrations of Ethiopia. The hospital has the mission to provide diagnostic, therapeutic and monitoring care for all referral TB cases in the country and in-patient care for both susceptible and resistant TB cases.
In this study, a total of 12,685 presumptive TB patients referred to St. Peter TB Specialized Hospital from January 2016 to December 2018 who have a complete record of the necessary data were included. A single sputum specimen from each patient was analyzed using the Gene Xpert® MTB/RIF assay (Cepheid, USA) following the manufacturer’s instructions by trained medical laboratory technologists working in the medical laboratory department of the Hospital.
Study Instrument and Variables
Variables such as sociodemographics, date of registration, medical history, laboratory results and other information including HIV/AIDS status and previous tuberculosis treatment status were recorded from the patients’ medical register. Participants’ data were collected using a pre-tested structured data extraction format specifically prepared for this study.
Data Analysis
Data were entered and analyzed using Statistical Package for Social Sciences (SPSS) for Windows version 20.0 statistical software. Descriptive statistics such as frequency and percentage count were used to summarize data; tables and figures to present data. The chi-square test was used to test the association of MTB and RR-MTB with independent variables. A P-value of <0.05 was considered statistically significant.
Ethical Consideration
The study protocol was ethically approved by the Research Ethics Committee of Addis Ababa University, College of Health Sciences, Department of Medical Laboratory Sciences with protocol approval number DRERC/356/18/UG to conduct the study. In this study, we have collected data of patients retrospectively from medical records after getting permission to conduct the study from St. Peter Tuberculosis Specialized Hospital. Informed consent was not sought from study participants since the study was retrospective and we have used the clinical data obtained for routine medical services. All patient identifiers were removed and only code numbers were used throughout the study to keep confidentiality of data.
Result
In this study a total of 12,685 patients were involved; 7040 males and 5634 females. Among these, about 88.8% had unknown HIV sero-status (not available in the record) and 6.1% were HIV-positive. About 43.6% of the study participants were within the age group between 21 and 40 years and the mean age was 40.3±18.7 years. The new cases comprise 85% of study participants, followed by the relapse cases (13.5%) (Table 1).
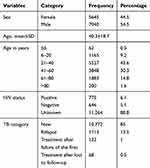 |
Table 1 Demographic and Clinical Characteristics of Study Participants at St. Peter Tuberculosis Specialized Hospital, Addis Ababa, Ethiopia, from 2016 to 2018 |
Among the total presumptive TB study participants, the overall prevalence of MTB was 13.5% (MTB was detected among 1714 patients from a total of 12,685 participants). It was 15.1% and 11.5% among male and female study participants, respectively, and gender did not show a statistically significant association with the prevalence of MTB infection (P=0.782). Patients within the age category of 6–20 years were found to have a higher proportion of MTB cases (22.7%), followed by 21–40 years (18.7%) and >80 years (16.5%) old, whereas it is relatively lower among children aged ≤5 years (8.5%); MTB prevalence showed a statistically significant difference over the different age groups (P=0.039) (Table 2).
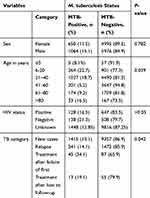 |
Table 2 Association of Clinical and Sociodemographic Factors with M. Tuberculosis Among Presumptive TB Patients Who Visited St. Peter Tuberculosis Specialized Hospital from 2016 to 2018 (n=12,685) |
Similarly, among presumptive TB patients the proportion of MTB was 34.1%, 19.1%, 14.1% and 13.1% among study participants coming for treatment after failure of the first treatment, for treatment after lost to follow-up, for lapsing cases and for new cases, respectively. Mycobacterium tuberculosis infection showed a statistically significant variation among study participants with different treatment categories (P=0.042). Among HIV sero-positive presumptive TB patients, MTB was detected among 16.5% of the participants; however, it did not show a significant difference with HIV sero-status of the participants (P˃0.05) (Table 2).
Among 1714 MTB confirmed cases, RR was detected among 169 patients (9.8%) and 20 cases were indeterminate (1.17%). Rifampicin resistance was a bit higher among female patients (10.3%) than males (9.6%) (P=0.81). Although the participants were few in number, RR was detected in one child among five MTB-confirmed children within 5 years of age; and RR showed a significant difference among the different age categories (P≤0.001). Similarly, among new, relapse, treatment after failure of the first treatment and treatment after lost to follow-up cases were 8.3%, 15.76%, 22% and 23%, respectively, revealing a significant difference of RR among the different categories of MTB cases (P=0.025). In relation to HIV sero-status of MTB cases, RR was 12.5%, 9.1% and 7.2% among HIV sero-positive, unknown and sero-negative patients, respectively, showing a significant difference with HIV sero-status of the patients (P=0.032) (Table 3).
 |
Table 3 Association of RR-MTB with Sociodemographic and Clinical Factors of Patients |
In this study area, prevalence of MTB had shown a slight decline through time; 16.7% in 2016 to 12.2% in 2018. Similarly, RR among MTB patients showed a declining trend from 12.9% in 2016 to 7.9% in 2018 (Figure 1).
 |
Figure 1 Trend of MTB and rifampicin resistance from 2016 to 2018 at St. Peter Tuberculosis Specialized Hospital, Addis Ababa, Ethiopia. |
Discussion
Tuberculosis is largely a disease of poverty which disproportionately affects peoples in resource-scarce settings, particularly in Africa and Asia. More than 90% of new TB cases and deaths occur in developing countries.6 The risk of tuberculosis is higher among people living in low socioeconomic status, the immune-suppressed, extreme age (older and children) groups, smokers and those having close contact with active tuberculosis cases.12,13 On top of being the major infectious killer worldwide, the emergence of drug-resistant forms of TB which need more resources to detect, to successfully treat and to effectively reduce the burden is among the top challenges we are facing.3,14
This study revealed an overall 13.5% prevalence of MTB among presumptive TB patients. This finding is lower compared to previous studies conducted in different parts of Ethiopia as well as in different countries around the world; 15.11% in Addis Ababa,15 20% in Gambella (Southwest Ethiopia),16 24.3% in Bahirdar (Northwest Ethiopia),17 21.32% in Kathmandu, Nepal,18 23% in Northern Nigeria (23%),19 27.6% in India,20 26% in South Africa21 and 37.7% in Lagos, Nigeria.22 The probable reason for this lower prevalence of MTB might have been geographical and demographical variation in the study population as well as the decreasing trend of tuberculosis which is in line with the WHO estimates.
According to the 2019 WHO Global Tuberculosis Report, TB affects people of both sexes in all age groups but the highest burden was in adult men, who accounted for 57% of all TB cases in 2018.3 In the current study, it was observed that MTB was more frequent among males (15.1%) compared to females (11.5%); this was in agreement with studies such as Datiko and Lindtjorn,23 Jaleta et al24 and Jetan et al.25 This difference in incidence of tuberculosis among gender might be due to differences in social roles, risk behaviors and activities. Males may travel more frequently; have more social contacts; spend more time in settings that may be conducive to transmission, such as bars; and engage in professions associated with a higher risk for tuberculosis.26 Women may also underreport their disease and seek care outside health institutions because of socioeconomic constraints.
Age remains an important variable influencing the risk of disease progression following a primary infection with Mycobacterium tuberculosis. Disease manifestation patterns show clear associations with age at the time of primary infection.27 Despite the fact that TB is an important cause of childhood morbidity and mortality worldwide, childhood tuberculosis has been neglected by health programmers and academics because children frequently have paucibacillary disease and are not thought to be infectious from a public health perspective.28,29 Childhood tuberculosis is an indicator of a recent transmission of the disease in the community.30 Although the true burden of childhood TB is underestimated because of the challenge in diagnostic accuracy, children accounted for 11% of the overall global TB cases that occurred in 2018.3 A mathematical modeling study of burden of childhood TB in 22 high-burden countries showed that the predicted proportion of tuberculosis burden in children for each country is correlated with incidence, varying between 4% and 21%.28 Although the national burden of childhood tuberculosis is not known in Ethiopia, retrospective studies conducted in Tigray region, Northern Ethiopia31 and Southern Ethiopia32 showed 8.1% and 13% among all notified tuberculosis cases, respectively.
Even though it is difficult to compare with previous studies directly, as different studies used different cutoff points for age groups, in this study TB was more prevalent among participants within age groups of 6–20 years (22.7%) and 21–40 years (18.7%); and lower within age groups of 41-60 years. This was in agreement with previous studies conducted in different parts of Ethiopia such as Gambela,16 Gondar,24 Debre Markos33 and Southern Ethiopia.34 The prevalence of TB in under-5 children was 8.1%, which was relatively lower when compared to the adolescent group. This is a good indicator for the impact of TB on the young age group of the society and this could be likely due to high mobility from place to place, increased risky sexual behaviors, high workload and environmental exposure that increases acquisition of TB infection. This could also add up on the economic burden of society in developing countries.
Ethiopia is among the countries which are endemic with TB, MDR-TB and TB-HIV co-infection.6 It has 1% overall national adult HIV/AIDS prevalence35 and 22% pooled prevalence of TB-HIV co-infection.36 Although the majority of presumptive TB patients (88.8%) in this study had unknown HIV status (not tested), the prevalence of TB-HIV co-infection was 16.5%. Our finding was higher than previous studies in Addis Ababa (6.7%)15 and Nigeria (12.5%),37 but relatively lower than the WHO 2016 estimate of TB/HIV co-infection in Ethiopia (19.1%).4 This discrepancy might be due to the epidemiological variation of HIV in different communities and the level of HIV testing performed in different studies.
The prevalence of RR-MTB in this study was 9.8%, which was comparable to the 9.9% prevalence reported from Addis Ababa,15 10.3% prevalence from Debre Markos33 and 9.9% from Kenya.38 However, our finding was lower compared to reports by Mesfin et al from Addis Ababa (39.4%),39 Jaleta et al from Gondar (15.8%),24 Hordofa from Southern Ethiopia (3.4% of RR-MTB from 16.5% total MTB cases, or 16.3%)40 and Adejumo et al from Lagos, Nigeria (23.4%).22 Meanwhile, it was higher than findings from previous studies conducted in Gambella (4.9%),16 in Afar region Dubti (4.3%),41 in Eastern Ethiopia (1.7%),42 in East Gojjam (2.59%)43 and in Nigeria (6.9%).37 These differences from place to place could be due to differences in patient selection, TB case management, diagnosis and treatment compliance. Additionally, rifampicin has several adverse effects that could result in patient non-adherence, and hence may lead to an increase in resistant strains.
It is known that MDR-TB can result from either being infected by a drug-resistant MTB from index patients of a primary drug resistance or drug-susceptible MTBC strains can develop resistance to anti-TB drugs resulting in acquired drug resistance.44 Drug-resistant TB is largely the consequence of human error as a result of poor supply management and quality of anti-TB drugs and inadequate or improper treatment which is further exacerbated by HIV.45 Studies have reported that retreatment MTB cases are more likely to harbor strains with full or partial drug resistance for drugs used in previous treatment so that acquired drug resistance is mostly observed among them.43,46 Recent review articles from Ethiopia have revealed a history of previous treatment is the major determinant for MDR-TB after reviewing 34 and 16 articles10,11. Globally in 2017, an estimated 3.5% (95% confidence interval [CI]: 2.5–4.7%) of new cases and 18% (95% CI: 6.3–34%) of previously treated cases had MDR/RR-TB.6 The current study showed 23%, 22.2%, 15.76% and 8.3% prevalence of RR-MTB among patients treated after lost to follow-up, treatment after failure of first treatment, relapse cases and new cases, respectively (P=0.025), so that majority were retreatment cases. This was in agreement with previous studies conducted in different areas16,24 as well as with the Ethiopian national prevalence for previously treated cases.6
A study conducted among smear-positive pulmonary tuberculosis patients in Eastern Ethiopia has reported that any drug resistance was associated with HIV infection (COR=3.7, 95% CI: 1.905–7.222) (P=0.000).42 Our study showed a higher rifampicin resistance among HIV sero--positive tuberculosis patients (12.5%) than sero-negative patients (7.2%) (P=0.032). In support of our finding, whether it is statistically significant or not, several studies revealed that TB patients with concurrent HIV infection were at higher risk of acquiring rifampicin resistance.46–49
The WHO Global Tuberculosis Report indicated that the average rate of decline in the TB incidence rate was 1.6% per year in the period 2000−2018, and 2.0% between 2017 and 2018; and the cumulative reduction between 2015 and 2018 was only 6.3%. Ethiopia is among the 30 high-TB-burden countries, those on track to reach the 2020 milestone of a 20% reduction in the TB incidence rate.3 The country has been practicing educational, managerial and regulatory interventions for prevention of TB, containment of antimicrobial resistance in general and MDR-TB in particular to curb the problem. Among the measures taken were client and community empowerment and awareness using adherence counseling, group educations and mass media broadcasts; capacity building as pre-service and in-service training of health practitioners; standardization of practices and antimicrobial use policy; improving access to antimicrobials, case detection through quality assured laboratories, monitoring and evaluation system with TB infection control impact measure and establishing/strengthening of antimicrobial stewardship committees.45,50,51 In line with this, our study showed a declining trend of MTB from 16.7% to 12.8% and 12.2% in 2016, 2017 and 2018, respectively. Similarly, prevalence of rifampicin RR-MTB was declining from 12.9% to 9.0% and to 7.9%, respectively. Our finding was also supported by other studies done in Southern Ethiopia,52 Western Ethiopia16 and in Jigjiga district53 revealing declining patterns of MTB/RR. The probable reasons for this decrease in MTB or RR-MTB incidence might be the continuous implementation of DOTS which is vital for effective adherence of patients to treatment, increased availability of health facilities and health service delivery improvement and increased awareness of the public about the disease.52
The strength of our study was the use of routine clinical data collected from a real-life clinical setting to describe the prevalence and trend of MTB and RR-MTB among presumptive tuberculosis patients, who underwent Gene Xpert® testing at a Tuberculosis Specialized Referral Hospital, on which information was limited among Ethiopian participants. However, the findings of this study should be considered alongside its limitations. The study was conducted by retrospectively reviewing medical records of patients who underwent Gene Xpert® test for MTB/RR. The majority of presumptive TB patients had unknown HIV status (not tested) which could be a limitation since HIV infection is an important determinant for incidence of TB and its drug resistance. The study did not include mycobacteriological laboratory works such as sputum smear examination using acid fast bacilli stain and culture as well as chest X-ray results as comparators for Gene Xpert® findings. In addition, we could not identify whether the rifampicin-resistant cases were tuberculosis or non-tuberculosis mycobacteria since the Gene Xpert® test result was a sole diagnostic for TB and RR for eligible cases in the study setting.
The recent unprecedent COVID-19 crisis may have an impact on TB prevention and control systems in different aspects. The stay at home measure taken to prevent the spread of COVID-19 might facilitate the household transmission of TB. The disruption of the healthcare settings due to the workload and service shift by COVID-19 might compromise treatment and the diagnostics service of TB. On top of this, the enormous damage of global and national economies by the pandemic will have an ultimate impact on TB prevention and control systems especially in resource-scarce high-TB-burden developing countries like Ethiopia; which might affect the WHO End TB Strategy by 2035.
Conclusion
Generally, the study has shown a decreasing trend of both incidence of MTB and RR-MTB from 2016 to 2018 in an MTB, MDR-MTB and TB/HIV co-infection high-burden setting, Addis Ababa, Ethiopia. Occurrence of MTB and RR-MTB was found associated with previous treatment history. Therefore, strict adherence to follow-up in identified cases would be helpful to prevent emergence or re-emergence of MTB and RR-MTB.
Abbreviations
TB, tuberculosis; MTB, Mycobacterium tuberculosis; RR, rifampicin resistance; RR-MTB, rifampicin-resistant Mycobacterium tuberculosis; HIV, human immune deficiency virus; DOTS, directly observed treatment, short course.
Data Sharing Statement
The data sets used or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for Publication
Not applicable as details like videos or images related to study subjects were not recorded for this study.
Acknowledgments
We are indebted to all study participants whose data were used in this study. We would like to thank St. Peter Tuberculosis Specialized Hospital management and staff for all the support given to carry out this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
We authors declare that there is no conflict of interest in this work.
References
1. World Health Organization. Global Tuberculosis Report 2017. Geneva: World Health Organization; 2017.
2. Banuls AL, Sanou A, Van Anh NT, Godreuil S. Mycobacterium tuberculosis: ecology and evolution of a human bacterium. J Med Microbiol. 2015;64(11):1261–1269. doi:10.1099/jmm.0.000171
3. World Health Organization. Global Tuberculosis Report 2019. Switzerland Geneva: WHO; 2019.
4. World Health Organization. Global Tuberculosis Report 2016. Geneva: World Health Organization; 2016.
5. World Health Organization. Rapid Implementation of the Xpert MTB/RIF Diagnostic Test: Technical and Operational ‘How-To’; Practical Considerations. Geneva: WHO; 2011.
6. World Health Organization. Global Tuberculosis Report 18. Geneva: World Health Organization; 2018.
7. Vassall A, van Kampen S, Sohn H, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med. 2011;8(11):e1001120. doi:10.1371/journal.pmed.1001120
8. Hamusse SD, Teshome D, Hussen MS, Demissie M, Lindtjorn B. Primary and secondary anti-tuberculosis drug resistance in Hitossa District of Arsi Zone, Oromia Regional State, Central Ethiopia. BMC Public Health. 2016;16:593. doi:10.1186/s12889-016-3210-y
9. Mulisa G, Workneh T, Hordofa N, Suaudi M, Abebe G, Jarso G. Multidrug-resistant Mycobacterium tuberculosis and associated risk factors in Oromia Region of Ethiopia. Int J Infect Dis. 2015;39:57–61. doi:10.1016/j.ijid.2015.08.013
10. Girum T, Muktar E, Lentiro K, Wondiye H, Shewangizaw M. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: a systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Trop Dis Travel Med Vaccines. 2018;4:5. doi:10.1186/s40794-018-0065-5
11. Eshetie S, Gizachew M, Dagnew M, et al. Multidrug resistant tuberculosis in Ethiopian settings and its association with previous history of anti-tuberculosis treatment: a systematic review and meta-analysis. BMC Infect Dis. 2017;17(1):219. doi:10.1186/s12879-017-2323-y
12. Gebretsadik D, Ahmed N, Kebede E, Mohammed M, Belete MA. Prevalence of tuberculosis by automated GeneXpert rifampicin assay and associated risk factors among presumptive pulmonary tuberculosis patients at Ataye District Hospital, North East Ethiopia. Infect Drug Resist. 2020;13:1507–1516. doi:10.2147/IDR.S248059
13. Gelaw SM. Socioeconomic factors associated with knowledge on tuberculosis among adults in Ethiopia. Tuberc Res Treat. 2016;2016:6207457.
14. Ministry of Health, Ethiopian Public Health Institute. ImplementatIon GuIdelIne for GeneXpert Mtb/Rif Assay in ethIopIa. Addis Ababa; 2014.
15. Arega B, Menbere F, Getachew Y. Prevalence of rifampicin resistant Mycobacterium tuberculosis among presumptive tuberculosis patients in selected governmental hospitals in Addis Ababa, Ethiopia. BMC Infect Dis. 2019;19(1):307. doi:10.1186/s12879-019-3943-1
16. Ejeta E, Beyene G, Bonsa Z, Abebe G. Xpert MTB/RIF assay for the diagnosis of mycobacterium tuberculosis and rifampicin resistance in high human immunodeficiency virus setting in Gambella regional state, southwest Ethiopia. J Clin Tuberc Other Mycobact Dis. 2018;12:14–20. doi:10.1016/j.jctube.2018.06.002
17. Mekonnen D, Derbie A, Desalegn E. TB/HIV co-infections and associated factors among patients on directly observed treatment short course in Northeastern Ethiopia: a 4 years retrospective study. BMC Res Notes. 2015;8:666. doi:10.1186/s13104-015-1664-0
18. Shrestha P, Arjyal A, Caws M, et al. The Application of GeneXpert MTB/RIF for smear-negative TB diagnosis as a fee-paying service at a South Asian General Hospital. Tuberc Res Treat. 2015;2015:102430.
19. Aliyu G, El-Kamary SS, Abimiku A, et al. Mycobacterial etiology of pulmonary tuberculosis and association with HIV Infection and multidrug resistance in Northern Nigeria. Tuberc Res Treat. 2013;2013:650561.
20. Alvarez-Uria G, Azcona JM, Midde M, Naik PK, Reddy S, Reddy R. Rapid diagnosis of pulmonary and extrapulmonary tuberculosis in HIV-infected patients. Comparison of LED fluorescent microscopy and the GeneXpert MTB/RIF assay in a District Hospital in India. Tuberc Res Treat. 2012;2012:932862.
21. Cox HS, Mbhele S, Mohess N, et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLoS Med. 2014;11(11):e1001760. doi:10.1371/journal.pmed.1001760
22. Adejumo OA, Olusola-Faleye B, Adepoju V, et al. Prevalence of rifampicin resistant tuberculosis and associated factors among presumptive tuberculosis patients in a secondary referral hospital in Lagos Nigeria. Afr Health Sci. 2018;18(3):472–478. doi:10.4314/ahs.v18i3.2
23. Datiko DG, Lindtjorn B. Tuberculosis recurrence in smear-positive patients cured under DOTS in southern Ethiopia: retrospective cohort study. BMC Public Health. 2009;9:348. doi:10.1186/1471-2458-9-348
24. Jaleta KN, Gizachew M, Gelaw B, Tesfa H, Getaneh A, Biadgo B. Rifampicin-resistant Mycobacterium tuberculosis among tuberculosis-presumptive cases at University of Gondar Hospital, northwest Ethiopia. Infect Drug Resist. 2017;10:185–192. doi:10.2147/IDR.S135935
25. Jetan CA, Jamaiah I, Rohela M, Nissapatorn V. Tuberculosis: an Eight Year (2000–2007) Retrospective Study at the University of Malaya Medical Centre (Ummc), Kuala Lumpur, Malaysia. Southeast Asian J Trop Med Public Health. 2010;41(2):378–384.
26. Nhamoyebonde S, Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis. 2014;209(suppl 3):S100–S6. doi:10.1093/infdis/jiu147
27. Ramos JM, Pérez-Butragueño M, Tesfamariam A, et al. Comparing tuberculosis in children aged under 5 versus 5 to 14 years old in a rural hospital in southern Ethiopia: an 18-year retrospective cross-sectional study. BMC Public Health. 2019;19(1):856. Published 2019 Jul 2. doi:10.1186/s12889-019-7206-2.
28. Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. 2014;2(8):e453–e9. doi:10.1016/S2214-109X(14)70245-1
29. Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis. 2004;8(5):636–647.
30. Tilahun G, Gebre-Selassie S. Treatment outcomes of childhood tuberculosis in Addis Ababa: a five-year retrospective analysis. BMC Public Health. 2016;16:612. doi:10.1186/s12889-016-3193-8
31. Mirutse G, Fang M, Kahsay AB, Ma X. Epidemiology of childhood tuberculosis and factors associated with unsuccessful treatment outcomes in Tigray, Ethiopia: a ten-year retrospective cross sectional study. BMC Public Health. 2019;19(1367):1–7. doi:10.1186/s12889-019-7732-y
32. Dangisso MH, Datiko DG, Lindtjorn B. Low case notification rates of childhood tuberculosis in southern Ethiopia. BMC Pediatr. 2015;15:142. doi:10.1186/s12887-015-0461-1
33. Mulu W, Abera B, Yimer M, Hailu T, Ayele H, Abate D. Rifampicin-resistance pattern of Mycobacterium tuberculosis and associated factors among presumptive tuberculosis patients referred to Debre Markos Referral Hospital, Ethiopia: a cross-sectional study. BMC Res Notes. 2017;10(1):8. doi:10.1186/s13104-016-2328-4
34. Ramos JM, Fernandez Munoz M, Tisiano G, et al. Use of Xpert MTB/RIF assay in rural health facilities in Southern Ethiopia. Arch Clin Microbiol. 2018;09(01). doi:10.4172/1989-8436.100077.
35. UNAIDS. UNAIDS Data 2019. Geneva, Switzerland: UNAIDS Joint United Nations Programme on HIV/AIDS; 2019.
36. Teweldemedhin M, Asres N, Gebreyesus H, Asgedom SW. Tuberculosis-Human Immunodeficiency Virus (HIV) co-infection in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2018;18(1):676. doi:10.1186/s12879-018-3604-9
37. Okonkwo R, Onwunzo M, Chukwuka C, et al. The use of the Gene Xpert mycobacterium tuberculosis/Rifampicin (MTB/Rif) assay in detection of multi-drug resistant tuberculosis (MDRTB) in Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria. J HIV Retro Virus. 2017;3(1):1–5.
38. Nyang’au LO, Amukoyeb E, Ng’ang’a Z. Determining first line anti-tuberculosis drug resistance among new and re-treatment tuberculosis/human immunodeficiency virus infected patients, Nairobi Kenya. Int J Sci Basic Appl Res. 2015;19(2):426–437.
39. Mesfin EA, Beyene D, Tesfaye A, et al. Drug-resistance patterns of Mycobacterium tuberculosis strains and associated risk factors among multi drug-resistant tuberculosis suspected patients from Ethiopia. PLoS One. 2018;13(6):e0197737. doi:10.1371/journal.pone.0197737
40. Hordofa MW. Prevalence of refampcin mono resistant mycobacterium tuberculosis among suspected cases attending at Yirgalem Hospital. Clin Med Res. 2015;4(3):75. doi:10.11648/j.cmr.20150403.13
41. Gebrehiwet GB, Kahsay AG, Welekidan LN, Hagos AK, Abay GK, Hagos DG. Rifampicin resistant tuberculosis in presumptive pulmonary tuberculosis cases in Dubti Hospital, Afar, Ethiopia. J Infect Dev Ctries. 2019;13(1):21–27. doi:10.3855/jidc.10462
42. Seyoum B, Demissie M, Worku A, Bekele S, Aseffa A. Prevalence and drug resistance patterns of mycobacterium tuberculosis among new smear positive pulmonary tuberculosis patients in Eastern Ethiopia. Tuberc Res Treat. 2014;2014:753492.
43. Adane K, Ameni G, Bekele S, Abebe M, Aseffa A. Prevalence and drug resistance profile of Mycobacterium tuberculosis isolated from pulmonary tuberculosis patients attending two public hospitals in East Gojjam zone, northwest Ethiopia. BMC Public Health. 2015;15:572. doi:10.1186/s12889-015-1933-9
44. Odone A, Calderon R, Becerra MC, et al. Acquired and transmitted multidrug resistant tuberculosis: the role of social determinants. PLoS One. 2016;11(1):e0146642. doi:10.1371/journal.pone.0146642
45. Biadglegne F, Sack U, Rodloff AC. Multidrug-resistant tuberculosis in Ethiopia: efforts to expand diagnostic services, treatment and care. Antimicrob Resist Infect Control. 2014;3(31):1–10. doi:10.1186/2047-2994-3-31
46. Abdella K, Abdissa K, Kebede W, Abebe G. Drug resistance patterns of Mycobacterium tuberculosis complex and associated factors among retreatment cases around Jimma, Southwest Ethiopia. BMC Public Health. 2015;15:599. doi:10.1186/s12889-015-1955-3
47. Abate D, Taye B, Abseno M, Biadgilign S. Epidemiology of anti-tuberculosis drug resistance patterns and trends in tuberculosis referral hospital in Addis Ababa, Ethiopia. BMC Res Notes. 2012;5:462. doi:10.1186/1756-0500-5-462
48. Hirpa S, Medhin G, Girma B, et al. Determinants of multidrug-resistant tuberculosis in patients who underwent first-line treatment in Addis Ababa: a case control study. BMC Public Health. 2013;13(782):1–9. doi:10.1186/1471-2458-13-782
49. Deressa MA, Demissie M. Risk factors of multi-drug resistant tuberculosis in Addis Ababa, Ethiopia: a matched case-control study. OALib. 2014;01(03):1–8. doi:10.4236/oalib.1100489
50. Ethiopian Food Medicine and Healthcare Administration and Control Authority. Strategy for the Prevention and Containment of Antimicrobial Resistance for Ethiopia. Addis Ababa, Ethiopia; 2015.
51. Ethiopian Food MaHAaCA. A Practical Guide to Antimicrobial Stewardship Program in Ethiopian Hospitals. Addis Ababa, Ethiopia; 2018.
52. Shargie EB, Lindtjorn B. DOTS improves treatment outcomes and service coverage for tuberculosis in South Ethiopia: a retrospective trend analysis. BMC Public Health. 2005;5:62. doi:10.1186/1471-2458-5-62
53. Geleta DA, Megerssa YC, Gudeta AN, Akalu GT, Debele MT, Tulu KD. Xpert MTB/RIF assay for diagnosis of pulmonary tuberculosis in sputum specimens in remote health care facility. BMC Microbiol. 2015;15:220. doi:10.1186/s12866-015-0566-6
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
