Back to Journals » Journal of Inflammation Research » Volume 14
Rheumatoid Arthritis Relapse and Remission – Advancing Our Predictive Capability Using Modern Imaging
Authors Terslev L, Ostergaard M
Received 14 April 2021
Accepted for publication 27 May 2021
Published 16 June 2021 Volume 2021:14 Pages 2547—2555
DOI https://doi.org/10.2147/JIR.S284405
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Lene Terslev,1,2 Mikkel Ostergaard1,2
1Copenhagen Center for Arthritis Research, Center for Rheumatology and Spine Diseases, Glostrup, Denmark; 2Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
Correspondence: Lene Terslev
Copenhagen Center for Arthritis Research, Center for Rheumatology and Spine Diseases, Rigshospitalet, Valdemar Hansens Vej 17, Glostrup, 2600, Denmark
Tel +45-38632616
Email [email protected]
Abstract: Clinical remission has become an achievable target for the majority of patients with rheumatoid arthritis, but subclinical inflammation as assessed by ultrasound and magnetic resonance imaging (MRI) has been demonstrated to be frequent in patients in clinical remission. Subclinical synovitis has been shown to be linked to both subsequent structural damage progression and a risk of flare, demonstrating that subclinical synovitis represents incomplete suppression of inflammation and questions whether it is appropriate only to use clinical composite scores as treatment target in clinical practice. Maintaining a state of remission has proven important as sustained clinical remission impacts long-term outcome regarding joint damage progression, physical function and quality of life. Treating subclinical inflammation has been attempted and has led to more frequent strict clinical remission and better physical function, but also to more adverse events. Thus, an overall benefit of incorporating imaging goals in treat-to-target strategies has not been documented. However, in patients in clinical remission on biological disease-modifying anti-rheumatic drugs, both ultrasound and MRI may aid in the clinical decision regarding whether drug tapering or even discontinuation should be attempted.
Keywords: ultrasound, magnetic resonance imaging, clinical remission, flare, tapering, subclinical synovitis
Introduction
As emphasized by the 2010 treat-to-target (T2T) recommendations, the treatment goal in patients with rheumatoid arthritis (RA) is to suppress inflammation and thereby prevent pain and joint destruction, and improve functional ability and quality of life. This should be obtained as quickly as possible and preferably within 6 months. Frequent clinical monitoring is suggested, ideally with an interval of 2–3 months, until the goal is achieved, which has been recommended to be set as clinical remission or at least low disease activity (LDA), assessed by the use of composite measures.1 The T2T recommendations are further supported by the 2019 European League Against Rheumatism (EULAR) recommendations for the management of RA with conventional synthetic (cs) and biological (b) disease-modifying anti-rheumatic drugs (DMARDs).65
Different composite measures for determining remission exist, and for many years the most frequently applied in routine care has been the composite Disease Activity Score based on 28-joint count (DAS28). The use of DAS28 <2.6 as a criterion for clinical remission has proved to be better than a conventional strategy not using a DAS28-targeted strategy.2,3 It does, however, allow for residual disease activity to be present despite fulfilling the definition of clinical remission. Therefore, the updated 2016 T2T recommendations and the EULAR treatment recommendations from 2019 now favour American College of Rheumatology (ACR)/EULAR Boolean remission or the simplified or clinical disease activity index (SDAI or CDAI) over DAS28 remission.4,5 In particular, the stringent ACR/EULAR Boolean remission seems better to reflect the clinical perception of remission, ie the absence of any signs and symptoms of significant inflammatory disease activity. However, the fact that patient-reported pain is incorporated in the composite remission criteria may, in patients without clinical signs of disease activity, impact their ability to fulfil composite remission criteria.6 Although clinical remission has become an achievable target for the majority of RA patients, defining “true” remission can be difficult as the current composite criteria do not take physical function and structural damage progression into consideration. Regarding the latter, it has been demonstrated that erosive progression still occurs in 20−30% of patients in clinical remission, regardless of the composite remission criteria used (DAS28, CDAI, SDAI or ACR/EULAR Boolean remission criteria).7–11 Currently, imaging is not part of any composite scores for remission.
Is Clinical Remission a Sufficient Goal?
The discrepancy between ensuring clinical remission and still seeing continued structural deterioration in some RA patients has led to the exploration of potential persistent silent inflammation, also called subclinical inflammation. Both ultrasound and magnetic resonance imaging (MRI) may be used to assess subclinical signs of inflammatory activity, as both imaging techniques are more sensitive than clinical evaluation for inflammation in joints, tendons and tendon sheaths.12–26 This is also the reason for acknowledging the use of ultrasound and MRI for joint assessment in the 2010 ACR/EULAR classification criteria for RA, facilitating an earlier fulfilment of the classification criteria.27
A high number of studies has been able to demonstrate that subclinical inflammation seen by ultrasound and MRI is present in a substantial proportion of RA patients in clinical remission, in both joints and tendons12–21,24,25,28 (Figures 1 and 2). Subclinical synovitis is present independent of using DAS28, CDAI, SDAI or even the more stringent ACR/EULAR Boolean remission criteria for determining clinical remission, and its presence is found to be independent of the type of treatment given, ie csDMARD or bDMARD.19,23,25,29–32
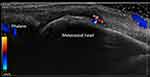 |
Figure 1 Patient with rheumatoid arthritis in clinical remission. The ultrasound image shows the third metacarpophalangeal joint with grade 2 synovial hypertrophy and grade 2 Doppler activity. |
In the assessment of subclinical synovitis by ultrasound, the majority of studies applies a grey-scale synovial hypertrophy score >1 as a sign of pathology,21,24 as grade 1 synovial hypertrophy itself is a frequent finding in healthy controls33,34 and in RA patients with diverging ability to improve.34,35 However, most emphasis has been placed on the presence of Doppler activity, although this component is very dependent on the equipment used.36 For optimal assessment of inflammation by MRI, the Outcome Measures in Rheumatology (OMERACT) group recommends that T1-weighted sequences are obtained before and after intravenous injection of gadolinium-containing contrast agent (optimal for assessment of synovitis and tenosynovitis), supplemented with a T2-weighted, fat-suppressed (T2FS) sequence or short tau inversion recovery (STIR), reflecting water content (optimal for bone marrow oedema/osteitis and also well suited for tenosynovitis).37,38 Synovitis scores of 1 are frequently seen in healthy controls, while bone marrow oedema is absent or very rare, when the most appropriate T2FS/STIR sequences are used, while less suitable sequences may provide different results.39–41
In subclinical synovitis, the presence of Doppler activity in particular, but also the presence of grey-scale synovial hypertrophy, is related to erosive progression in csDMARD-treated patients, and the absence of ultrasound inflammation (no Doppler signal, grey-scale score ≤2) is associated with no radiographic progression.42 Furthermore, both subclinical synovitis and tenosynovitis in cs/bDMARD-treated patients have been shown to be related to a risk of flare.7,13,14,20,32,43–45 Finally, subclinical synovitis is also related to unsuccessful tapering of bDMARD treatment.45,46 In most studies, only the hands were assessed for signs of subclinical synovitis, but some studies have included both large and small joints – up to a total of 42 joints.13,14,21,23,30–32,46 Currently, there is no agreement on a reduced joint set for assessing RA patients in remission; however, a study from 2017 found that by performing ultrasound examination of the hands only, it was possible to capture ≥90% of patients with subclinical inflammation, and this approach appears feasible for use in clinical practice for evaluating the disease state in RA patients in remission.24 The ability for MRI to predict erosive progression seems to be related less to the presence of subclinical synovitis than to the presence of osteitis and tenosynovitis, which have both been demonstrated to be independent predictors of 2-year MRI damage progression in RA patients in clinical remission.13,17,45,48
Can Subclinical Synovitis in Remission Be Prevented?
Research has investigated whether adding imaging to the T2T regimen in RA patients could abrogate subclinical disease activity in the state of remission. The randomized controlled ARCTIC trial49 investigated whether adding ultrasound information to the treatment decisions in early RA was better than clinical strategy alone, and found that the majority of patients in remission in both groups had subclinical synovitis by ultrasound and MRI after 1 year.28 However, a randomized controlled trial in patients in clinical remission on csDMARDs investigated an MRI T2T strategy, aiming at eliminating osteitis, compared with a clinical T2T strategy.50 The study found statistically significantly higher improvements in osteitis, tenosynovitis and total inflammation, and a trend towards higher improvement in synovitis, in the MRI T2T arm compared to the clinical T2T arm,50 although subclinical inflammation was not totally eliminated.
MRI and ultrasound currently appear to have limited validated value as an addition to routine clinical examinations for preventing subclinical synovitis in patients in remission.
Defining Imaging Remission
There is no international agreement on what constitutes imaging remission. For ultrasound, remission is generally perceived as an ultrasound disease state without Doppler activity (Doppler remission) and has been reported to be related to stable remission,43 but different studies put varying emphasis on the presence of synovial hypertrophy by grey-scale ultrasound. Strict ultrasound remission (ie no synovial hypertrophy and no Doppler activity in any joint) has been reported in 7–35% of patients in remission, whereas Doppler remission is more frequent and has been reported in 46–76% of patients.19,29,31,32 This indicates that clinical treatment with or without a T2T approach is more likely to result in Doppler remission than a complete absence of imaging signs of synovial hypertrophy.
Also, for MRI, there is no international consensus on how remission should be defined. It could be based on the absence of certain imaging findings, such as absence of MRI synovitis,13 potentially not considering grade 1, or lack of bone marrow oedema,39,50 or it could be data driven, eg a state in which radiographic progression rarely occurs in RA patients in general (defined by Baker et al,51 and by Ahmad et al52 as synovitis score <3, osteitis score <3 or total inflammation score [osteitis double-weighted] <9) or in RA patients in clinical remission (defined by Gandjbakhch et al,17 in a cohort of 254 RA patients in clinical remission or LDA, as a synovitis score ≤5), or it could be defined as a state in which clinical flare is rare if a drug is tapered (defined by Brahe et al53 as a total inflammation score <3 based on a sum score of synovitis found in the wrist and metacarpophalangeal joints 2–5).
Treating Subclinical Synovitis in Remission
Treating subclinical inflammation should ideally halt the progression of structural joint destruction and, not least, improve patient outcome over and above a treatment strategy based on conventional clinical and biochemical assessments54 (see Box 1). Although it is recognized that subclinical synovitis is present in a large majority of patients in remission, only a few studies are available in which treatment of subclinical synovitis has been attempted.
 |
Box 1 Steps for Evidence-Based Documentation of the Relevance of Incorporating Imaging Goals into Treat-to-Target Goals in RA |
In 2015, a study evaluated the effects of intensifying treatment to prevent joint damage in RA patients in clinical remission (DAS28 <2.6) with a least one Doppler-positive joint.55 The patients were randomized 1:1 either to continue the existing treatment with methotrexate or to increase the methotrexate dose. The study found that the Doppler score decreased significantly and the modified total Sharp score (mTSS) on X-ray was significantly suppressed at 52-week follow-up in the group where treatment was intensified compared to the group that continued on the same dose. Although the data are promising, the study has still only been presented as a congress abstract. Ongoing studies (TURA and REVECHO) are currently exploring the role of ultrasound in treating subclinical synovitis, but no data are available at this time.
Regarding MRI, an MRI-guided T2T strategy, aiming at absence of osteitis, was investigated in RA patients in clinical remission and compared with a conventional T2T strategy, aiming at clinical remission. In this randomized controlled clinical trial (IMAGINE-RA), the MRI-guided T2T strategy did not result in improved DAS28 remission rates or reduce radiographic progression, compared with the conventional T2T strategy.50
However, patients who followed the MRI-targeted treatment strategy had improved chances of achieving more stringent remission across all definitions (such as CDAI, SDAI and ACR/EULAR Boolean remission) after 2 years. This also indicates that the achievement of a “deeper” (ie more stringent) state of remission is an achievable goal in patients in DAS28 remission.48,56 This is relevant since patients who achieve sustained SDAI and ACR/EULAR Boolean remission have a better long-term outcome (>10 years) regarding joint damage progression, physical function and quality of life.57–61 However, it should be noted that the MRI T2T treatment strategy used in the IMAGINE-RA study also led to a higher number of serious adverse events, which were likely to be related to the more intensive treatment administered.
Predicting Flare in Remission
Once remission has been achieved, it is important to maintain remission and avoid flares. Flares are episodes of increased disease activity and involve a deterioration in patient-reported outcomes, such as functional ability and general health, pain and morning stiffness.44,60–63 However, flares may also result in more objective changes such as structural damage.
The lowest risk of flares is seen in patients with persistent ACR/EULAR Boolean remission64 but, in general, patients with short-term remission (remission interrupted by flares) are more likely to experience radiographic erosive progression compared to patients who achieve persistent remission.65
Flares are frequent in RA patients, with 30–50% experiencing a disease flare within the first 2 years of remission.60,66 In a study published in 2020, patient-reported flares were associated with increased disease activity by clinical examination and by ultrasound, ie they were demonstrated to be true (objectively confirmed) flares.66 Serial imaging by ultrasound and MRI after self-reported flares found the flares to be related to synovial and tenosynovial inflammation, followed by delayed-onset bone marrow oedema.67
An important question is whether flares can be avoided. Several studies have investigated the ability of imaging modalities to predict flares in DMARD-treated RA patients.
One study demonstrated that the presence of subclinical tenosynovitis in RA patients in clinical remission was associated with flare. If the tenosynovitis had both grey-scale and Doppler activity present, it was associated with shorter duration of remission (<12 months).20
In another study, subclinical synovitis, defined as the presence of Doppler signal, was associated with an increased risk of flare,21 and the presence of concurrent Doppler-positive tenosynovitis and joint synovitis has been shown to predict flare in RA patients in remission, with an odds ratio (OR) and 95% confidence interval (95% CI) of 2.75 (1.45 to 5.20) in crude analyses and 2.09 (1.06 to 4.13) in adjusted analyses.21,68 Thus, Doppler-negative joints have been reported to increase the chance of not experiencing a flare,43,44 and Doppler-positive findings in at least one joint have been shown to be the main predictor of flare.43 This demonstrates that subclinical synovitis represents an incomplete suppression of inflammation.
Tapering Therapy in Patients in Remission
As remission has become an obtainable goal, there has been interest in assessing the ability to taper or even discontinue DMARD treatment. There are very scarce data in relation to stopping versus continuing cDMARDs in RA patients in clinical remission. One older trial assessed the ability to stop cDMARDs in RA patients in remission, and found that drug discontinuation was associated with a significant increase in flare rate compared to patients continuing their csDMARD treatment;69 furthermore, the study found that patients experiencing a flare had difficulties in regaining remission.69,70 Although tapering of csDMARDs may be considered,5 since it will be successful in some patients, the frequent difficulty in stopping csDMARDs illustrates that RA often is a life-long (incurable) disease requiring continuous therapy.
More attention has been given to the ability to taper bDMARDs, as this is relevant both in relation to reducing costs and regarding safety issues.71,72 Hence, according to the EULAR recommendations for the management of RA with csDMARDs and bDMARDs, it is suggested to taper bDMARDs before attempting to taper csDMARD treatment in RA patients on combination therapy.5
There is consensus that tapering or discontinuation of bDMARDs should only be attempted in patients in persistent remission, but no clear definition exists on what constitutes “persistent remission”.5 Successful dose tapering, and even discontinuation, has been reported in several clinical trials, but is not achievable in all patients73–79 but there is a risk of flare related to both tapering and discontinuation.73–80 However, discontinuing bDMARDs is also associated with radiographic progression,80,81 which has not been reported when tapering bDMARDs to a lower dose than the standard dose.53,81 In the latter study, comprising patients in low disease activity or remission on tumour necrosis factor (TNF)-inhibitor therapy, no radiographic progression (no increase in hands and feet Sharp–van der Heijde score) was found after 48 weeks in 90% of the patients continuing on standard dose and, similarly, no erosive progression was seen in 75% of the patients receiving half of standard dose and in 55% of the patients discontinuing TNF-inhibitor therapy (progression in the group with discontinued therapy was statistically significantly higher than in patients continuing the standard dose, even though all patients in tapering/discontinuation groups resumed the full standard dose in case of flare).81
Tapering may be obtained by spacing the treatment intervals or by reducing the actual drug dose. Both ultrasound and MRI, based on their ability to detect subclinical signs of inflammation, have been investigated for their value for selecting patients who could successfully taper or even discontinue bDMARD treatment. Most studies apply a tapering approach when investigating the possibility for discontinuation,46,47,53 but some studies discontinue treatment without prior tapering.82 In studies investigating tapering of bDMARDs in the attempt to discontinue, it has been shown that low baseline MRI combined inflammation score and low baseline MRI combined damage scores are independent predictors for successful tapering to half or two-thirds of standard dose at 2-year follow-up.53 The absence of Doppler activity in joints has been reported both to be predictive of successful tapering and to be without predictive value.23,46
Furthermore, a lower Doppler sum score of 24 joints prior to tapering may predict successful discontinuation of bDMARDs at 2-year follow-up.23 In this study, a one-unit increase in Doppler 24-joint sum score decreased the odds for achieving successful discontinuation at 2 years by 56%.23
Only one study attempted discontinuation of bDMARD treatment without prior tapering,82 and found a significant difference in the degree of residual inflammation at the time of discontinuing bDMARDs between patients who could successfully discontinue treatment and those who could not, with higher residual inflammation in the latter group. However, the predictive value could not be established owing to the too small sample size.
In the imaging studies where imaging parameters were incorporated as potential predictors, demographic data, such as short disease duration, a maximum of one previous bDMARD and male gender, have also been reported to be predictors of successful tapering.53,82–84 Furthermore, one study found the DAS28 level prior to tapering to be predictive of successful tapering.46
Conclusion
Ultrasound and MRI are sensitive imaging modalities, which have demonstrated that subclinical synovitis, tenosynovitis and osteitis are frequently present in RA patients in clinical remission and may impact erosive progression and the risk of flare. This questions whether it is appropriate to use clinical composite scores alone for establishing remission. Treating subclinical inflammation has led to more frequent strict clinical remission and better physical function, but also to more adverse events. Thus, an overall benefit of incorporating imaging goals in T2T strategies has not been documented. Both MRI and ultrasound appear promising in aiding in the decision on whether tapering or discontinuing bDMARD treatment will be successful.
Disclosure
Prof. Dr. Lene Terslev reports personal fees from Roche, Novartis and Pfizer, outside the submitted work; Prof. Dr. Mikkel Ostergaard reports grants, personal fees and non-financial support from AbbVie, BMS, Boehringer-Ingelheim and Eli Lilly; non-financial support from Janssen; grants from Amgen, Celgene, Merck, Pfizer, Regeneron, Roche, Sanofi and UCB; and grants and non-financial support from Novartis and Gilead, outside the submitted work.
References
1. Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631–637. doi:10.1136/ard.2009.123919
2. Schipper LG, Vermeer M, Kuper HH, et al. A tight control treatment strategy aiming for remission in early rheumatoid arthritis is more effective than usual care treatment in daily clinical practice: a study of two cohorts in the Dutch Rheumatoid Arthritis Monitoring registry. Ann Rheum Dis. 2012;71:845–850. doi:10.1136/annrheumdis-2011-200274
3. Gullick NJ, Oakley SP, Zain A, et al. Goal-directed therapy for RA in routine practice is associated with improved function in patients with disease duration up to 15 years. Rheumatology (Oxford). 2012;51:759–761. doi:10.1093/rheumatology/ker399
4. Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75:3–15. doi:10.1136/annrheumdis-2015-207524
5. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of RA with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–699. doi:10.1136/annrheumdis-2019-216655
6. Hammer HB, Uhlig T, Kvien TK, Lampa J. Pain catastrophizing, subjective outcomes, and inflammatory assessments including ultrasound: results from a longitudinal study of rheumatoid arthritis patients. Arthritis Care Res (Hoboken). 2018;70(5):703–712. doi:10.1002/acr.23339
7. Nguyen H, Ruyssen-Witrand A, Gandjbakhch F, Constantin A, Foltz V, Cantagrel A. Prevalence of ultrasound-detected residual synovitis and risk of relapse and structural progression in rheumatoid arthritis patients in clinical remission: a systematic review and meta-analysis. Rheumatology (Oxford). 2014;53(11):2110–2118. doi:10.1093/rheumatology/keu217
8. Lillegraven S, Prince FH, Shadick NA, et al. Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Ann Rheum Dis. 2012;71(5):681–686. doi:10.1136/ard.2011.154625
9. Paulshus Sundlisæter N, Olsen IC, Aga A-B, et al. Predictors of sustained remission in patients with early rheumatoid arthritis treated according to an aggressive treat-to-target protocol. Rheumatology. 2018;57:2022–2031. doi:10.1093/rheumatology/key202
10. Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70:404–413. doi:10.1136/ard.2011.149765
11. Sewerin P, Vordenbaeumen S, Hoyer A, et al. Silent progression in patients with rheumatoid arthritis: is DAS28 remission an insufficient goal in RA? Results from the German Remission-plus cohort. BMC Musculoskelet Disord. 2017;18(1):163. doi:10.1186/s12891-017-1528-y
12. Olmez MO, Gunal EK, Ureyen SB, et al. Comparison of composite indices with global synovitis score on ultrasound for detecting remission. Clin Rheumatol. 2018;37:1111–1114. doi:10.1007/s10067-017-3925-x
13. Brown AK, Quinn MA, Karim Z, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54(12):3761–3773. doi:10.1002/art.22190
14. Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58(10):2958–2967. doi:10.1002/art.23945
15. Wakefield RJ, Freeston JE, Hensor EM, Bryer D, Quinn MA, Emery P. Delay in imaging versus clinical response: a rationale for prolonged treatment with anti-tumor necrosis factor medication in early rheumatoid arthritis. Arthritis Rheum. 2007;57(8):1564–1567. doi:10.1002/art.23097
16. Zhang H, Xu H, Chen S, Mao X. The application value of MRI in the diagnosis of subclinical inflammation in patients with rheumatoid arthritis in remission. J Orthop Surg Res. 2018;13(1):164. doi:10.1186/s13018-018-0866-2
17. Gandjbakhch F, Haavardsholm EA, Conaghan PG, et al. Determining a magnetic resonance imaging inflammatory activity acceptable state without subsequent radiographic progression in rheumatoid arthritis: results from a follow-up MRI study of 254 patients in clinical remission or low disease activity. J Rheumatol. 2014;41(2):398–406. doi:10.3899/jrheum.131088
18. Zufferey P, Möller B, Brulhart L, et al. Persistence of ultrasound synovitis in patients with rheumatoid arthritis fulfilling the DAS28 and/or the new ACR/EULAR RA remission definitions: results of an observational cohort study. Joint Bone Spine. 2014;81(5):426–432. doi:10.1016/j.jbspin.2014.04.014
19. Terslev L, Brahe CH, Østergaard M, et al. Using a DAS28-CRP-steered treat-to-target strategy does not eliminate subclinical inflammation as assessed by ultrasonography in rheumatoid arthritis patients in longstanding clinical remission. Arthritis Res Ther. 2021;23(1):48. doi:10.1186/s13075-021-02426-w
20. Bellis E, Scirè CA, Carrara G, et al. Ultrasound-detected tenosynovitis independently associates with patient-reported flare in patients with rheumatoid arthritis in clinical remission: results from the observational study STARTER of the Italian Society for Rheumatology. Rheumatology (Oxford). 2016;55:1826–1836. doi:10.1093/rheumatology/kew258
21. Filippou G, Sakellariou G, Scirè CA, et al. The predictive role of ultrasound-detected tenosynovitis and joint synovitis for flare in patients with rheumatoid arthritis in stable remission. Results of an Italian multicentre study of the Italian Society for Rheumatology Group for Ultrasound: the STARTER study. Ann Rheum Dis. 2018;77(9):1283–1289. doi:10.1136/annrheumdis-2018-213217
22. Wakefield RJ, Green MJ, Marzo-Ortega H, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis. 2004;63(4):382–385. doi:10.1136/ard.2003.007062
23. Terslev L, Brahe CH, Hetland ML, et al. Doppler ultrasound predicts successful discontinuation of biological DMARDs in rheumatoid arthritis patients in clinical remission. Rheumatology (Oxford). 2021;keab276. doi:10.1093/rheumatology/keab276
24. Hammer HB, Kvien TK, Terslev L. Ultrasound of the hand is sufficient to detect subclinical inflammation in rheumatoid arthritis remission: a post hoc longitudinal study. Arthritis Res Ther. 2017;19(1):221. doi:10.1186/s13075-017-1428-4
25. Gandjbakhch F, Conaghan PG, Ejbjerg B, et al. Synovitis and osteitis are very frequent in rheumatoid arthritis clinical remission: results from an MRI study of 294 patients in clinical remission or low disease activity state. J Rheumatol. 2011;38(9):2039–2044. doi:10.3899/jrheum.110421
26. Szkudlarek M, Court-Payen M, Jacobsen S, Klarlund M, Thomsen HS, Østergaard M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003;48(4):955–962. doi:10.1002/art.10877
27. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/art.27584
28. Sundin U, Sundlisater NP, Aga AB, et al. Value of MRI and ultrasound for prediction of therapeutic response and erosive progression in patients with early rheumatoid arthritis managed by an aggressive treat-to-target strategy. RMD Open. 2021;7(1):e001525. doi:10.1136/rmdopen-2020-001525
29. Saleem B, Brown AK, Keen H, et al. Disease remission state in patients treated with the combination of tumor necrosis factor blockade and methotrexate or with disease-modifying antirheumatic drugs: a clinical and imaging comparative study. Arthritis Rheum. 2009;60(7):1915–1922. doi:10.1002/art.24596
30. Spinella A, Sandri G, Carpenito G, Belletti L, Mascia MT. The discrepancy between clinical and ultrasonographic remission in rheumatoid arthritis is not related to therapy or autoantibody status. Rheumatol Int. 2012;32(12):3917–3921. doi:10.1007/s00296-011-2259-2
31. Geng Y, Han J, Deng X, Zhang Z. Presence of power Doppler synovitis in rheumatoid arthritis patients with synthetic and/or biological disease-modifying anti-rheumatic drug-induced clinical remission: experience from a Chinese cohort. Clin Rheumatol. 2014;33(8):1061–1066. doi:10.1007/s10067-014-2634-y
32. Cruces M, Al Snih S, Serra-Bonett N, Rivas JC. Subclinical synovitis measured by ultrasound in rheumatoid arthritis patients with clinical remission induced by synthetic and biological modifying disease drugs. Reumatol Clin. 2019;15(4):218–222. doi:10.1016/j.reuma.2017.08.004
33. Padovano I, Costantino F, Breban M, D’Agostino MA. Prevalence of ultrasound synovial inflammatory findings in healthy subjects. Ann Rheum Dis. 2016;75:1819–1823. doi:10.1136/annrheumdis-2015-208103
34. Witt M, Mueller F, Nigg A, et al. Relevance of grade 1 gray-scale ultrasound findings in wrists and small joints to the assessment of subclinical synovitis in rheumatoid arthritis. Arthritis Rheum. 2013;65:1694–1701. doi:10.1002/art.37954
35. Terslev L, Østergaard M, Sexton J, Hammer HB. Is synovial hypertrophy without Doppler activity sensitive to change? Post-hoc analysis from a rheumatoid arthritis ultrasound study. Arthritis Res Ther. 2018;20(1):224. doi:10.1186/s13075-018-1709-6
36. Torp-Pedersen S, Christensen R, Szkudlarek M, et al. Power and color Doppler ultrasound settings for inflammatory flow: impact on scoring of disease activity in patients with rheumatoid arthritis. Arthritis Rheumatol. 2015;67(2):386–395. doi:10.1002/art.38940
37. Østergaard M, Peterfy C, Conaghan P, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheum. 2003;30:1385–1386.
38. Østergaard M, Peterfy CG, Bird P, et al. The OMERACT rheumatoid arthritis Magnetic Resonance Imaging (MRI) scoring system: updated recommendations by the OMERACT MRI in arthritis working group. J Rheumatol. 2017;44(11):1706–1712. doi:10.3899/jrheum.161433
39. Ejbjerg E, Narvestad E, Rostrup E, et al. Magnetic resonance imaging of wrist and finger joints in healthy subjects occasionally shows changes resembling erosions and synovitis as seen in rheumatoid arthritis. Arthritis Rheumatism. 2004;50:1097–1106. doi:10.1002/art.20135
40. Mangnus L, van Steenbergen HW, Reijnierse M, van der Helm-van Mil AHM. Magnetic resonance imaging–detected features of inflammation and erosions in symptom-free persons from the general population. Arthritis Rheumatol. 2016;68(11):2593–2602. doi:10.1002/art.39749
41. Østergaard M, Haavardsholm E. MRI in healthy volunteers — important to do, and do correctly. Nat Rev Rheum. 2016;12(10):563–564. doi:10.1038/nrrheum.2016.142
42. Paulshus Sundlisæter N, Aga AB, Olsen IC, et al. Clinical and ultrasound remission after 6 months of treat-to-target therapy in early rheumatoid arthritis: associations to future good radiographic and physical outcomes. Ann Rheum Dis. 2018;77(10):1421–1425. doi:10.1136/annrheumdis-2017-212830
43. Scirè CA, Montecucco C, Codullo V, Epis O, Todoerti M, Caporali R. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology (Oxford). 2009;48(9):1092–1097. doi:10.1093/rheumatology/kep171
44. Saleem B, Brown AK, Quinn M, et al. Can flare be predicted in DMARD treated RA patients in remission, and is it important? A cohort study. Ann Rheum Dis. 2012;71(8):1316–1321. doi:10.1136/annrheumdis-2011-200548
45. Foltz V, Gandjbakhch F, Etchepare F, et al. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low levels of disease activity. Arthritis Rheum. 2012;64(1):67–76. doi:10.1002/art.33312
46. Naredo E, Valor L, De la Torre I, et al. Predictive value of doppler ultrasound-detected synovitis in relation to failed tapering of biologic therapy in patients with rheumatoid. Arthritis Rheumatol (Oxford). 2015;54(8):1408–1414. doi:10.1093/rheumatology/kev006
47. Alivernini S, Peluso G, Fedele AL, Tolusso B, Gremese E, Ferraccioli G. Tapering and discontinuation of TNF-α blockers without disease relapse using ultrasonography as a tool to identify patients with rheumatoid arthritis in clinical and histological remission. Arthritis Res Ther. 2016;18:3. doi:10.1186/s13075-016-0927-z
48. Møller-Bisgaard S, Georgiadis S, Hørslev-Petersen K, et al. Predictors of joint damage progression and stringent remission in patients with established rheumatoid arthritis in clinical remission. Rheumatology (Oxford). 2021;60(1):380–391. doi:10.1093/rheumatology/keaa496
49. Haavardsholm EA, Aga A-B, Olsen IC, et al. Ultrasound in management of rheumatoid arthritis: arctic randomised controlled strategy trial. BMJ. 2016;354:i4205. doi:10.1136/bmj.i4205
50. Møller-Bisgaard S, Hørslev-Petersen K, Ejbjerg B, et al. Effect of magnetic resonance imaging vs conventional treat-to-target strategies on disease activity remission and radiographic progression in rheumatoid arthritis: the IMAGINE-RA randomized clinical trial. JAMA. 2019;321(5):461–472. doi:10.1001/jama.2018.21362
51. Baker JF, Østergaard M, Emery P, Baker DG, Conaghan P. Development and validation of rheumatoid arthritis magnetic resonance imaging inflammation thresholds associated with lack of damage progression. Clin Exp Rheumatol. 2017;35(4):607–613.
52. Ahmad HA, Baker JF, Østergaard M, Ye J, Emery P, Conaghan PG. Determining MRI inflammation targets when considering a rheumatoid arthritis treat-to-target strategy: results of a randomized, placebo-controlled trial. Adv Ther. 2019;36:2384–2393. doi:10.1007/s12325-019-01020-6
53. Brahe CH, Krabbe S, Østergaard M, et al. Dose tapering and discontinuation of biological therapy in rheumatoid arthritis patients in routine care - 2-year outcomes and predictors. Rheumatology (Oxford). 2019;58(1):110–119. doi:10.1093/rheumatology/key244
54. Østergaard M, Møller-Bisgaard S. Optimal use of MRI in clinical trials, clinical care and clinical registries of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2014;32(Suppl 85):S17–S22.
55. Okano T, Inui K, Tada M, et al. Additional intensive treatment for rheumatoid arthritis patients with positive power doppler signals reduce the radiological joint damage even after achieving clinical remission -SCRUM study- [abstract]. Arthritis Rheumatol. 2015;67(suppl 10).
56. Møller-Bisgaard S, Østergaard M. Treat-to-target strategies in rheumatoid arthritis. Reply to comment by van der Helm-van Mil et al. JAMA. 2019;322:83–84. doi:10.1001/jama.2019.5856
57. Kavanaugh A, Fleischmann RM, Emery P, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis. 2013;72:64–71. doi:10.1136/annrheumdis-2011-201247
58. Linde L, Sørensen J, Østergaard M, Hørslev-Petersen K, Hetland ML. Does clinical remission lead to normalization of EQ-5D in patients with rheumatoid arthritis and is selection of remission criteria important? J Rheumatol. 2010;37:285–290. doi:10.3899/jrheum.090898
59. Radner H, Alasti F, Smolen JS, Aletaha D. Physical function continues to improve when clinical remission is sustained in rheumatoid arthritis patients. Arthritis Res Ther. 2015;17:203. doi:10.1186/s13075-015-0719-x
60. Molenaar ET, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, Dijkmans BA. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 2004;50:36–42. doi:10.1002/art.11481
61. Markusse IM, Dirven L, Gerards AH, et al. Disease flares in rheumatoid arthritis are associated with joint damage progression and disability: 10-year results from the BeSt study. Arthritis Res Ther. 2015;17:232.
62. Bartlett SJ, Hewlett S, Bingham CO, et al. Identifying core domains to assess flare in rheumatoid arthritis: an OMERACT international patient and provider combined Delphi consensus. Ann Rheum Dis. 2012;71:1855–1860. doi:10.1136/annrheumdis-2011-201201
63. Lie E, Woodworth TG, Christensen R, et al. Validation of OMERACT preliminary rheumatoid arthritis flare domains in the NOR-DMARD study. Ann Rheum Dis. 2014;73:1781–1787. doi:10.1136/annrheumdis-2013-203496
64. Smolen JS, Pedersen R, Jones H, Mahgoub E, Marshall L. Impact of flare on radiographic progression after eternacpet continuation, tapering or withdrawal in patients with rheumatoid arthritis. Rheumatology(Oxford). 2020;59:153–164. doi:10.1093/rheumatology/kez224
65. Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum. 2005;52(9):2625–2636. doi:10.1002/art.21235
66. Kuettel D, Terslev L, Weber U, et al. Flares in rheumatoid arthritis: do patient-reported swollen and tender joints match clinical and ultrasonography findings? Rheumatology (Oxford). 2020;59(1):129–136. doi:10.1093/rheumatology/kez231
67. Kuettel D, Glinatsi D, Østergaard M, et al. Serial magnetic resonance imaging and ultrasound examinations demonstrate differential inflammatory lesion patterns in soft tissue and bone upon patient-reported flares in rheumatoid arthritis. Arthritis Res Ther. 2020;22(1):19. doi:10.1186/s13075-020-2105-6
68. Peluso G, Michelutti A, Bosello S, Gremese E, Tolusso B, Ferraccioli G. Clinical and ultrasonographic remission determines different chances of relapse in early and long standing rheumatoid arthritis. Ann Rheum Dis. 2011;70(1):172–175. doi:10.1136/ard.2010.129924
69. Ten Wolde S, Breedveld FC, Hermans J, et al. Randomised placebo-controlled study of stopping second-line drugs in rheumatoid arthritis. Lancet. 1996;347:347–352. doi:10.1016/S0140-6736(96)90535-8
70. Ten Wolde S, Hermans J, Breedveld FC, Dijkmans BA. Effect of resumption of second line drugs in patients with rheumatoid arthritis that flared up after treatment discontinuation. Ann Rheum Dis. 1997;56:235–239. doi:10.1136/ard.56.4.235
71. Ollendorf DA, Klingman D, Hazard E, et al. Differences in annual medication costs and rates of dosage increase between tumor necrosis factor-antagonist therapies for rheumatoid arthritis in a managed care population. Clin Ther. 2009;31:825–835. doi:10.1016/j.clinthera.2009.04.002
72. Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi:10.1001/jama.295.19.2275
73. Smolen JS, Nash P, Durez P, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet. 2013;381:918–929. doi:10.1016/S0140-6736(12)61811-X
74. Van Vollenhoven RF, Østergaard M, Leirisalo-Repo M, et al. Full dose, reduced dose or discontinuation of etanercept in rheumatoid arthritis. Ann Rheum Dis. 2016;75:52–58. doi:10.1136/annrheumdis-2014-205726
75. Van den Broek M, Klarenbeek NB, Dirven L, et al. Discontinuation of infliximab and potential predictors of persistent low disease activity in patients with early rheumatoid arthritis and disease activity score-steered therapy: subanalysis of the BeSt study. Ann Rheum Dis. 2011;70:1389–1394. doi:10.1136/ard.2010.147751
76. Smolen JS, Emery P, Fleischmann R, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomized controlled OPTIMA trial. Lancet. 2014;383:321–332. doi:10.1016/S0140-6736(13)61751-1
77. Den Broeder AA, van Herwaarden N, van der Maas A, et al. Dose REduction strategy of subcutaneous TNF inhibitors in rheumatoid arthritis: design of a pragmatic randomised non inferiority trial, the DRESS study. BMC Musculoskelet Disord. 2013;14:299. doi:10.1186/1471-2474-14-299
78. Haschka J, Englbrecht M, Hueber AJ, et al. Relaps rates in patients with rheumatoid arthritis in stable remission tapering or stopping antirheumatic therapy: interim results from the prospective randomised controlled RETRO study. Ann Rheum Dis. 2016;75:45–51. doi:10.1136/annrheumdis-2014-206439
79. Tanaka Y, Takeuchi T, Mimori T, et al. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann Rheum Dis. 2010;69:1286–1291. doi:10.1136/ard.2009.121491
80. Henaux S, Ruyssen-Witrand A, Cantagrel A, et al. Risk of losing remission, low disease activity or radiographic progression in case of bDMARD discontinuation or tapering in rheumatoid arthritis: systematic analysis of the literature and meta-analysis.Ann. Rheum Dis. 2018;77(4):515–522. doi:10.1136/annrheumdis-2017-212423
81. Østergaard M, Leirisalo-Repo M, Uhlig T, et al. In rheumatoid arthritis patients with stable low disease activity on methotrexate plus etanercept, continuation of etanercept is superior both clinically and radiographically to discontinuation: results from a randomized, 3-armed, double-blind clinical trial. Arthritis Rheum. 2013;65(10):S1017–8.
82. Iwamoto T, Ikeda K, Hosokawa J, et al. Prediction of relapse after discontinuation of biologic agents by ultrasonographic assessment in patients with rheumatoid arthritis in clinical remission: high predictive values of total gray-scale and power Doppler scores that represent residual synovial inflammation before discontinuation. Arthritis Care Res (Hoboken). 2014;66(10):1576–1581. doi:10.1002/acr.22303
83. Saleem B, Keen H, Goeb V, et al. Patients with RA in remission on TNF blockers: when and in whom can TNF blocker therapy be stopped? Ann Rheum Dis. 2010;69(9):1636–1642. doi:10.1136/ard.2009.117341
84. Lamers-Karnebeek FB, Luime JJ, Ten Cate DF, et al. Limited value for ultrasonography in predicting flare in rheumatoid arthritis patients with low disease activity stopping TNF inhibitors. Rheumatology (Oxford). 2017;56(9):1560–1565. doi:10.1093/rheumatology/kex184
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.