Back to Journals » Clinical Epidemiology » Volume 12
Rheumatoid Arthritis as a Risk Factor for Coronary Artery Calcification and Obstructive Coronary Artery Disease in Patients with Chest Pain: A Registry Based Cross-Sectional Study
Authors Tinggaard AB , de Thurah A , Andersen IT, Riis AH , Therkildsen J , Winther S , Hauge EM , Bøttcher M
Received 24 February 2020
Accepted for publication 11 May 2020
Published 24 June 2020 Volume 2020:12 Pages 679—689
DOI https://doi.org/10.2147/CLEP.S251168
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Vera Ehrenstein
Andreas Bugge Tinggaard,1 Annette de Thurah,2 Ina Trolle Andersen,3 Anders Hammerich Riis,3 Josephine Therkildsen,1 Simon Winther,4 Ellen-Margrethe Hauge,2 Morten Bøttcher1
1Department of Cardiology, Hospital Unit West, Herning, Denmark; 2Department of Rheumatology, Aarhus University Hospital, Aarhus, Denmark; 3Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark; 4Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark
Correspondence: Andreas Bugge Tinggaard Tel +0045 30272717
Email [email protected]
Purpose: To examine the occurrence and severity of coronary artery disease (CAD) in patients with rheumatoid arthritis (RA) compared to non-RA patients in a population referred for coronary computed tomography angiography (CTA) due to chest pain.
Patients and Methods: In this cross-sectional study, 46,210 patients from a national CTA database were included. Patients with RA were stratified on serology, treatment with conventional synthetic or biological disease-modifying antirheumatic drugs (DMARDs), and the need for relapse or flare treatment with intraarticular or -muscular glucocorticoid injections (GCIs). Primary outcomes were coronary artery calcium score (CACS) > 0 and CACS ≥ 400, and secondary outcome was obstructive CAD. Associations between RA and outcomes were examined using logistic regression and results were adjusted for age, sex, cardiovascular risk factors and comorbidities.
Results: A total of 395 (0.9%) RA patients were identified. In overall RA, crude odds ratio (OR) for having CACS > 0 was 1.48 (1.21– 1.82) and 1.52 (1.15– 2.01) for CACS ≥ 400, whereas adjusted ORs were 1.08 (0.86– 1.36) and 1.21 (0.89– 1.65), respectively. Seropositive RA patients had adjusted OR of 1.16 (0.89– 1.50) for CACS > 0 and 1.37 (0.98– 1.90) for CACS ≥ 400. Patients who had received ≥ 1 GCI in the period of 3 years prior to the CTA had an adjusted OR of 1.37 (0.94– 2.00) for having CACS > 0 and 1.46 (0.92– 2.31) for CACS ≥ 400.
Conclusion: This is the first large-scale, CTA-based study examining the occurrence and severity of CAD in RA patients with symptoms suggestive of cardiovascular disease. A higher prevalence of coronary artery calcification was found in RA patients. After adjusting for age, sex, cardiovascular risk factors and comorbidities, the tendency was less pronounced. We found a trend for increased coronary calcification in RA patients being seropositive or needing treatment with GCI for a relapse or flare.
Keywords: rheumatoid arthritis, computed tomography angiography, coronary artery disease, coronary artery calcium score, cross-sectional
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by systemic inflammation with a prevalence of 0.5–1% in the adult population.1 RA patients have an increased mortality compared to the general population, regardless of gender, in every age group except for >85 years,2 and cardiovascular (CV) disease is the main reason for this increased mortality in RA patients.3–5 An increased prevalence of traditional CV risk factors is found in RA patients,6–8 but the increased risk of CV disease does not seem to be completely explained by traditional CV risk factors.3 The evidence for systemic inflammation as an atherogenic factor contributing to accelerated development of CAD is increasing,9–11 and in RA patients residual inflammation has been proposed as an independent risk factor for CV disease.12
Coronary computed tomography angiography (CTA) is an accurate and well-established method as the primary investigation of suspected CAD.13 CTA consists of a non-enhanced and a contrast-enhanced CT scan. The non-contrast procedure provides a coronary artery calcium score (CACS), which is a strong predictor of CV events,14,15 and the contrast-enhanced procedure provides detailed information in the presence of coronary artery stenoses.16,17 A previous CTA study demonstrated that residual disease activity was associated with higher prevalence and severity of coronary plaques in a cohort of RA patients without symptoms of CAD compared to healthy controls,18 and in follow-up data, inflammation was identified as an independent predictor of coronary atherosclerosis progression.19 A recent meta-analysis found an increased prevalence of asymptomatic CAD in RA patients based on CTA data.20 Thus, existing evidence proposes RA to be an independent risk factor for CV disease driven by increased inflammatory burden.
Previous CTA studies have examined RA patients without symptoms suggestive of CAD and, to our knowledge, no studies have investigated the occurrence and severity of CAD among RA patients with symptoms suggestive of CAD. Hence, in this cross-sectional study based on data from the Western Denmark Cardiac Computed Tomography Registry (WDHR), we aimed to examine the occurrence and severity of CAD in RA patients compared to non-RA patients referred for CTA due to suspected CAD. Furthermore, we aimed to examine CAD in RA subgroups based on serology, treatment with conventional synthetic or biological disease-modifying antirheumatic drugs (DMARDs), and the need for relapse or flare treatment with intraarticular or -muscular glucocorticoid injections (GCIs) to identify high-risk RA subgroups. In addition to the WDHR, data was retrieved from the National Patient Registry (NPR) and the Danish National Prescription Registry (DNPR).
Patients and Methods
Data Sources, Study Design and Study Population
This was a cross-sectional study based on data from the WDHR, the NPR and the DNPR. Using the unique personal identification number assigned to all Danish citizens, data from the WDHR was linked to nationwide administrative registries regarding vital status, International Classification of Diseases (ICD) diagnosis codes and treatment codes from the NPR and prescribed medication from the DNPR.
The WDHR is a semi-national, multicenter-based registry with longitudinal registration of patient data and clinical procedures since 1999. As of December 31, 2017 the WDHR contains CTA information from almost 76,000 patients. Data was collected from all thirteen clinical WDHR centers between January 1, 2008 and December 31, 2017 within a population of approximately 3.3 million individuals (55% of the total Danish population). The WDHR is a mandatory database, which by legislation requires all physicians to report findings in all CTAs including registration of up to 40 variables for each procedure to enable evaluation of clinical performance. According to international guidelines, CTA is recommended as the primary diagnostic modality for patients having a low-to-intermediate a-priori risk of CAD.21 Hence, the database reflects a population of patients with de-novo chest pain symptoms with no prior CTA examinations.
The NPR includes data on all somatic hospital admissions from patients since 1977 and outpatients since 1995, including information on discharge diagnosis, hospital department, and dates of admission and discharge.22 Patients treated by rheumatologists in the private sector are not included in the NPR. Discharge diagnoses in the NPR are, since 1994, classified according to the World Health Organization’s ICD-10.
Since 1995 the DNPR has recorded detailed individual-level information on all dispensed prescriptions in Denmark.23,24 Drugs prescribed to nursing-home resident are also included. The registry contains 46 variables that characterize each redeemed prescription. Drugs used during hospital admissions, drugs supplied directly by hospitals or treatment centers (eg chemotherapeutic agents) and over-the-counter drugs are not recorded in the DNPR.
Compliance with Ethical Standards
This study has been approved by the Danish Data Protection Agency. By Danish law, no approval from the national committee on health research ethics was required in this register-based study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in an a-priori approval by the institution’s human research committee.
Exposure: Identification of Rheumatoid Arthritis Patients
Within the WDHR, RA patients were identified through the NPR. ICD-8 and ICD-10 codes used for identification of RA patients are shown in Supplementary Table 1. In order to validate the diagnosis of RA, we linked the codes for RA diagnoses from the NPR to relevant treatment codes (ATC codes) in the DNPR. A previous study assessed the positive predictive value of identifying RA patients to be 87.7% (95% CI; 82.5–91.5) when linking the NPR to relevant ATC codes in the DNPR.25 Only ATC codes for csDMARDs were used for the validation, since all patients receiving bDMARDs have already been treated with csDMARDs at some point.26 ATC codes for csDMARDs are shown in Supplementary Table 1. Next, RA patients were subgrouped based on serology, treatment with biological DMARDs (bDMARDs) and the need for relapse or flare treatment with intraarticular or -muscular CGIs.
Rheumatoid Arthritis Serology
Serology of RA is classified based on seropositivity of rheumatoid factor and anti-citrullinated protein antibodies. In this study, serology was classified by ICD-10 codes of seropositive RA (M05) and other RA (M06), if recorded as a primary or secondary diagnosis. Referral diagnoses were not used. Patients registered with one of the M05 diagnostic sub-codes at the first registration were defined as having “seropositive RA”, and otherwise, patients were defined as having “other RA”. This was done in order to avoid misclassification of seronegative patients.25
Rheumatoid Arthritis, Disease-Modifying Antirheumatic Drugs
In accordance with international guidelines RA patients are recommended treatment with bDMARDs when: 1) present disease activity has lasted for more than three months without improvement, verified by at least two consecutive consultations or treatment goal or remission has not been reached after six months verified by frequent controls, 2) ongoing treatment with a combination of conventional synthetic DMARDs (csDMARDs) (triple treatment) and 3) temporary treatment with glucocorticoids, systemic or as intra-articular injection, has proven ineffective.26 Hence, we used treatment with bDMARD as a surrogate marker for disease severity. We defined two treatment levels: “csDMARD” (treatment with csDMARDs alone) and “bDMARD” (treatment with bDMARD at any time during the disease course with or without a combination with csDMARDs). Information on csDMARDs was retrieved through the DNPR using the ATC codes mentioned in Supplementary Table 1. Information on bDMARDs was identified through the NPR using treatment procedure codes shown in Supplementary Table 1.
Rheumatoid Arthritis Disease Activity
In RA, the disease fluctuates between long periods of remission and short periods with disease flares. In Denmark, RA relapses (return of disease activity) and flares (substantial increase of disease activity) are generally treated with intra-articular or intra-muscular GCIs supplementary to DMARDs and DMARD escalation.27 Since inflammation is involved in atherogenesis, information about RA disease activity is valuable. Therefore, we used information about GCIs in RA patients three years prior to CTA as a surrogate marker for disease activity. Treatment codes were obtained from the NPR and are shown in Supplementary Table 1. We defined two disease activity groups based on the number of GCIs: “0 injections” and “≥ 1 injections”. As patients often receive multiple GCIs during the first year following initial RA diagnosis, the first year after diagnosis was ignored.
Outcomes: Coronary Artery Calcium Score and Obstructive Coronary Artery Disease
Using non-contrast CT data CACS was obtained by a semi-automated algorithm in accordance with the Agatston method.28 Absence of coronary artery calcium obtained by the Agatston method has a negative predictive value of 98% for luminal stenosis > 50%, and the presence of luminal stenosis > 50% on invasive coronary angiography is associated with a positive CACS with a sensitivity of 98%.29
Primary outcomes were CACS > 0 and CACS ≥ 400.
Based on contrast-enhanced CT data, the coronary tree was divided into the three major arteries: The right coronary artery (RCA), the left anterior descending artery (LAD) and the left circumflex artery (Cx).30 Possible plaques were analyzed and categorized as; no plaque (0% stenosis), mild stenosis (1–49%), moderate stenosis (50–69%) and severe stenosis (70–99%).30 No plaques present in the coronary tree or diffuse atherosclerosis without severe stenosis was categorized as “non-obstructive CAD”. Based on the number of major vessels (RCA, LAD and Cx) affected by severe stenosis, patients were categorized as having 1-, 2- or 3-vessel disease, all defined as “obstructive CAD”. Secondary outcome was obstructive CAD.
All CT data was interpreted by trained cardiologists.
Exclusion of Patients
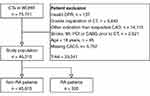
A total of 75,751 CTAs were identified in the WDHR. We considered only the first CTA for each patient with a valid personal identification number. Next, we restricted to those referred for CTA due to chest pain or shortness of breath. Subsequently, we excluded all patients younger than 18 or patients who had prior CV diseases, ie had a history of known CAD, stroke, acute myocardial infarction, percutaneous coronary intervention or coronary artery bypass grafting. From these exclusions we identified 52,972 relevant CTAs. A total of 6762 patients had missing CACS in the WDHR, and thus, 46,210 patients were included in the study. Figure 1 shows a flowchart of patient inclusion.
Covariates: Comorbidity, Cardiovascular Risk Factors and Concurrent Medication
A Charlson Comorbidity Index (CCI)31 score was completed for each patient based on data from the NPR at any time before the date of CTA. The CCI was originally developed to predict the risk of 1-year mortality attributable to comorbidity in a longitudinal study of 604 hospitalized patients. The CCI has, however, also been useful to predict mortality in a group of patients with RA.32 Based on the CCI a morbidity score of 0, 1 or 2+ was identified for all patients in the study population. Diabetes is included in the CCI, but is a well-known CV risk factor. To avoid incorrect adjustment for diabetes in this study, it was removed from the CCI score and included as an independent CV risk factor.
Information on traditional CV risk factors was retrieved from the WDHR, the NPR and the DNPR. Smoking, BMI and a family history of CV disease were retrieved from the WDHR. Dyslipidemia was identified in the WDHR and by redeemed prescriptions of lipid-lowering drugs in the DNPR with the treatment code for statin (ATC: C10AA). Hypertension was identified in the WDHR and by redeemed prescriptions of antihypertensive drugs in the DNPR with the following treatment codes: Angiotensin-converting-enzyme or angiotensin II inhibitors (ATC: C09A, C09B, C09C), beta-blockers (ATC: C07A), calcium channel blockers (ATC: C08CA) and bendroflumethiazide (ATC: C03AB01). Diabetes was identified in the WDHR, by following ICD10 codes in the NPR: E10-E14, O24 and H36, and by redeemed prescriptions of antidiabetic drugs in the DNPR (ATC: A10A, A10B). For lipid-lowering treatment, antihypertensive and antidiabetic drugs only prescriptions 180 days prior to CTA was used for risk factor identification.
Referral Symptoms
In international guidelines, traditional clinical classification of suspected anginal symptoms is defined as follows: “Typical angina” meets all three of the characteristics; 1) constricting discomfort in the front of the chest or in the neck, jaw, shoulder or arm; 2) precipitated by physical exertion; 3) relieved by rest or nitrates within 5 minutes.21 “Atypical angina” meets two of these characteristics and “non-anginal chest pain” meets only one or none of the characteristics. In order to refer a patient with symptoms suggestive of CAD to CTA, the referring physician reports the indication for the CTA and characterization of chest pain. Additionally, “shortness of breath” can indicate a CTA as a possible angina equivalent.
Statistical Methods
In the main analysis, we compared RA patients to non-RA patients in the WDHR population for the two primary outcomes; CACS > 0 and CACS ≥ 400, and the secondary outcome; obstructive CAD. Furthermore, we subgrouped RA patients by serology, DMARD treatment and disease activity as described and compared the subgroups to non-RA patients. We tabulated all relevant characteristics of the RA patients. The association between each RA exposures and outcomes was examined using logistic regression. We computed crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs), adjusted for the potential confounders: sex, age, comorbidity score (0, 1, 2+), hypertension (yes/no), dyslipidemia (yes/no), diabetes (yes/no), BMI (<18.5, 18.5–25, 25–30, >30) and smoking (never, former, current). All analyses were conducted using the SAS software v9.4 (SAS Institute Inc., Cary, NC, USA).
We used multiple imputation to handle missing values of the confounders; height (15% missing) and weight (14% missing) to calculate BMI, and smoking (7% missing). The missing at random assumption for this method was questioned for CACS (13% missing), thus, we restricted the analysis to the complete cases of this variable.
Results
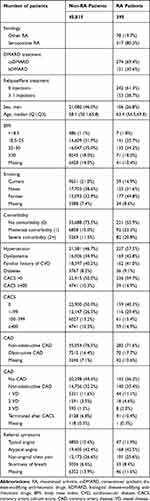
In total, 395 RA patients (0.9%) with registered CACS were identified. At CTA index date, RA patients tended to be older (median age 63.4 [IQR 56.5;69.8] vs 58.1 [IQR 50.1;66.8]) and to have more comorbidities (prevalence of comorbidity score 2+ was 20.8% vs 11.5%) compared to non-RA patients. A higher prevalence of the traditional CV risk factors such as dyslipidemia (42.8% vs 34.9%) and hypertension (57.5% vs 46.7%) was found in RA patients compared to non-RA patients, but similar prevalence of diabetes (9.1% vs 8.2) was found. In general, RA is more frequent in females than males, which was also found in our study population (73.2% females in the RA group vs 54.0% in the non-RA group). Table 1 shows RA subgroups and the distributions of demographic characteristics among included patients.
Coronary Artery Calcium Score and Obstructive Coronary Artery Disease in Rheumatoid Arthritis
Table 1 also contains descriptive CTA data in RA patients and non-RA patients. The primary outcome, CACS > 0, was found in 59.7% of the RA patients compared to 50.0% of the non-RA patients, corresponding to a crude OR of 1.48 (95% CI; 1.21–1.82) and an adjusted OR of 1.08 (95% CI; 0.86–1.36). CACS ≥ 400 was found in 14.9% of the RA patients compared to 10.3% of the non-RA, corresponding to a crude OR of 1.52 (95% CI; 1.15–2.01) and an adjusted OR of 1.21 (95% CI; 0.89–1.65). The secondary outcome, obstructive CAD, was found in 17.7% of the RA patients and 16.4% of the non-RA patients, corresponding to a crude OR of 1.15 (95% CI; 0.89–1.50) and an adjusted OR of 1.05 (95% CI; 0.80–1.37). The results are shown inTable 2.
Rheumatoid Arthritis Subgroups
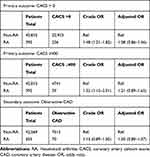
Table 3 presents crude and adjusted results for CACS > 0, CACS ≥ 400 and obstructive CAD in RA patients subgrouped based on serology, DMARD treatment and treatment of relapse or flare as previously described. In the subgroup analysis, non-RA patients were used as reference group.
A tendency of increased odds was found in seropositive RA patients, most pronounced was; crude OR of 1.56 (95% CI; 1.24–1.95) and adjusted OR of 1.16 (0.89–1.50) for CACS > 0, and crude OR of 1.66 (95% CI; 1.23–2.24) and adjusted OR of 1.37 (0.98–1.90) for CACS ≥ 400.
Similarly, a tendency of increased odds was found in patients with ≥ 1 CGIs, most pronounced was; crude OR of 1.89 (95% CI; 1.35–2.63) and adjusted OR of 1.37 (95% CI; 0.94–2.00), and crude OR of 1.77 (95% CI; 1.16–2.71) and adjusted OR of 1.46 (95% CI; 0.92–2.31), for CACS>0 and CACS ≥ 400, respectively.
For patients only treated with csDMARDs, a tendency of increased odds was found for CACS ≥ 400; crude OR of 1.66 (95% CI; 1.20–2.29) and adjusted OR of 1.28 (95% CI; 0.90–1.83), and for obstructive CAD; crude OR of 1.39 (95% CI; 1.03–1.88) and adjusted OR of 1.24 (95% CI; 0.91–1.69). The bDMARD group did not show the same tendencies.
Discussion
In this large-scale, population-based cohort study we found a higher prevalence of coronary artery calcification in RA patients compared to non-RA patients, referred to CTA due to symptoms suggestive of CAD. After adjusting for age, sex, traditional CV risk factors and comorbidities the association was less pronounced and not statistically significant. The strongest associations with increased coronary artery calcification were seen in seropositive RA patients and in RA patients requiring CGI treatment, Also, in RA patients on csDMARD treatment, a tendency of increased odds for CACS > 400 and obstructive CAD was found.
An increased risk of CV morbidity and mortality in RA patients is established,3–5 and is mainly explained by an increased prevalence of CV risk factors.6–8 Previous CTA studies have shown a higher prevalence and burden of asymptomatic CAD in RA patients.18,20 The setup of this study is clinically relevant since it covers a population of patients referred to diagnostic testing due to chest pain. The study shows that RA patients are approximately 5 years older at CTA referral and have more dyslipidemia and hypertension, which possibly derives from the age difference. Non-contrast CT data shows a higher prevalence of both CACS > 0 and CACS ≥ 400 in RA patients – a clinically relevant finding even though adjusting for sex, age, comorbidities and CV risk factors even out the difference. Interestingly, the increased coronary artery calcification does not seem to be reflected in a higher prevalence of obstructive CAD in RA patients.
Despite the delayed referral and increased coronary calcification, we found no difference in typical angina as referral symptom in RA patients. Rollefstad et al have reported a weak association of chest pain and CAD in RA.33 Our findings emphasize that management of traditional risk factors and careful examination of RA patients is imperative including timely referral for diagnostic testing when CAD is suspected.
The subgroup analysis showed a trend of increased odds for both CACS > 0 and ≥ 400 in seropositive RA patients compared to non-RA patients. This trend was not found in “other RA” patients. These results might support previous studies suggesting that seropositive RA causes an increased inflammatory drive and consequently an accelerated atherosclerotic process.34,35 Furthermore, it supports the widely recognized opinion that seropositive and seronegative RA are two different, heterogeneous diseases driven by different mechanisms.36
RA is a fluctuating disease with an unpredictable course characterized by long periods of remission and short periods with disease flares. In this study, we used relapse or flare treatment with CGIs as a surrogate marker for disease activity. Our results showed a tendency of increased odds of CACS > 0 and ≥ 400 in patients who had received ≥ 1 GCI three years prior to the CTA, and this tendency was not found for RA patients without GCI treatment prior to the CTA.
A recent study by Karpouzas et al identified inflammation measured by time-averaged CRP and cumulative prednisone dose to be independent predictors of coronary atherosclerosis progression in RA patients without symptoms suggestive of CAD.19 Our results might support these findings suggesting more advanced CAD in subgroups of RA patients with seropositivity and higher disease activity.
Another recent CTA study by Karpouzas et al, in the same population, proposes bDMARD use to slow the coronary atherosclerosis in RA patients.37 In the present study, we found a tendency of increased odds for CACS ≥ 400 and obstructive CAD in RA patients on csDMARD treatment, which was not seen in patients on bDMARD treatment. However, due to few patients, the estimates for bDMARDs presented with 95% CIs ranging from a small negative to a strong positive association.
Strengths and Limitations
To our knowledge, this is the first large-scale, population-based CTA study to investigate CAD in RA patients with symptoms suggestive of CAD. The quantity of clinical information from the WDHR enabled adjustment for potential confounders such as smoking and BMI, which are normally not available in data from administrative databases. Linkage of the WDHR data to the NPR and the DNPR data allowed optimal adjustment for traditional CV risk factors, which has been a challenge in previous registry-based studies. The study cohort consist of a population of patients with de-novo chest pain with no prior CTA examinations and crude results represents the prevalence of coronary artery calcification in this population. This knowledge is highly relevant to guide physicians in a clinical setting of angina evaluation.
The validity of the RA diagnosis was doubly verified through the NPR and the DNPR; a approach of identifying RA patients previously validated with a high positive predictive value.25 Stratification of RA patients into “DMARD treatment” and “relapse/flare treatment” subgroups to assess disease severity and activity contributed to utilizing the potential of the data from the WDHR. This approach has, to our knowledge, not previously been performed in cross-sectional cohort studies of RA patients using administrative registries.
To identify RA diagnosis we linked the RA diagnosis codes from the NPR to relevant treatment codes (ATC codes) in the DNPR. A possible selection bias is introduced since some RA patients attend private rheumatologists and are therefore not registered in the NPR. In Denmark, however, this is only a small population since the rheumatologic expertise is mainly centered in the hospitals, and thus, the majority of RA patients are referred to the hospitals. Consequently, we expect any potential selection bias to have little impact on our study estimates their generalizability.
A possible limitation of the study is the referral of high-risk patients to invasive coronary angiography rather than CTA. Clinicians could be more inclined to refer patients with an additional risk factor, being RA, to invasive coronary angiography instead of CTA. Consequently, this could have resulted in a selection bias and an underestimation of coronary calcification in RA patients.
We had no access to a continuous measure of disease activity in RA patients such as DAS28-CRP. Instead we used GCIs prior to CTA as a surrogate marker for disease activity. Thus, RA patients who presented with high disease activity, but were not offered GCI treatment could have been wrongly classified. This bias would have made us underestimate the influence of RA disease activity on the risk of coronary calcification. Since we found a tendency of increased coronary calcification in the RA subgroup receiving ≥ 1 GCI three years prior to CTA, we do not consider this bias substantial in our study. Evans et al have shown an association of higher prednisone dose to a greater incidence of CV events in RA,38 and Karpouzas et al found cumulative prednisone dose to be an independent predictor of coronary atherosclerosis progression in RA.19 Both studies were conducted in the United States, where RA treatment with orally administered glucocorticoids is common. None of the studies reported glucocorticoid administration route. In Denmark, RA relapses and flares are generally treated with intra-articular or intra-muscular GCIs, and treatments with oral glucocorticoids are less frequent and transitory. We do not consider a possible atherogenic adverse effect of oral glucocorticoid treatment to be a bias in our study.
Non-steroidal anti-inflammatory drugs (NSAIDs) have been associated with an increased risk of CAD.39 In Denmark, many NSAIDs are over-the counter drugs, and therefore, we had no access to this information through the registers. Consequently, we were unable to control for this confounder.
In our main analysis, 13% of the patients, both non-RA and RA, had missing CACS and were consequently removed, as we did not believe we could model the missing information in a satisfactory way. In contrast, we used multiple imputation to assess the missing comorbidity data of smoking and BMI. In a sensitivity analysis, where CACS was included in the imputation model, no considerable changes where found.
After confounder adjustment, most results ranged from small negative to relative strong positive associations. Furthermore, the RA subgroups had in some cases quite few outcomes. Thus, a larger dataset is essential to strengthen the observed tendencies. This will be possible in future analyses within the WDHR.
Conclusion
In a population of patients with suspected CAD, RA patients have a higher prevalence of coronary artery calcification compared to non-RA patients. At referral for CTA, RA patients were older and had more dyslipidemia and hypertension. After adjusting for age, sex, traditional CV risk factors and comorbidities, a trend towards increased odds for coronary artery calcification was still demonstrated in seropositive RA patients and RA patients with the need of relapse or flare treatment. A tendency of increased odds for obstructive CAD was found in RA patients on csDMARD treatment, but not in RA patients on bDMARD treatment. Further research in a larger study population is, however, needed in order to examine this association more closely.
Acknowledgments
The abstract of this paper was presented at the ESC Congress 2018, the 2018 ACR/ARHP Annual Meeting and EULAR 2019 as a poster presentation with interim findings.
Disclosure
Professor Ellen-Margrethe Hauge reports personal fees from Sanofi, Sobi, AbbVie, UCB, grants from Novartis, and received travel reimbursement from Sobi and Celgene, outside the submitted work. The authors received no financial support for the research, authorship or publication of this article. The authors report no other conflicts of interest in this work.
References
1. Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. doi:10.1038/nrdp.2018.1
2. Widdifield J, Bernatsky S, Paterson JM, et al. Trends in excess mortality among patients with rheumatoid arthritis in Ontario, Canada. Arthritis Care Res (Hoboken). 2015;67(8):1047–1053. doi:10.1002/acr.22553
3. Gabriel SE. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med. 2008;121(Suppl 10):S9–14. doi:10.1016/j.amjmed.2008.06.011
4. Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71(9):1524–1529. doi:10.1136/annrheumdis-2011-200726
5. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26(5 Suppl 51):S35–61.
6. Jafri K, Bartels CM, Shin D, Gelfand JM, Ogdie A. Incidence and management of cardiovascular risk factors in psoriatic arthritis and rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken). 2017;69(1):51–57. doi:10.1002/acr.23094
7. Romano S, Salustri E, Ruscitti P, Carubbi F, Penco M, Giacomelli R. Cardiovascular and metabolic comorbidities in rheumatoid arthritis. Curr Rheumatol Rep. 2018;20(12):81. doi:10.1007/s11926-018-0790-9
8. Jagpal A, Navarro-Millan I. Cardiovascular co-morbidity in patients with rheumatoid arthritis: a narrative review of risk factors, cardiovascular risk assessment and treatment. BMC Rheumatol. 2018;2:10. doi:10.1186/s41927-018-0014-y
9. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–979. doi:10.1056/NEJM199704033361401
10. Golia E, Limongelli G, Natale F, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. 2014;16(9):435. doi:10.1007/s11883-014-0435-z
11. Raggi P, Genest J, Giles JT, et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98–108. doi:10.1016/j.atherosclerosis.2018.07.014
12. Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52(3):722–732. doi:10.1002/art.20878
13. Nielsen LH, Norgaard BL, Tilsted HH, et al. The Western Denmark cardiac computed tomography registry: a review and validation study. Clin Epidemiol. 2015;7:53–64. doi:10.2147/CLEP.S73728
14. Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49(18):1860–1870. doi:10.1016/j.jacc.2006.10.079
15. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi:10.1056/NEJMoa072100
16. Schroeder S, Kuettner A, Leitritz M, et al. Reliability of differentiating human coronary plaque morphology using contrast-enhanced multislice spiral computed tomography: a comparison with histology. J Comput Assist Tomogr. 2004;28(4):449–454. doi:10.1097/00004728-200407000-00003
17. Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol. 2014;11(7):390–402. doi:10.1038/nrcardio.2014.60
18. Karpouzas GA, Malpeso J, Choi TY, Li D, Munoz S, Budoff MJ. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis. 2014;73(10):1797–1804. doi:10.1136/annrheumdis-2013-203617
19. Karpouzas GA, Ormseth SR, Hernandez E, Budoff MJ. Impact of cumulative inflammation, cardiac risk factors, and medication exposure on coronary atherosclerosis progression in rheumatoid arthritis. Arthritis Rheum. 2020;72(3):400–408. doi:10.1002/art.41122
20. Hansen PR, Feineis M, Abdulla J. Rheumatoid arthritis patients have higher prevalence and burden of asymptomatic coronary artery disease assessed by coronary computed tomography: a systematic literature review and meta-analysis. Eur J Intern Med. 2019;62:72–79. doi:10.1016/j.ejim.2019.02.018
21. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477.
22. Mosbech J, Jorgensen J, Madsen M, Rostgaard K, Thornberg K, Poulsen TD. [The national patient registry. Evaluation of data quality]. Ugeskr Laeger. 1995;157(26):3741–3745. Danish.
23. Kildemoes HW, Sorensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7 Suppl):38–41. doi:10.1177/1403494810394717
24. Pottegard A, Schmidt SAJ, Wallach-Kildemoes H, Sorensen HT, Hallas J, Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017;46(3):798–798f. doi:10.1093/ije/dyw213
25. Linauskas A, Overvad K, Johansen MB, Stengaard-Pedersen K, de Thurah A. Positive predictive value of first-time rheumatoid arthritis diagnoses and their serological subtypes in the Danish national patient registry. Clin Epidemiol. 2018;10:1709–1720. doi:10.2147/CLEP.S175406
26. Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509. doi:10.1136/annrheumdis-2013-204573
27. Hetland ML, Horslev-Petersen K. The CIMESTRA study: intra-articular glucocorticosteroids and synthetic DMARDs in a treat-to-target strategy in early rheumatoid arthritis. Clin Exp Rheumatol. 2012;30(4 Suppl 73):S44–49.
28. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi:10.1016/0735-1097(90)90282-T
29. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675–688. doi:10.1016/j.jcmg.2008.12.031
30. Raff GL, Abidov A, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3(2):122–136. doi:10.1016/j.jcct.2009.01.001
31. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
32. England BR, Sayles H, Mikuls TR, Johnson DS, Michaud K. Validation of the rheumatic disease comorbidity index. Arthritis Care Res (Hoboken). 2015;67(6):865–872. doi:10.1002/acr.22456
33. Rollefstad S, Ikdahl E, Hisdal J, Kvien TK, Pedersen TR, Semb AG. Association of chest pain and risk of cardiovascular disease with coronary atherosclerosis in patients with inflammatory joint diseases. Front Med. 2015;2:80. doi:10.3389/fmed.2015.00080
34. Hjeltnes G, Hollan I, Forre O, Wiik A, Mikkelsen K, Agewall S. Anti-CCP and RF IgM: predictors of impaired endothelial function in rheumatoid arthritis patients. Scand J Rheumatol. 2011;40(6):422–427. doi:10.3109/03009742.2011.585350
35. Arnab B, Biswadip G, Arindam P, Shyamash M, Anirban G, Rajan P. Anti-CCP antibody in patients with established rheumatoid arthritis: does it predict adverse cardiovascular profile? J Cardiovasc Dis Res. 2013;4(2):102–106. doi:10.1016/j.jcdr.2012.09.003
36. Ajeganova S, Huizinga TW. Rheumatoid arthritis: seronegative and seropositive RA: alike but different? Nat Rev Rheumatol. 2015;11(1):8–9. doi:10.1038/nrrheum.2014.194
37. Karpouzas GA, Ormseth SR, Hernandez E, Budoff MJ. Biologics may prevent cardiovascular events in rheumatoid arthritis by inhibiting coronary plaque formation and stabilizing high-risk lesions. Arthritis Rheum. 2020. doi:10.1002/art.41293
38. Evans MR, Escalante A, Battafarano DF, Freeman GL, O'Leary DH, del Rincon I. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63(5):1211-1220.
39. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76(1):17–28. doi:10.1136/annrheumdis-2016-209775
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.