Back to Journals » Drug Design, Development and Therapy » Volume 17
Review of the Protective Mechanism of Paeonol on Cardiovascular Disease
Authors Yang C, Cheng J, Zhu Q, Pan Q, Ji K, Li J
Received 28 March 2023
Accepted for publication 19 June 2023
Published 26 July 2023 Volume 2023:17 Pages 2193—2208
DOI https://doi.org/10.2147/DDDT.S414752
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Chunkun Yang,1,* Jiawen Cheng,1,* Qinwei Zhu,2 Qingquan Pan,2 Kui Ji,2 Jun Li1
1Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 2Department of Emergency, Weifang Hospital of Traditional Chinese Medicine, Weifang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Li, Email [email protected]
Abstract: Cardiovascular disease (CVD) is one of the leading causes of death in the world. Paeonol(Pae) is a phenolic component extracted from peony bark, peony root and Xu Changqing. Studies have shown that Pae can protect cardiomyocytes by inhibiting oxidative stress, promoting mitochondrial fusion, regulating mitochondrial autophagy and inhibiting inflammation. In addition, Pae improves ventricular remodeling by inhibiting myocardial apoptosis, hypertrophy and fibrosis. Pae also has a good protective effect on blood vessels by inhibiting vascular inflammation, reducing the expression of adhesion molecules, inhibiting vascular proliferation, and inhibiting oxidative stress and endoplasmic reticulum stress(ERS). Pae also has the effect of anti-endothelial cell senescence, promoting thrombus recanalization and vasodilating. In conclusion, the molecular targets of Pae are very complex, and the relationship between different targets and signaling pathways cannot be clearly explained, which requires us to use systems biology methods to further study specific molecular targets of Pae. It has to be mentioned that the bioavailability of Pae is poor, and some nanotechnology-assisted drug delivery systems improve the therapeutic effect of Pae. We reviewed the protective mechanism of paeonol on the cardiovascular system, hoping to provide help for drug development in the treatment of CVD.
Keywords: paeonol, oxidative stress, inflammation, mitochondrial fusion, adhesion, autophagy
Introduction
With the change of living habits and environment, the spectrum of human diseases is also gradually changing.Epidemiological studies have shown that with the ageing of the population, increasing environmental pollution and poor lifestyle habits, all contribute to chronic diseases, especially cardiovascular diseases, which are the major causes of morbidity and mortality worldwide.1 Cardiovascular disease (CVD) has become one of the leading causes of death in the world.The World Health Organization estimates that 30,000 people die every day from CVD and that more than 40% of non-communicable disease deaths each year are attributed to CVD.2
Paeonol (Pae) is a phenolic component extracted from peony bark, paeonia rubra and Xu Changqing. It has a wide range of biological activities, including antioxidant, anti-inflammatory, anti-cancer and apoptosis inhibition.3 In recent years, studies have shown that Pae has a good protective effect on the cardiovascular system. In China, Pae is used alone or in combination with other traditional Chinese medicines to effectively protect the cardiovascular system, suggesting that Pae may be used as an alternative or supplement to compensate for the limited efficiency of modern drugs in treating CVD. A previous paper4 on advances in the bioactivity of Pae in CVD was published in 2021, but this paper included only 20 basic studies. When writing this review, we searched relevant studies in CNKI and pubmed, including a total of 57 basic studies on Pae’s improvement of CVD. In this review, we have more comprehensively summarized the improvement mechanism of Pae on CVD (Figure 1).
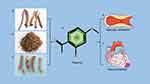 |
Figure 1 (a) Peony bark. (b) Xu Changqing. (c) Paeonia rubra. (d) Chemical structure of Paeonol. (e and f) Pharmacological effects of Paeonol. |
Pae Protects the Heart
Pae Reduces Oxidative Stress
Myocardial ischemia/reperfusion (I/R) injury is a significant clinical problem, resulting in increased production of reactive oxygen species (ROS), lipid peroxidation of myocardial membrane, inactivation of key enzymes, and interference with nucleic acid synthesis. This leads to dysfunction of heart muscle cells.5 Breast cancer type 1 sensitive protein (BRCA1) gene, as a regulator of genomic stability, is involved in DNA repair and transcription.6 BRCA1 has been shown to protect vascular smooth muscle cells (VSMC) from oxidative stress.7 Zheng et al found that after myocardial anoxia/reoxygenation (A/R) injury, Pae (10 µM) can improve the activity of antioxidant enzymes and reduce the production of ROS by up-regulating the expression of BRCA1. Zheng et al8 found that after myocardial A/R injury, Pae (10 µM) can improve the activity of antioxidant enzymes and reduce the production of ROS by up-regulating the expression of BRCA1. Furthermore, the formation of nod-like receptor protein 3 (NLRP3) inflammasome and the activity of nuclear factor κB (NF-κB) were inhibited. Ultimately, the secretion of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β was reduced (Figure 2 and Table 1).
 |
Table 1 Protective Mechanism of Pae on the Myocardium |
Silent information regulator 1 (SIRT1) is a protein that encodes gene localization to chromosome 10q21.3. Liu et al9 found that Pae (12 mg/kg/d) alleviated oxidative stress and myocardial damage caused by I/R in rat myocardium, improved cardiac function and reversed ventricular remodeling by up-regulating the expression of SIRT1. Xu et al10 used Pae (4, 8, 16 mg/kg) to intervene acute myocardial infarction (AMI) rats. It was found that Pae could improve myocardial cell edema, inhibit cardiomyopathy, increase left ventricular ejection fraction (LVEF), decrease serum levels of IL-6, IL-1β and TNF-α, and increase the level of superoxide dismutase (SOD) in myocardial tissue. The mechanism may be related to the regulation of SIRT3/β-catenin/PPARγ signaling pathway in myocardial tissue (Figure 2 and Table 1).
Doxorubicin (DOX), an anthracycline derivative, is used clinically to treat various cancers, but its use is limited by the fact that DOX can cause irreversible myocardial damage and heart failure (HF). S-phase kinase-associated protein 2 (SKP2), a member of the F-box family, improves cardiac function after ischemia.27 Chen et al11 established a rat model of chronic heart failure (CHF) with DOX induction, and found that Pae (25 and 50 mg/kg/d) could alleviate myocardial histopathological damage, inhibit myocardial cell apoptosis and ROS production. Its mechanism is related to the regulation of miR-21-5p/SKP2 axis (Figure 2 and Table 1).
Pae Promotes Mitochondrial Fusion
The heart is a highly energy-demanding organ that relies heavily on the function of mitochondria, which provide about 90% of the energy. Unbalanced mitochondrial fusion/division dynamics have been found to be an early cause of increased mitochondrial ROS production and induction of mitochondrial dysfunction.28,29 Studies30 have found that promoting mitochondrial fusion through upregulation of Mfn2 expression does not impair cardiac mitochondrial function, while complete inhibition of Drp1-mediated mitochondrial division does. This suggests that promoting mitochondrial fusion is a safer strategy than suppressing mitochondrial division. Liu et al12 found that Pae (100 μmol/L) promoted mitochondrial fusion and improved mitochondrial function in cardiomyocytes treated with high glucose. In animal studies, Pae (300 mg/kg/d) increased LVEF and left ventricular fractional shortening(LVFS), decreased left ventricular end systolic diameter (LVESD), improved cardiac diastolic function, and inhibited myocardial hypertrophy and interstitial fibrosis in diabetic rats. Further studies found that the expression of the fusion-related protein Opa1 was decreased in the hearts of diabetic rats, while Pae administration attenuated the decrease in the expression of Opa1. In addition, deletion of Opa1 attenuates the protective effect of Pae on diabetic hearts. These results suggest that Pae may at least improve diabetic cardiomyopathy(DCM) by up-regulating Opa1 expression and increasing mitochondrial fusion. Liu et al also found that the up-regulation of Opa1 expression was achieved by activation of the CK2α/Jak2/Stat3 signaling pathway (Figure 2 and Table 1).
In the process of mitochondrial fusion, the outer membrane of mitochondria fused first, and then the inner membrane fused. Mfn1 and Mfn2 are essential proteins for mitochondrial outer membrane fusion, and Opa1 is located in the inner membrane of mitochondria and is a key protein responsible for inner membrane fusion.31 Ding et al13 found that Pae (50 μmol/L) restored mitochondrial fusion and enhanced mitochondrial function in DOX-treated cardiomyocytes. In animal experiments, Pae (150 mg/kg/d) increased LVEF and LVFS, decreased LVESD, and inhibited myocardial apoptosis and oxidative stress in DOX treated rats. The protective mechanism may be that Pae promotes mitochondrial fusion through PKCε/Stat3/Mfn2 signaling pathway (Figure 2 and Table 1).
Pae Regulates Mitochondrial Autophagy
Autophagy is an intracellular catabolic process in which long-lived proteins are recycled and damaged organelles are removed.32 However, excessive activation of autophagy degrades essential proteins and organelles, leading to progressive consumption of proteins or organelles and cell death during I/R.33 Autophagy and apoptosis are mediated by the interaction between Beclin-1 and B-cell lymphoma-2 (Bcl-2) proteins. After their interaction, autophagy is inhibited, while Bcl-2 maintains its anti-apoptotic potential.34 Tsai et al14 found that Pae (1 mg/kg/d) significantly improved cardiac function after myocardial I/R injury, and reduced arrhythmia and mortality induced by myocardial I/R. The mechanism is related to the up-regulation of Bcl-2 protein and the down-regulation of caspase-9 and caspase-3 expression, and the down-regulation of Beclin-1 protein (Figure 2 and Table 1).
As mentioned above, moderate autophagy facilitates the clearance of damaged organelles. Wu et al15 found that Pae (50 mg/kg/d) improved cardiac dysfunction in BALB/c mice induced by DOX and reduced myocardial cell damage. In cell experiments, Pae (100 μM) upregulated miR-1, thereby inhibiting PI3K/AKT/mTOR signaling pathway to up-regulate autophagy, and inhibited PI3K/AKT/NF-κB signaling pathway to reduce myocardial apoptosis. In another study, Liu et al16 found that Pae (12 mg/kg/d) promoted myocardial mitochondrial autophagy in AMI rats, thus protecting cardiac function. In cell experiments, Pae (100 μM) reduced hypoxic-induced cardiomyocyte injury by activating Pink1/Parkin signaling pathway (Figure 2 and Table 1).
Pae Inhibits Inflammation
Toll-like receptor 4 (TLR4) is an important member of the TLR family, which can activates NF-κB and promotes the production of inflammatory mediators.35 Methotrexate (MTX) is a cytotoxic chemotherapy drug with folate antagonistic activity, which can cause certain damage to the heart.36 Al-Taher et al17 found that Pae (100 mg/kg/d) may reduce the cardiotoxicity induced by MTX, inhibit the production of TNF-α and IL-6, and reduce the apoptosis of cardiomyocytes in rats by inhibiting the TLR4/NF-κB signaling pathway. Chen et al18 injected Escherichia coli into the rat abdomen to establish a rat model of septic shock, and found that Pae (5 mg/kg/d) treatment could inhibit the levels of inflammatory cell infiltration, inflammatory factors and cardiomyocyte apoptosis in the heart tissue. In vitro experiments, Pae (5 μmol/L) reduced the apoptotic level and inflammatory cytokine release of H9C2 cardiomyocytes treated with lipopolysaccharide (LPS), and the mechanism is related to the activation of PI3K/AKT pathway (Figure 2 and Table 1).
High mobility group protein B1 (HMGB1) is an important inflammatory mediator, and plays a certain role in the mechanism of myocardial I/R injury.37 Huang et al19 established the rat myocardial I/R injury model by ligating the left anterior descending (LAD) for 30min and then reperfusion for 2h. It was found that Pae (15, 30 mg/kg/d) reduced the expression of IL-1β and HMGB1, thereby alleviating myocardial cell damage.In another study, Zhao et al20 found that Pae (4, 6, 8 mg/kg/d) improved ventricular remodeling in rats after AMI by inhibiting NF-κB signaling pathway and reducing IL-1 (Figure 2 and Table 1).
Pae Inhibits Myocardial Apoptosis, Hypertrophy and Fibrosis
MicroRNAs (miRNAs) are short strands of RNA that are about 20–24 bases long and do not encode proteins. In one study, Liu et al21 found that Pae (100 μmol/L) attenuates A/R-induced H9C2 cell apoptosis by down-regulating the level of miR-155-5p. DCM refers to myocardial disease caused solely by hyperglycemia in diabetic patients, excluding hypertension and coronary heart disease. Among the factors causing DCM, cardiomyocyte apoptosis is an important pathogenesis. Wu et al22 found that Pae (40 μg/mL) effectively inhibited the apoptosis of cardiomyocytes induced by high glucose, which may be achieved by activating PI3K/Akt signaling pathway and thereby up-regulating the expression of anti-apoptotic genes and inhibiting pro-apoptotic genes (Figure 2 and Table 1).
HF is often accompanied by ventricular hypertrophy, cardiomyocyte apoptosis, cardiac fibrosis and inflammation. Chen et al23 studied the protective mechanism of Pae against HF induced by transverse aortic coarction(TAC) in mice, and found that Pae (50 mg/kg) alleviated myocardial injury and inhibited myocardial apoptosis and hypertrophy by regulating ERK1/2/JNK signaling pathway. In another study, Thabassum et al24 found that Pae (2.5 mM) protected H9C2 cardiomyocytes from DOX-induced apoptosis, collagen deposition, and hypertrophy by activating Notch1. Myocardial fibrosis (MF) is an important aspect of ventricular remodeling after AMI and a key factor affecting the prognosis of patients.38 Shi et al25,26 found that Pae (8, 12 and 16 mg/kg/d) reduced MF in AMI rats, and the mechanism was related to the inhibition of TGF-β/Smads signaling pathway and the decrease of Collagen III. Table 1 summarizes the research objects, intervention methods and protective mechanisms of pae in the myocardial protection experiment (Figure 2 and Table 1).
Protective Mechanism of Pae on Blood Vessels
Inhibit Vascular Inflammation
Atherosclerosis (AS) is the major cause of morbidity and mortality globally. The injury and dysfunction of vascular endothelial cell(VEC) are the driving factors for the development and progression of AS. Previous studies39 have shown that phosphatase and tensin homolog (PTEN) are associated with VEC inflammation. Liu et al40 found that Pae (2.5 μmol/L) reduces VEC damage induced by oxidized low-density lipoprotein(ox-LDL) by modulating miR-21/PTEN pathway and down-regulating TNF-α release. NOD-Like receptor thermal protein domain associated protein 3 (NLRP3) inflammatories play an important role in regulating the inflammatory response, has been identified as a direct target of miR-223.41 Shi et al42 found that Pae (75, 150 or 300 mg/kg/d) alleviated vascular damage by increasing plasma exosome miRNA-223 in hyperlipidemia rats. In cell experiments, pae (20, 40, and 80 μ g/mL) alleviates the inflammatory damage of rat aortic endothelial cells (RAEC) through miRNA-223/NLRP3 signal pathway. In another study, Liu et al43 found that Pae (10 mg/kg/d) increased the level of miR-223 in aorta of ApoE−/− mice and inhibited the expression of STAT3 and pSTAT3. In vitro studies, Pae (30, 60, and 120 µM) enhanced miR-223 in human umbilical vein endothelial cells (HUVECs) co-culture system with THP-1 cells, thereby inhibiting STAT3. Furthermore, the LPS-induced secretion of IL-1β, IL-6, intercellular adhesion molecule-1 (ICAM-1) and vascular intercellular adhesion molecule-1 (VCAM-1) in HUVECs was down-regulated, and the adhesion between monocytes and HUVECs was decreased (Figure 3 and Table 2).
 |
Table 2 Protective Mechanism of Pae on Blood Vessels |
Caveolin-1 is a special vesicular depression structure widely expressed in the cell membrane of VECs, which can negatively regulate cell signaling pathways by binding and inactivating some important molecules of intracellular signal transduction.72 Liu et al44 found that Pae (75, 150 and 300 mg/kg) significantly reduced aortic plaque area, intimal hyperplasia and lipid deposition in vascular walls, and increased aortic caveolin-1 expression in rats. In vitro studies, Pae (30, 60 and 120 μmol/L) significantly reduced the levels of TNF-α, IL-6 and VCAM-1 secreted by LPS-induced VEC through the caveolin-1/NF-κB signaling pathway. Hu et al45 observed the protective effect of Pae on LPS-induced VEC co-cultured with VSMC, and found that Pae (15, 30 and 60 μmol/L) could reduce LPS damage to VEC by inhibiting the PI3K/Akt/NF-κB pathway (Figure 3 and Table 2).
T helper type 17(Th17) cells are characterized by the production of pro-inflammatory cytokine IL-17, which is a major factor in the pathogenesis of AS.73 In contrast, regulatory T(Treg) cells have been identified as specialized inhibitors of multiple immune responses and inflammation through the secretion of the anti-inflammatory cytokine IL-10.74 Th17 and Treg cells play an important role in regulating extracellular matrix (ECM) composition and vascular fibrosis of vascular plaques by regulating the expression of collagen and matrix metalloproteinases (MMP).75 Shi et al46 found that Pae (400 mg/kg) increased the production of short-chain fatty acids (SCFA) by regulating intestinal flora, up-regulated Treg cells, down-regulated Th17 cells, reduced the levels of pro-inflammatory cytokines IL-1β, IL-6, TNF-α and IL-17 and increased the levels of anti-inflammatory factor IL-10 in aorta. Furthermore, the protein expression levels of lysyloxidase (LOX), MMP-2/9 and collagen I/III were down-regulated, and the level of vascular fibrosis was reduced (Figure 3 and Table 2).
Macrophages in AS plaques are prone to be polarized into classical activation type (M1) and alternative activation type (M2) with the change of microenvironment. M1-type macrophages mainly secrete pro-inflammatory factors to exacerbate the instability of AS plaques, while M2-type macrophages mainly secrete anti-inflammatory cytokines to help stabilize AS plaques. Sun et al47 have found that Pae (30 μmol/L) can inhibit M1-type polarization of macrophages induced by co-stimulation of LPS and interferon-γ (IFN-γ), thereby reducing the secretion of inflammatory cytokines IL-6 and TNF-α. The mechanism is related to the down-regulation of miR-155, thereby increasing the expression of suppressor of cytokine signaling 1 (SOCS1), and inhibiting the JAK1/STAT1 pathway (Figure 3 and Table 2).
Adhesion Reduction
Cell adhesion molecules are membrane receptor glycoproteins that play a very important role in cell adhesion to ECM and VECs. Yuan et al48 found that VCAM-1 is a specific target gene of miR-126, and Pae (120 µM) can inhibit the expression of VCAM-1 by up-regulating the level of miR-126 in ox-LDL damaged VECs. Activation of NF-κB is required to induce the expression of adhesion molecules such as VCAM-1. Yuan et al also found that Pae blocked the activation of PI3K/Akt/NF-κB signaling pathway through upregulation of miR-126, thereby inhibiting the expression of VCAM-1 and inhibiting the adhesion of monocytes to ox-LDL damaged VECs. Mitogen-activated protein kinase (MAPK) is involved in signal transduction of growth factors, cytokines, stress stimuli, and various pharmacological compounds.76 Pan et al49 found that Pae (3, 6, and 12 µM) down-regulates the expression of VCAM-1 by inhibiting p38 and extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathways, thereby inhibiting TNF-α-induced monocytes adhesion to RAEC. In another study, Nizamutdinova et al50 found that Pae (50 µM) inhibited the expression of ICAM-1 in TNF-α-stimulated HUVEC by blocking the p38, ERK, and NF-κB signaling pathways, thereby inhibiting the adhesion of monocytes to HUVEC. Wang et al51 found that Pae (21.6 and 25.2 μM) also inhibited ox-LDL-induced monocyte adhesion to VEC by inhibiting the c-Jun N-terminal kinase (JNK) pathway (Figure 3 and Table 2).
LPS, one of the outer membrane components of Gram-negative bacteria, is a strong inflammatory promoter, which can cause the activation and injury of VEC and lead to pathophysiological changes of AS. Yang et al52 found that Pae (10 μg/mL) can reduce TNF-α release and VCAM-1 level in rat VEC induced by LPS by inhibiting TLR4/NF-κB signaling pathway. In addition, Pae (2.5, 5 and 10 μg/mL) effectively inhibited the adhesion of LPS-induced monocytes to VEC and significantly improved the survival rate of LPS-induced VEC.77 Zhang et al78 also found that Pae (15, 30 and 60 μmol/L) could effectively inhibit LPS-induced VEC damage in co-culture with VSMC, and reduce the levels of IL-1β, TNF-α and the expression of ICAM-1 (Figure 3 and Table 2).
Inhibition of Vascular Proliferation and Stenosis
Abnormal proliferation, migration and phenotypic transformation of VSMC are prominent manifestations of VSMC dysfunction, leading to the formation of neointima and the development of AS.79 Zhang et al80 found that the local application of Pae could prevent restenosis in the early stage of rabbit vein transplantation, and its mechanism was related to the inhibition of VSMC proliferation, VCAM-1 expression, intima and middle media hyperplasia, and anti-VEC apoptosis. Xu et al53 found that Pae (30 mg/kg/d) could reduce the formation of new intima after ligation of the left common carotid artery in mice. In vitro studies, Pae (50 ng/mL) inhibited VSMC proliferation and migration in rat aorta, and inhibited rapamycin induced VSMC phenotype transition from differentiation to dedifferentiation.
Dysregulation of the gut microbiome may affect the progression of AS by producing metabolites that increase the inflammatory response. In particular, high levels of LPS have been found in patients with AS, and LPS promotes the proliferation of VSMC.81 Osteopontin (OPN), a secreted proinflammatory protein involved in the proliferation of VSMC, is an important driver of AS.82 Recent studies have shown that LPS acute stimulation enhances OPN expression in mouse peripheral blood mononuclear cells.83 Shi et al54 found that Pae (40 and 20 mg/kg/d) inhibited AS progression and VSMC proliferation in high-fat ApoE−/− mice, and the mechanism was related to changing the composition of intestinal microbiota and reducing the production of LPS by intestinal microbiota. In vitro studies, Pae inhibited LPS-induced proliferation of VSMC in a co-culture system of THP-1 cells and VSMC by a mechanism related to inhibition of the OPN/NF-κB signaling pathway (Figure 3 and Table 2).
Platelet-derived growth factor (PDGF) is a strong and potent growth factor that promotes mitosis. Among them, PDGF-BB promoted the proliferation and migration of VSMC cultured in vitro, activated cell signaling cascade reaction, and thus promoted cell growth.84 Lin et al55 found that Pae (100, 125, 150 mg/L) inhibited the proliferation of VSMC induced by PDGF-BB in a concentration-dependent way and prevented VSMC from entering the S phase from G0/G1 phase. In another study, Chen et al56 found that Pae (60 and 120 μM) could reduce the damage of high glucose on VEC, and reduce the release of vascular endothelial growth factor (VEGF) and PDGF from VEC. Thus, the proliferation of VSMCs co-cultured with VECs was inhibited. Chen et al also found that Pae could inhibit the proliferation of VSMC by blocking Ras/Raf/ERK1/2 signaling pathway in VSMC (Figure 3 and Table 2).
Inflammation is an important factor leading to the proliferation of VSMC. Meng et al57 found that Pae (25 μM) inhibited TNF-α-induced proliferation of VSMC, which may be achieved by promoting the apoptosis of VSMC. In addition, Pae also inhibits VSMC migration by down-regulating the expression of MMP-2 and MMP-9, and inhibits the production of pro-inflammatory cytokines IL-6 and IL-1β. The expression of HMGB1 in AS plaques increased significantly. Liu et al58 found that Pae (100 nmol/L) alleviated the inflammatory response of VSMC caused by ox-LDL by down-regulating HMGB1, and therefore inhibited the proliferation and migration of VSMC (Figure 3 and Table 2).
Mitochondrial autophagy can inhibit the proliferation of VSMC. For example, drug-eluting stents are loaded with rapamycin to induce autophagy, thereby reducing VSMC proliferation and promoting contractile phenotypes.85 Wu et al59 found that Pae(30 μM) could up-regulate autophagy by activating AMPK/mTOR signaling pathway, thus inhibiting ox-LDL-induced VSMC proliferation. In another study, Liu et al60 found that Pae (60 µmol/L) could induce autophagy activity of VSMC by activating class III PI3K/Beclin-1 signaling pathway (Figure 3 and Table 2).
Peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α) is a transcriptional coactivator that is highly expressed in mitochondria.86 Wang et al61 found that Pae (100 mg/kg/d) could prevent hypertrophy of rat pulmonary middle media and mitochondrial damage of VSMC caused by hypoxia in vivo. In vitro studies, Pae (200 μM) alleviated inhibition of ATP production, mitochondrial morphological abnormalities, and increase of ROS in pulmonary artery smooth muscle cells (PASMC) under hypoxia. In addition, Pae also stimulates PASMC apoptosis by inhibiting PGC-1α expression, thus reducing proliferation (Figure 3 and Table 2).
Inhibiting Oxidative Stress and Endoplasmic Reticulum Stress
Forkhead box O3a (FOXO3a) is an important transcription factor that plays an important role in the regulation of oxidative stress.87 Tang et al62 found that Pae (10 and 50 μM) increased cell viability, SOD activity, and inhibited apoptosis, ROS, and inflammatory cytokines in HUVEC treated with high glucose/high palmitic acid by regulating SIRT1/FOXO3a/NF-kB signaling pathway. Tet methylcytosine dioxygenase 2 (TET2), a member of the TET family, is an enzyme that works by catalyzing the oxidation of DNA 5-methylcytosine.88 Yu et al63 found that Pae (120 μM) inhibited ox-LDL-induced VEC apoptosis, inflammation and oxidative damage in mice by down-regulating miR-338-3p and increasing TET2 expression. Aspartate-β-hydroxylase (ASPH) belongs to the group of type II transmembrane proteins that can not only enhance cell proliferation, migration and invasion, but also promote tumor growth by stimulating angiogenesis and immunosuppression.89 Liu et al64 found that Pae (120 μmol/L) inhibited ox-LDL-induced apoptosis of mouse VEC cells and the levels of IL-1β, IL-6 and ROS by regulating the miR-152-3p/ASPH signaling pathway (Figure 4 and Table 2).
ERS can trigger oxidative stress outbreaks by targeting mitochondria.90 Choy et al65 found that Pae (0.1 μM/mL) reversed the ERS, oxidative stress and nitric oxide (NO) bioavailability reduction induced by lamycin in HUVEC, C57BJ-6J and PPARδ mice aorta, thereby alleviating vascular endothelial dysfunction. The mechanism is related to the up-regulation of the expression and activity of 5′ adenosine monophosphate-activated protein kinase (AMPK) and peroxisome proliferator-activated receptorδ (PPARδ) (Figure 4 and Table 2).
Promote Lipid Metabolism
Macrophage-derived foam cells play a key role in the development of AS, a chronic arterial disease characterized by lipid deposition and inflammation in blood vessel walls. The formation of foam cells is mainly caused by impaired cholesterol efflux from macrophages or uncontrolled uptake of ox-LDL by macrophages. There are several types of scavenger receptors (SR) on the cell membrane of macrophages, among which class A (SR-A) and cluster of differentiation 36 (CD36) are mainly responsible for ox-LDL uptake. However, ATP-binding cassette transporter A1 (ABCA1), ABCG1 and Class B (SR-B) were mainly responsible for intracellular lipid efflux.91,92 Li et al66 found that Pae (5, 10, and 50 μM) reduced the accumulation of cholesterol in RAW264.7 macrophages by promoting ABCA1-dependent cholesterol effluence and inhibiting CD36-dependent ox-LDL uptake. In animal experiments, Pae (150 mg/kg) increased the expression of ABCA1 protein and decreased the expression of CD36 protein in the aorta of ApoE−/− mice by promoting the expression of heme oxygenase-1(HO-1), thus alleviating the lesion of AS (Figure 4 and Table 2).
Bile acids (BA) are the main downstream products of cholesterol catabolism and play an important role in the occurrence of AS. BA can activate farnesoid x receptor (FXR), a key nuclear receptor that regulates lipid metabolism, and further promote the expression of intestinal fibroblast growth factor 15(FGF15), inhibit BA synthesis and increase liver cholesterol content. He et al67 found that Pae (200 mg/kg/d) could reduce aortic plaque area, liver lipid deposition, and significantly reduce serum and liver total cholesterol levels in AS model mice. Its mechanism is related to inhibiting FXR/FGF15 signaling pathway and promoting BA synthesis and excretion (Figure 4 and Table 2).
Other Protective Mechanisms for Blood Vessels
Studies have shown that autophagy can help cells adapt to different pathological conditions, but excessive autophagy can lead to the degradation of basic cell components and promote cell death. miR-30a can negatively regulate the expression of Beclin-1, a key autophagy promoting gene.93 Li et al68 found that ox-LDL (100 mg/L) up-regulated the formation of autophagy vacuoles in VEC and inhibited the growth of VEC, while Pae (60µM) inhibited excess autophagy through the miR-30a/Beclin-1 pathway to counteract ox-LDL-induced VEC damage (Figure 4 and Table 2).
Cell senescence can be defined as an irreversible state of growth arrest in cells that proliferate normally. Jamal et al69 found that Pae (30 μM) inhibited HUVEC irreversible growth arrest induced by hydrogen peroxide (H2O2) by down-regulating p53 protein and up-regulating silencing regulatory protein 1 (Sirt1) expression (Figure 4 and Table 2).
VEGF is a specific endothelial cell mitogen and chemokine that stimulates angiogenesis in vivo during myocardial ischemia. VEGF165 has also been shown to increase thrombus recirculation shortly after thrombus formation.70 Ye et al94 found that Pae (1.25 mg/kg) could improve the prothrombotic state and increase the expression of VEGF165 in the rat model of thrombosis. In cell experiments, Pae (0.5 µmol/L) activated ERK1/2 phosphorylation of HUVEC and upregulated VEGF165 expression, thereby promoting thrombus recirculation (Figure 4 and Table 2).
It is well known that von willebrand factor (vWF) is involved in coagulation and reflects the extent of endothelial dysfunction. Angiotensin receptors 1 (AT1) and endothelin (ET)-1 are closely associated with blood pressure contraction, whereas NO is an important vasodilator synthesized by EVCs. Gai et al71 found that Pae (2 and 5mg/kg/d) significantly reduced serum vWF, AT1 and ET-1 levels, improved serum NO levels and reduced vascular endothelial cell damage in spontaneously hypertensive rats (SHR). Table 2 summarizes the research objects, intervention methods and protective mechanisms of pae in vascular protection experiments (Figure 4 and Table 2).
Discussion
Pae is a phenolic component extracted from peony bark, peony root and Xu Changqing, which has a protective effect on the cardiovascular system. This paper reviews the cardiovascular protective mechanism of Pae. Pae can protect the heart by inhibiting oxidative stress, promoting mitochondrial fusion, regulating mitochondrial autophagy, inhibiting inflammation, inhibiting myocardial apoptosis, hypertrophy and fibrosis. In addition, Pae can also play a protective role in blood vessels by inhibiting vascular inflammation, reducing adhesion, inhibiting vascular proliferation, inhibiting oxidative stress and ERS.
It’s important to point out that some of these experiments do not show exactly the same results. For example, Chen et al18 found that Pae (5 μmol/L) reduced the apoptosis level of H9C2 cardiomyocytes and the release of inflammatory factors by activating the PI3K/AKT pathway. However, Wu et al15 found that Pae (100 μM) inhibited PI3K/AKT/NF-κB signaling pathway through upregulation of miR-1 to reduce Dox-induced apoptosis of cardiocytes. The two researchers came to different conclusions about the activation or inhibition of PI3K/AKT pathway by Pae, which may be related to intervention factors and different doses. The molecular targets of Pae are very complex, and the relationship between different targets and signaling pathways cannot be clearly explained, which requires us to use systems biology methods to further study specific molecular targets of Pae. For example, a photoactive probe was used to label metformin and finally PEN2 was identified as the direct target of metformin.95
After oral administration, Pae can be absorbed into the intestinal tract and quickly enter the portal vein circulation.96 Along with the blood flow, Pae can be rapidly transported to multiple target organs such as the heart, liver, kidney and brain. Pae has a short half-life and can be eliminated from the body in a short time, which makes the safety of Pae very high.97 It has been reported that paeonol absorption is primary metabolism, which does not depend on concentration. Under acidic conditions, especially in hypertonic solutions, Pae is easily absorbed.98 Pae is excreted mainly by the urinary system, and its excretion in urine is higher than in stool.99 However, the rapid and complete first metabolism of Pae, as well as its low water solubility and high volatility, determine its poor bioavailability. The level and duration of Pae in the heart and brain can be significantly increased by co-administration with other phytochemicals, such as Danshentsu. In addition, several nanotechnology-assisted drug delivery systems have been developed, such as microemulsion gels, transglycosomes, porous microspheres, liquid crystal nanoparticles, and microsponges, which greatly enhance the therapeutic efficacy of Pae due to their high encapsulation capacity and stability, as well as better control over the release and residence time of the drug in the target tissue.
At present, Pae dosage forms approved by China’s National Medical Products Administration(NMPA) mainly include tablets, ointments and injections, which are mainly used in clinical dermatology. According to the NMPA, the label for Pae covers a number of areas, for example, inflammation or pain-related indications, including fever, headache, neuralgia, myalgia, and rheumatoid arthritis.
To sum up, the mechanism of Pae in the treatment of CVD is complex. We should pay attention to the reliability of these basic experiments and the possibility that poor methodology may lead to results bias. It is suggested that future researchers should further study specific molecular targets of Pae by using systems biology methods, so that the fundamental mechanism of Pae can be elaborated fundamentally. In recent years, clinical studies on the treatment of CVD by Pae are still lacking. Large randomized, controlled trials are urgently needed to evaluate the efficacy and safety of Pae in the treatment of CVD. It should be noted that Pae is a complementary or alternative medicine, not a replacement for the main treatment. Pae should be used under the guidance of a physician.
Abbreviations
Pae, Paeonol; ERS, endoplasmic reticulum stress; CVD, cardiovascular disease; I/R, ischemia/reperfusion; ROS, reactive oxygen species; BRCA1, breast cancer type 1 sensitive protein; VSMC, vascular smooth muscle cells; A/R, anoxia/reoxygenation; NLRP3, nod-like receptor protein 3; NF-κB, nuclear factor κB; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; SIRT1, silent information regulator 1; AMI, acute myocardial infarction; LVEF, left ventricular ejection fraction; SOD, superoxide dismutase; DOX, doxorubicin; HF, heart failure; SKP2, s-phase kinase-associated protein 2; CHF, chronic heart failure; LVFS, left ventricular fractional shortening; LVESD, left ventricular end systolic diameter; DCM, diabetic cardiomyopathy; Bcl-2, B-cell lymphoma-2; TLR4, toll-like receptor 4; MTX, methotrexate; LPS, lipopolysaccharide; HMGB1, high mobility group protein B1; LAD, left anterior descending; miRNAs, microRNAs; TAC, transverse aortic coarction; MF, myocardial fibrosis; AS, atherosclerosis; VEC, vascular endothelial cell; PTEN, phosphatase and tensin homolog; ox-LDL, oxidized low-density lipoprotein; NLRP3, NOD-Like receptor thermal protein domain associated protein 3; RAEC, rat aortic endothelial cells; HUVECs, human umbilical vein endothelial cells; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular intercellular adhesion molecule-1; Th17, T helper type 17; Treg, regulatory T cells; ECM, extracellular matrix; MMP, matrix metalloproteinases; SCFA, short-chain fatty acids; LOX, lysyloxidase; IFN-γ, interferon-γ; SOCS1, suppressor of cytokine signaling 1; MAPK, mitogen-activated protein kinase; ERK1/2, extracellular signal-regulated kinase 1/2; JNK, c-Jun N-terminal kinase; OPN, osteopontin; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor; PDGF, platelet derived growth factor; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator 1α; PASMC, pulmonary artery smooth muscle cells; FOXO3a, forkhead box O3a; TET2, tet methylcytosine dioxygenase 2; ASPH, aspartate-β-hydroxylase; NO, nitric oxide; AMPK, 5′ adenosine monophosphate-activated protein kinase; PPARδ, peroxisome proliferator-activated receptorδ; SR, scavenger receptors; CD36, cluster of differentiation 36; ABCA1, ATP-binding cassette transporter A1; HO-1, heme oxygenase-1; BA, bile acids; FXR, farnesoid x receptor; FGF15, fibroblast growth factor 15; H2O2, hydrogen peroxide; Sirt1, silencing regulatory protein 1; vWF, von willebrand factor; ET-1, endothelin-1; AT1, angiotensin receptors 1; SHR, spontaneously hypertensive rats.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by grants from General Program of National Natural Science Foundation of China (Grant no. 81973836), Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (Grant no. CI2021A00902).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Clark H. NCDs: a challenge to sustainable human development. Lancet. 2013;381(9866):510–511.
2. Mendis S, Davis S, Norrving B. Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke. 2015;46(5):e121–2.
3. Horng CT, Shieh PC, Tan TW, et al. Paeonol suppresses chondrosarcoma metastasis through up-regulation of miR-141 by modulating PKCδ and c-Src signaling pathway. Int J Mol Sci. 2014;15(7):11760–11772.
4. Vellasamy S, Murugan D, Abas R, et al. Biological activities of paeonol in cardiovascular diseases: a review. Molecules. 2021;26(16):4976.
5. Moens AL, Claeys MJ, Timmermans JP, et al. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100(2):179–190.
6. Leung JW, Makharashvili N, Agarwal P, et al. ZMYM3 regulates BRCA1 localization at damaged chromatin to promote DNA repair. Genes Dev. 2017;31(3):260–274.
7. Lovren F, Pan Y, Quan A, et al. BRCA1 shields vascular smooth muscle cells from oxidative stress. J Thorac Cardiovasc Surg. 2014;147(6):1946–1955.
8. Zheng J, Mao Z, Zhang J, et al. Paeonol Pretreatment Attenuates Anoxia-Reoxygenation Induced Injury in Cardiac Myocytes via a BRCA1 Dependent Pathway. Chem Pharm Bull (Tokyo). 2020;68(12):1163–1169.
9. Liu C, Wang MJ, Fan YF, et al. Paeonol alleviates myocardial ischemia-reperfusion injury in rats by upregulation of SIRT1. Hebei Med J. 2017;06:917–921.
10. Xu M, Hou J, Zhao LN. Effect of paeonol on acute myocardial infarction model rats based on SIRT3/β-catenin/PPARγ signaling pathway. J Cardio-Cerebrovascular Dis Integr Trad Chine Western Med. 2022;20:3704–3708+3716.
11. Chen C, Liu S, Cao G, et al. Cardioprotective Effect of Paeonol on Chronic Heart Failure Induced by Doxorubicin via Regulating the miR-21-5p/S-Phase Kinase-Associated Protein 2 Axis. Front Cardiovasc Med. 2022;9:695004.
12. Liu C, Han Y, Gu X, et al. Paeonol promotes Opa1-mediated mitochondrial fusion via activating the CK2α-Stat3 pathway in diabetic cardiomyopathy. Redox Biol. 2021;46:102098.
13. Ding M, Shi R, Fu F, et al. Paeonol protects against doxorubicin-induced cardiotoxicity by promoting Mfn2-mediated mitochondrial fusion through activating the PKCε-Stat3 pathway. J Adv Res. 2022:456.
14. Tsai CF, Su HH, Chen KM, et al. Paeonol protects against myocardial ischemia/reperfusion-induced injury by mediating apoptosis and autophagy crosstalk. Front Pharmacol. 2021;11:586498.
15. Wu J, Sun C, Wang R, et al. Cardioprotective effect of paeonol against epirubicin-induced heart injury via regulating miR-1 and PI3K/AKT pathway. Chem Biol Interact. 2018;286:17–25.
16. Liu C, Wang MJ, Fan YF, et al. Effects of paeonol on cardiac function and Pink1/Parkin after myocardial infarction in rats. World Sci Technol. 2020;5:1428–1436.
17. Al-Taher AY, Morsy MA, Rifaai RA, et al. Paeonol Attenuates Methotrexate-Induced Cardiac Toxicity in Rats by Inhibiting Oxidative Stress and Suppressing TLR4-Induced NF-κB Inflammatory Pathway. Mediators Inflamm. 2020;2020:8641026.
18. Chen JH, Zhou R, Dai L, et al. Study on the protective effect of paeonol activation of PI3K/AKT on myocardial injury induced by septicaemia. Chinese Pharmacist. 2002;06:987–994.
19. Huang X, Ding HS. Effects of paeonol on high mobility group protein B1 and Interleukin 1β and myocardial apoptosis during myocardial ischemia-reperfusion injury in rats. Chine J Geriatric Cardio-Cerebrovascular Dis. 2019;05:522–524.
20. Zhao JY, Dong HY, Zhou XH, et al. Effects of paeonol on ventricular remodeling and expression of NF-κBp65, IL-1 mrna in rats with acute myocardial infarction. Chine J Exp Formulae. 2014;15:177–180.
21. Liu C, Qu J, Wang MJ, et al. Paeonol alleviates hypoxia/reoxygenation injury in cultured cardiomyocytes by inhibiting miR-155-5p. J Chongqing Med Univ. 2011;2:156–161.
22. Wu DD, Zhou XH. Effects of paeonol on PI3K/Akt Signaling Pathway in cardiomyocytes induced by high glucose. Chine Patent Med. 2022;2:597–601.
23. Chen X, Zhang Z, Zhang X, et al. Paeonol attenuates heart failure induced by transverse aortic constriction via ERK1/2 signalling. Pharm Biol. 2022;60(1):562–569.
24. Thabassum AIS, Tirupathi Pichiah PB, Raja S, et al. Paeonol Reverses Adriamycin Induced Cardiac Pathological Remodeling through Notch1 Signaling Reactivation in H9c2 Cells and Adult Zebrafish Heart. Chem Res Toxicol. 2020;33(2):312–323.
25. Shi ZP, Zhou XH, Xu Q, et al. Effects of paeonol on mRNA expression of Smad2, Smad3 and Smad7 in rats with acute myocardial infarction. Chine J Exp Formulae. 2015;2:146–150.
26. Shi ZP, Cao K, Zhao JY, et al. Effects of paeonol on myocardial pathological changes and expression of TGF-β1 and CollagenIII in acute myocardial infarction model rats. Chine J Exp Formulae. 2015;24:98–103.
27. Tamamori-Adachi M, Takagi H, Hashimoto K, et al. Cardiomyocyte proliferation and protection against post-myocardial infarction heart failure by cyclin D1 and Skp2 ubiquitin ligase. Cardiovasc Res. 2008;80(2):181–190.
28. Yu T, Sheu SS, Robotham JL, et al. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79(2):341–351.
29. Marín-García J, Akhmedov AT, Moe GW. Mitochondria in heart failure: the emerging role of mitochondrial dynamics. Heart Fail Rev. 2013;18(4):439–456.
30. Qin Y, Li A, Liu B, et al. Mitochondrial fusion mediated by fusion promotion and fission inhibition directs adult mouse heart function toward a different direction. FASEB J. 2020;34(1):663–675.
31. Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8(11):870–879.
32. Lv XC, Zhou HY. Resveratrol protects H9c2 embryonic rat heart derived cells from oxidative stress by inducing autophagy: role of p38 mitogen-activated protein kinase. Can J Physiol Pharmacol. 2012;90(5):655–662.
33. Zeng M, Wei X, Wu Z, et al. Simulated ischemia/reperfusion-induced p65-Beclin 1-dependent autophagic cell death in human umbilical vein endothelial cells. Sci Rep. 2016;6:37448.
34. Ciechomska IA, Goemans GC, Skepper JN, et al. Bcl-2 complexed with Beclin-1 maintains full anti-apoptotic function. Oncogene. 2009;28(21):2128–2141.
35. Roy A, Srivastava M, Saqib U, et al. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int Immunopharmacol. 2016;40:79–89.
36. Perez-Verdia A, Angulo F, Hardwicke FL, et al. Acute cardiac toxicity associated with high-dose intravenous methotrexate therapy: case report and review of the literature. Pharmacotherapy. 2005;25(9):1271–1276.
37. Ding HS, Yang J, Chen P, et al. The HMGB1-TLR4 axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte apoptosis. Gene. 2013;527(1):389–393.
38. Elias N, Tarasoutchi F, Spina GS, et al. Myocardial fibrosis and ventricular remodeling in severe chronic aortic regurgitation. Arq Bras Cardiol. 2009;92(1):63–67.
39. Wang M, Liu M, Ni T, et al. miR-214 mediates vascular inflammation and apoptosis via PTEN expression. Mol Med Rep. 2018;18(2):2229–2236.
40. Liu YR, Chen JJ, Dai M. Paeonol protects rat vascular endothelial cells from ox-LDL-induced injury in vitro via downregulating microRNA-21 expression and TNF-α release. Acta Pharmacol Sin. 2014;35(4):483–488.
41. Hoseini Z, Sepahvand F, Rashidi B, et al. NLRP3 inflammasome: its regulation and involvement in atherosclerosis. J Cell Physiol. 2018;233(3):2116–2132.
42. Shi X, Xie X, Sun Y, et al. Paeonol inhibits NLRP3 mediated inflammation in rat endothelial cells by elevating hyperlipidemic rats plasma exosomal miRNA-223. Eur J Pharmacol. 2020;885:173473.
43. Liu Y, Li C, Wu H, et al. Paeonol Attenuated Inflammatory Response of Endothelial Cells via Stimulating Monocytes-Derived Exosomal MicroRNA-223. Front Pharmacol. 2018;9:1105.
44. Liu YR, Shao Q, Zhang HH, et al. Upregulation of paeonol on small concave protein-1 inhibits NF-κB pathway and reduces endothelial inflammation in aorta of atherosclerotic model rats. Chine J Traditional Chine Med. 2011;11:2578–2585.
45. Hu WJ, Zhang Z, Dai M. Protective effect of paeonol on LPS-induced vascular endothelial cells cocultured with smooth muscle cells by inhibiting PI3K/AKT-NF-κB pathway. Chine J Traditional Chine Med. 2016;12:2298–2302.
46. Shi X, Huang H, Zhou M, et al. Paeonol Attenuated Vascular Fibrosis Through Regulating Treg/Th17 Balance in a Gut Microbiota-Dependent Manner. Front Pharmacol. 2021;12:765482.
47. Sun Y, Liu L, Shi XY, et al. Paeonol inhibits M1 polarization in macrophages by down-regulating miR-155/JAK1-STAT1 pathway. Chine J Traditional Chine Med. 2010;9:2158–2164.
48. Yuan X, Chen J, Dai M. Paeonol promotes microRNA-126 expression to inhibit monocyte adhesion to ox-LDL-injured vascular endothelial cells and block the activation of the PI3K/Akt/NF-κB pathway. Int J Mol Med. 2016;38(6):1871–1878.
49. Pan LL, Dai M. Paeonol from Paeonia suffruticosa prevents TNF-alpha-induced monocytic cell adhesion to rat aortic endothelial cells by suppression of VCAM-1 expression. Phytomedicine. 2009;16(11):1027–1032.
50. Nizamutdinova IT, Oh HM, Min YN, et al. Paeonol suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells by blocking p38, ERK and nuclear factor-kappaB signaling pathways. Int Immunopharmacol. 2007;7(3):343–350.
51. Wang YQ, Dai M, Zhong JC, et al. Paeonol inhibits oxidized low density lipoprotein-induced monocyte adhesion to vascular endothelial cells by inhibiting the mitogen activated protein kinase pathway. Biol Pharm Bull. 2012;35(5):767–772.
52. Yang L, Dai M, Chen P. Effects of paeonol on the release of VCAM-1, TNF-α and TLR4/NF-κB signaling Pathway in rat vascular endothelial cells induced by Lipopolysaccharide. J Anhui Univ Traditional Chine Med. 2015;1:46–50.
53. Xu H, Wu Z, Jin Z, et al. Paeonol Suppresses Vasculogenesis Through Regulating Vascular Smooth Muscle Phenotypic Switching. J Endovasc Ther. 2022;29(1):117–131.
54. Shi X, Wu H, Liu Y, et al. Inhibiting vascular smooth muscle cell proliferation mediated by osteopontin via regulating gut microbial lipopolysaccharide: a novel mechanism for paeonol in atherosclerosis treatment. Front Pharmacol. 2022;13:936677.
55. Lin HX. Effects of paeonol on the proliferation cycle of vascular smooth muscle cells induced by platelet-induced growth factor BB type. Lingnan J Cardiovascular Dis. 2013;05:621–625.
56. Chen J, Dai M, Wang Y. Paeonol Inhibits Proliferation of Vascular Smooth Muscle Cells Stimulated by High Glucose via Ras-Raf-ERK1/2 Signaling Pathway in Coculture Model. Evid Based Complement Alternat Med. 2014;2014:484269.
57. Meng L, Xu W, Guo L, et al. Paeonol Inhibits the Proliferation, Invasion, and Inflammatory Reaction Induced by TNF-α in Vascular Smooth Muscle Cells. Cell Biochem Biophys. 2015;73(2):495–503.
58. Liu C, Wang MJ, Shan WC, et al. Effects of paeonol on proliferation and migration of vascular smooth muscle cells and its mechanism of action. J Clinical Exp Med. 2018;8:822–826.
59. Wu H, Song A, Hu W, et al. The Anti-atherosclerotic Effect of Paeonol against Vascular Smooth Muscle Cell Proliferation by Up-regulation of Autophagy via the AMPK/mTOR Signaling Pathway. Front Pharmacol. 2018;8:948.
60. Liu Y, Song A, Wu H, et al. Paeonol inhibits apoptosis of vascular smooth muscle cells via up-regulation of autophagy by activating class III PI3K/Beclin-1 signaling pathway. Life Sci. 2021;264:118714.
61. Wang D, Du Y, Xu H, et al. Paeonol protects mitochondrial injury and prevents pulmonary vascular remodeling in hypoxia. Respir Physiol Neurobiol. 2019;268:103252.
62. Tang H, Li K, Zhang S, et al. Inhibitory Effect of Paeonol on Apoptosis, Oxidative Stress, and Inflammatory Response in Human Umbilical Vein Endothelial Cells Induced by High Glucose and Palmitic Acid Induced Through Regulating SIRT1/FOXO3a/NF-κB Pathway. J Interferon Cytokine Res. 2021;41(3):111–124.
63. Yu Y, Yan R, Chen X, et al. Paeonol suppresses the effect of ox-LDL on mice vascular endothelial cells by regulating miR-338-3p/TET2 axis in atherosclerosis. Mol Cell Biochem. 2020;475(1–2):127–135.
64. Liu Y, An S, Shi YJ, et al. Paeonol inhibits ox-LDL-induced apoptosis, inflammation and oxidative stress of mouse vascular endothelial cells by regulating the miR-152-3p/ASPH axis in atherosclerosis. J Shenyang Pharm Univ. 2013;1–13.
65. Choy KW, Mustafa MR, Lau YS, et al. Paeonol protects against endoplasmic reticulum stress-induced endothelial dysfunction via AMPK/PPARδ signaling pathway. Biochem Pharmacol. 2016;116:51–62.
66. Li X, Zhou Y, Yu C, et al. Paeonol suppresses lipid accumulation in macrophages via upregulation of the ATP-binding cassette transporter A1 and downregulation of the cluster of differentiation 36. Int J Oncol. 2015;46(2):764–774.
67. He H, Huang HW, Shi XY, et al. Paeonol regulates bile acid metabolism in ApoE−/− mice and plays an anti-atherosclerosis role. Chine J Arteriosclerosis. 2011;2:123–128+165.
68. Li C, Yang L, Wu H, et al. Paeonol Inhibits Oxidized Low-Density Lipoprotein-Induced Vascular Endothelial Cells Autophagy by Upregulating the Expression of miRNA-30a. Front Pharmacol. 2018;9:95.
69. Jamal J, Mustafa MR, Wong PF. Paeonol protects against premature senescence in endothelial cells by modulating Sirtuin 1 pathway. J Ethnopharmacol. 2014;154(2):428–436.
70. Waltham M, Burnand KG, Collins M, et al. Vascular endothelial growth factor enhances venous thrombus recanalisation and organisation. Thromb Haemost. 2003;89(1):169–176.
71. Gai Z, Wang Z, Zhang L, et al. Paeonol protects against hypertension in spontaneously hypertensive rats by restoring vascular endothelium. Biosci Biotechnol Biochem. 2019;83(11):1992–1999.
72. Yang C, Liu XZ. Research progress of small concave protein-1 in atherosclerosis formation. Hainan Med. 2013;03:437–439.
73. Taleb S, Tedgui A, Mallat Z. IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler Thromb Vasc Biol. 2015;35(2):258–264.
74. Ou HX, Guo BB, Liu Q, et al. Regulatory T cells as a new therapeutic target for atherosclerosis. Acta Pharmacol Sin. 2018;39(8):1249–1258.
75. Slobodin G, Rimar D. Regulatory T Cells in Systemic Sclerosis: a Comprehensive Review. Clin Rev Allergy Immunol. 2017;52(2):194–201.
76. English J, Pearson G, Wilsbacher J, et al. New insights into the control of MAP kinase pathways. Exp Cell Res. 1999;253(1):255–270.
77. Chen JJ, Dai M, Chen P. Effects of paeonol on vascular endothelial cell adhesion induced by lipopolysaccharide in rats. Chine Materia Med. 2013;03:433–437.
78. Zhang Z, Dai M. Effects of paeonol on the adhesion function of vascular endothelial cells cocultured with smooth muscle cells after lipopolysaccharide-induced injury. Chine J Traditional Chine Med. 2014;06:1058–1063.
79. Lacolley P, Regnault V, Segers P, et al. Vascular Smooth Muscle Cells and Arterial Stiffening: relevance in Development, Aging, and Disease. Physiol Rev. 2017;97(4):1555–1617.
80. Zhang JY, Lei L, Shang J, et al. Local application of paeonol prevents early restenosis: a study with a rabbit vein graft model. J Surg Res. 2017;212:278–287.
81. Loffredo L, Ivanov V, Ciobanu N, et al. Is There an Association Between Atherosclerotic Burden, Oxidative Stress, and Gut-Derived Lipopolysaccharides? Antioxid Redox Signal. 2020.
82. Zhang X, Eirin A, Lerman A, et al. Osteopontin: an emerging therapeutic target in uraemic vascular disease. Cardiovasc Res. 2013;98(3):332–333.
83. Fatkhullina AR, Peshkova IO, Dzutsev A, et al. An Interleukin-23-Interleukin-22 Axis Regulates Intestinal Microbial Homeostasis to Protect from Diet-Induced Atherosclerosis. Immunity. 2018;49(5):943–957.e9.
84. Ho HC, Chang HC, Ting CT, et al. Caffeic acid phenethyl ester inhibits proliferation and migration, and induces apoptosis in platelet-derived growth factor-BB-stimulated human coronary smooth muscle cells. J Vasc Res. 2012;49(1):24–32.
85. Grube E, Buellesfeld L. Rapamycin analogs for stent-based local drug delivery. Everolimus- and tacrolimus-eluting stents. Herz. 2004;29(2):162–166.
86. Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol. 2009;71:177–203.
87. Liu S, Zhang X, Sun M, et al. FoxO3a plays a key role in the protective effects of pomegranate peel extract against amikacin-induced ototoxicity. Int J Mol Med. 2017;40(1):175–181.
88. Ko M, Huang Y, Jankowska AM, et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468(7325):839–843.
89. Kanwal M, Smahel M, Olsen M, et al. Aspartate β-hydroxylase as a target for cancer therapy. J Exp Clin Cancer Res. 2020;39(1):163.
90. Bhandary B, Marahatta A, Kim HR, et al. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci. 2012;14(1):434–456.
91. Lin CY, Lee TS, Chen CC, et al. Endothelin-1 exacerbates lipid accumulation by increasing the protein degradation of the ATP-binding cassette transporter G1 in macrophages. J Cell Physiol. 2011;226(8):2198–2205.
92. Saeed O, Otsuka F, Polavarapu R, et al. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(2):299–307.
93. Zhu H, Wu H, Liu X, et al. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy. 2009;5(6):816–823.
94. Ye S, Liu X, Mao B, et al. Paeonol enhances thrombus recanalization by inducing vascular endothelial growth factor 165 via ERK1/2 MAPK signaling pathway. Mol Med Rep. 2016;13(6):4853–4858.
95. Ma T, Tian X, Zhang B, et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature. 2022;603(7899):159–165.
96. Adki KM, Kulkarni YA. Chemistry, pharmacokinetics, pharmacology and recent novel drug delivery systems of paeonol. Life Sci. 2020;250:117544.
97. Li P, Shen J, Wang Z, et al. Genus Paeonia: a comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J Ethnopharmacol. 2021;269:113708.
98. Chen X, Lu Y, Du S, et al. In situ and in vivo study of nasal absorption of paeonol in rats. Int J Mol Sci. 2010;11(12):4882–4890.
99. Gjertsen FB, Solheim E, Scheline RR. Metabolism of aromatic plant ketones in rats: acetovanillone and paeonol. Xenobiotica. 1988;18(2):225–234.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



