Back to Journals » OncoTargets and Therapy » Volume 13
Review of Adjuvant Therapies in Renal Cell Carcinoma: Evidence to Date
Authors Tacconi EMC , Tuthill M , Protheroe A
Received 9 June 2020
Accepted for publication 4 September 2020
Published 30 November 2020 Volume 2020:13 Pages 12301—12316
DOI https://doi.org/10.2147/OTT.S174149
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yao Dai
Eliana MC Tacconi, Mark Tuthill, Andrew Protheroe
Department of Oncology, Churchill Hospital, Oxford University Hospitals NHS Foundation Trust, Oxford OX3 7LE, UK
Correspondence: Andrew Protheroe
Department of Oncology, Churchill Hospital, Oxford University Hospitals NHS Foundation Trust, Oxford OX3 7LE, UK
Email [email protected]
Abstract: In 2018, there were 400,000 new cases of renal cell carcinoma (RCC) globally, with 175,000 deaths attributable to the disease. Three quarters of patients have potentially curable localised disease at diagnosis; however, recurrence rates are as high as 40% following surgery. There are currently no adjuvant therapies in routine clinical use which reliably improve outcomes. Effective adjuvant therapy is an urgent unmet need to reduce recurrence risk and improve outcomes. Early efforts explored chemotherapy, radiotherapy, cytokine therapy, hormonal treatments and tumour cell vaccines as adjuvant therapies, however, have yielded disappointing results. More recently, interest shifted to evaluating tyrosine kinase inhibitors (TKIs) in the adjuvant setting, as they improve outcomes in metastatic disease. Five phase III clinical trials testing adjuvant use of a range of TKIs have been performed, with the results of a sixth trial awaited. Unfortunately, these studies have thus far yielded conflicting and disappointing results, and there is currently no strong evidence for routine adjuvant TKI therapy. In parallel, novel immunotherapy treatment approaches have recently been developed, transforming the management of a range of malignancies, particularly through immune checkpoint inhibitors (ICIs). These approaches are well established in the metastatic context in RCC, as well as in the adjuvant treatment of melanoma. On this basis, five phase III trials are currently ongoing to test the efficacy of a range of ICIs in adjuvant RCC patients, with initial results expected over the next few years. In this article, we review the current evidence for adjuvant therapies in RCC, discuss ongoing clinical trials and suggest directions for future work to address this unmet need.
Keywords: renal cell carcinoma, adjuvant therapy, tyrosine kinase inhibitors, immune checkpoint inhibitors
Introduction
Approximately 13,100 people are diagnosed with renal cell carcinoma (RCC) in the UK each year, making it the seventh most common cancer affecting the UK population.1 A total of 4500 UK deaths are attributable to RCC annually and five-year survival is just 56%. In 2018, there were over 400,000 new cases globally and RCC was responsible for 1.8% of the cancer deaths worldwide, with 175,000 deaths attributable to the disease.2 Although 75% of newly diagnosed patients have potentially curable localised or locally advanced disease,3 recurrence rates in patients with stage II and III disease following nephrectomy are as high as 40%.4 Effective adjuvant therapies are therefore needed to reduce recurrence risk and improve outcomes. Targeted treatments including tyrosine kinase inhibitors (TKIs) and novel immunotherapies have significantly improved the outlook for patients with metastatic RCC in recent years,5–16 providing impetus for studies aimed at identifying an effective adjuvant therapy. Indeed, extensive efforts have been invested in attempts to translate clinical benefits from the metastatic to adjuvant setting. In this review, we examine current evidence for adjuvant therapies in RCC, discuss ongoing clinical trials and suggest future directions in the search for an effective adjuvant therapy.
Predicting Recurrence Risk in the Adjuvant Setting
RCC is currently staged using the eighth TNM staging system.17 Using this system, patients are grouped into prognostic stages based on features of the primary tumour and the presence or absence of nodal and distant metastases (Table 1).
 |
Table 1 UICC TNM Staging of Kidney Tumours |
Leibovich et al aimed to develop an algorithm to predict metastasis development following surgery.18 In a study of 1671 patients with localised clear cell RCC (ccRCC), metastasis development was associated with tumour stage, regional lymph node status, tumour size, nuclear grade and histologic tumour necrosis. The Leibovich Risk Score therefore incorporates these features to stratify patients into low, intermediate or high-risk groups, reliably predicting metastasis-free survival (Table 2).
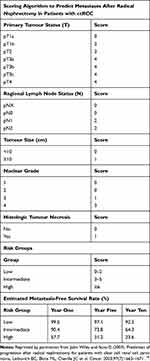 |
Table 2 Leibovich Risk Score |
The UCLA Integrated Staging System (UISS) similarly stratifies ccRCC patients into low, intermediate and high-risk groups to predict postoperative outcomes.19 By stratifying 814 patients based on primary tumour status, grade and Eastern Cooperative Oncology Group (ECOG) performance status, the authors reliably predicted overall survival (OS) and disease-specific survival (Table 3).
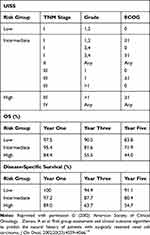 |
Table 3 UCLA Integrated Staging System (UISS) |
These approaches reliably predict recurrence risk in the adjuvant setting for ccRCC and are therefore important not only as prognostic tools to guide individual management, but also as a consistent framework for evaluating novel adjuvant therapies. However, there is currently no consensus for a specific prognostic model20 and this is likely responsible, at least in part, for discrepancies in results of contemporary studies. Development of novel biomarkers is needed to improve and unify existing prognostic models and this may, in turn, improve concordance between studies assessing novel adjuvant therapies.
Failure to Identify Effective Adjuvant Therapies in the Past
Following potentially curative surgery, adjuvant therapies are given with the aim of killing any residual microscopic disease and undetectable micro-metastases. Despite many approaches reaching phase III trials, early efforts failed to identify adjuvant therapies which improve RCC survival (Table 4).
 |
Table 4 Summary of Early Clinical Studies Investigating Adjuvant RCC Therapies |
Based on observations that interferon-alpha (INF-α) and interleukin-2 (IL-2) increase time to progression and OS in metastatic RCC,12,21,22 many early efforts have been invested in evaluating adjuvant cytokine therapy. A prospective phase III trial evaluating IL-2 versus observation in patients with high-risk RCC following surgical resection23 closed early when interim analysis provided no evidence that IL-2 improves disease-free survival (DFS). A study comparing IFN-α-NL versus observation similarly identified no benefit.24 In this phase III study, adjuvant IFN-α-NL failed to improve either OS or relapse-free survival (RFS) in 247 patients following resection of locally extensive RCC. Accordingly, a randomised study found no significant difference in five-year OS or event-free survival in patients treated with recombinant IFN-α2b versus observation following nephrectomy.25
Passalacqua et al assessed the combination of IL-2 plus IFN-α in a phase III randomised trial of 303 patients.26 RFS and OS were not significantly different between groups, consistent with the above studies. However, unplanned subgroup analysis suggested that patients with two of more of age 60 or younger, pN0, tumour grades 1–2 and stage pT3a were more likely to benefit from treatment, with improved RFS rates.
Atzpodien et al evaluated IL-2 and IFN-α2a in combination with 5-flurouracil (5-FU) in the adjuvant setting.27 In this prospective trial, 203 high-risk patients were randomised to either eight weeks of IL-2, IFN-α2a and 5-FU or observation. This study found no RFS benefit of treatment, and that OS was actually inferior to observation alone. In the more recent RE04 study from the UK community, Aitchison et al similarly compared IL-2, IFN-α and 5-FU to observation in high-risk RCC patients eight weeks following nephrectomy.28 While no significant difference was found in DFS or OS, treatment was associated with significant toxicity and worse quality of life. These studies support that there is no benefit of adjuvant cytokine therapy in RCC, either alone or in combination with chemotherapy, and that treatment may be associated with poorer quality of life and worse outcomes.
Another approach in the search for an effective adjuvant RCC therapy has been the development of tumour cell vaccines.12 No significant difference in DFS or OS was detected in a prospective study of 43 patients randomised to hormonal therapy alone or in combination with autologous irradiated tumour cells.29 However, this study was significantly underpowered and there was a trend towards improved DFS in patients with localised disease. Galligioni et al randomised 120 patients to receive either control or three intradermal injections of 107 autologous irradiated tumour cells following radical nephrectomy.30 Thirty-eight of 54 immunised patients showed a delayed-type cutaneous hypersensitivity reaction; however, despite this, there was no significant difference in DFS or OS between groups.
In a phase III trial of RCC patients who had undergone radical nephrectomy (pT2–3b pN0–3 M0), Jocham et al randomised 558 patients to receive no adjuvant treatment or autologous renal tumour cell vaccine (six intradermal applications at four-week intervals post-operatively).31 Progression-free survival (PFS) at five and 70 months was 77.4% and 72% respectively in the vaccine group, and 67.8% and 59.3% respectively in controls, a statistically significant difference. However, this study was unblinded and many baseline characteristics were unbalanced; this, coupled with the failure of similar studies to detect benefit, has meant that this approach has not translated into widespread clinical use. Furthermore, Wood et al performed a multicentre randomised phase III trial of an adjuvant autologous, tumour-derived heat-shock protein (glycoprotein 96)-peptide complex therapeutic vaccine in 818 patients.32 Participants were randomised to treatment or observation, and no significant difference was found in RFS between the groups, supporting the negative results from earlier autologous therapeutic vaccine trials.
More recently, Chamie et al evaluated weekly girentuximab in patients with high-risk ccRCC following partial or radical nephrectomy (ARISER).33 Girentuximab is a chimeric monoclonal antibody targeting carbonic anhydrase IX, a cell surface glycoprotein overexpressed in ccRCC but absent from normal renal tissue. In this randomised, double-blind, placebo-controlled phase III trial, no significant difference was detected in either DFS or OS, indicating that there is no clinical benefit of adjuvant girentuximab.
Compounding the disappointing results thus far, Massari et al performed a meta-analysis of 14 phase III clinical trials exploring adjuvant RCC therapies, in particular cytokine and vaccine-based therapies.34 This study found no statistically significant difference in five-year RFS and results suggested that cytokine therapy may have a detrimental effect on OS.
The studies discussed here failed to demonstrate consistent improvement in DFS or OS. Although they likely struggled with a lack of effective treatments, it is important to note that many of these trials were designed and completed before validated stratification systems for recurrence risk were established. As patient recruitment was mainly based on histopathological parameters, results may have been different had one of the above risk stratification systems been used.
Novel Approaches to Adjuvant Treatment: TKIs
TKIs have been shown to improve outcomes in patients with metastatic RCC.6–8,10–14 For example, sunitinib improved OS by almost five months compared to IFN-α in a phase III trial.8 Newer targeted therapies for metastatic RCC, in particular TKIs, arose through an improved understanding of its molecular pathogenesis.13 The tumour suppressor VHL (von Hippel–Lindau) is frequently mutated in RCC and encodes an E3 ubiquitin ligase that marks proteins including HIF1-α (hypoxia-inducible factor 1-alpha) for degradation, in turn regulating the activity of pro-angiogenic factors including VEGF (vascular endothelial growth factor). This discovery provided impetus to investigate VEGFR (vascular endothelial growth factor receptor) inhibitors such as sorafenib and sunitinib in metastatic RCC, and their use is now well established in this setting. The PI3K-AKT (phosphatidylinositol 3-kinase; RAC-α serine/threonine-protein kinase) signalling pathway has also been implicated in RCC through angiogenesis promotion and its regulation via mTOR (mechanistic target of rapamycin) has been exploited in metastatic RCC; mTOR inhibitors approved for clinical use include temsirolimus and everolimus. TKIs are now in widespread use as both first- and second-line agents for treatment of metastatic RCC13 and their mechanism of action is summarised in Figure 1.
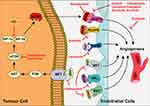 |
Figure 1 Mechanism of action of TKIs used in metastatic RCC. Kinases regulating angiogenesis are frequently overexpressed in RCC, culminating in increased tumour vascular supply. VHL inactivation leads to overexpression of pro-angiogenic factors including VEGF via increased HIFα expression. HIFα is also upregulated via PI3K/mTOR signalling. VEGF binds to VEGFR1/2/3 on endothelial cell surfaces, promoting angiogenesis. Additional cell surface receptors regulating angiogenesis include PDGFR (platelet-derived growth factor receptor), FGFR (fibroblast growth factor receptor), tyrosine-protein kinase MET (hepatocyte growth factor receptor), AXL oncogene (tyrosine-protein kinase receptor UFO) and proto-oncogene tyrosine-protein kinase receptor RET. A range of TKIs have been developed targeting various aspects of these signalling pathways. Adapted by permission from Springer Nature © (2017). Posadas EM, Limvorasak S, Figlin RA. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol. 2017;13(8):496–511.13 |
Having been established as effective therapies in metastatic disease, TKIs have been the focus of extensive efforts in developing an effective adjuvant RCC treatment. Several studies have been performed or are ongoing to assess TKIs in this setting (Table 5), including ASSURE, S-TRAC, PROTECT, ATLAS, SORCE and EVEREST.
 |
Table 5 Summary of Newer Generation Studies Testing Adjuvant TKIs |
ASSURE (adjuvant sorafenib or sunitinib for unfavourable renal carcinoma) was the first phase III trial assessing the efficacy of TKIs in adjuvant RCC patients.12–14,35 This double-blind, randomised study compared sorafenib or sunitinib for one year versus placebo on DFS in 1943 patients with high-risk RCC. Participants from 226 centres with high-grade pathological stage T1b or greater non-metastatic RCC who had undergone nephrectomy were randomised 1:1:1 to sunitinib (50mg; 647 patients), sorafenib (800mg; 649 patients) or placebo (647 patients). No significant difference was found in the primary endpoint of DFS for either sunitinib or sorafenib relative to placebo (median DFS: 5.8, 6.1 and 6.6 years for sunitinib, sorafenib and placebo, respectively; OS: 77.9%, 80.5% and 80.3%, respectively). Furthermore, treatment was associated with high rates of adverse events (AEs) including hypertension, rash, fatigue and hand-foot syndrome; 63% of the patients receiving sunitinib and 72% of the patients receiving sorafenib reported grade three or worse AEs, compared to 25% in the placebo group. Following dose reduction, this figure still exceeded 55%. In patients started on full dose, treatment discontinuation rates due to AEs or patient withdrawal were 44% and 45% in the sunitinib and sorafenib groups, respectively (11% in the placebo group). The disappointing results of this study suggested that TKIs would not be useful for preventing RCC recurrence in the adjuvant setting.
Shortly after publication of ASSURE, the findings of S-TRAC (Sunitinib Trial in Adjuvant Renal Carcinoma) were made available.12–14,36 This was a randomised, double-blind phase III study comparing sunitinib versus placebo for one year in 615 patients at high-risk of recurrence from 99 centres. This study considered a higher risk population than ASSURE, with eligibility criteria focusing on locoregional UISS high-risk T3-T4 disease. Patients were randomised to receive sunitinib (50mg) or placebo for one year. In contrast to ASSURE, a significant difference in DFS was detected (6.8 years for sunitinib and 5.6 years for placebo). This study was, therefore, the first to show benefit of adjuvant TKI therapy. Subgroup analyses confirmed a benefit of adjuvant sunitinib over placebo across subgroups, consistent with the primary analysis.37 While DFS was prolonged in the sunitinib group, this must be balanced against toxicity and quality of life considerations; 48% of the patients in the treatment group experienced grade three to four AEs (16% in the placebo group), with a discontinuation rate of 28%.
There are several possible explanations for the conflicting results of ASSURE and S-TRAC. While ASSURE included patients with stage I tumours, S-TRAC focused on a higher risk population as discussed above. Furthermore, S-TRAC included only ccRCC while ASSURE did not restrict their study population. Dose reductions were greater in ASSURE, with reductions to 25mg included while in S-TRAC maximum reduction was to 37.5mg, with fewer dose reductions. Furthermore, S-TRAC involved central radiograph review to confirm disease-free status and the primary endpoint of DFS was based on blinded central review while in ASSURE this was by investigators only. When both studies were included in a meta-analysis of 1909 patients38 (with sorafenib ASSURE arm excluded), a trend towards benefit of sunitinib was detected; however, there was no significant benefit of sunitinib on either DFS or OS; these findings did not change when only the ccRCC histology subgroup of ASSURE was included. Furthermore, the hazard ratio (HR) for OS was greater than one, suggesting harm of treatment, and risk of grade three or greater AEs increased by up to 2.63 times upon treatment. These results support that there is no evidence for the use of TKIs in the adjuvant setting at present; while there is no clear evidence of clinical benefit, there is increased risk of toxicity and possible harm to OS.
In sub-population analyses of S-TRAC, higher risk patients benefited more from treatment, with DFS of 6.2 years versus 4.0 years in the placebo group. This high-risk subgroup was defined as tumour stage III, no or undetermined nodal involvement, no metastasis, Fuhrman grade 2 or more and an ECOG score of 1 or more or tumour stage 4, local nodal involvement or both. Although there is no clear evidence for adjuvant TKIs as blanket treatment, it is important to note that there may be a benefit for higher risk patients or those with certain pathological features. Further studies should focus on identifying those most likely to benefit, in particular by identifying predictive biomarkers that can predict response to adjuvant therapies.
PROTECT was designed to evaluate efficacy and safety of pazopanib in 1538 patients with localised or locally advanced RCC at high-risk of relapse following nephrectomy.12–14,39 In this phase III trial, patients with resected pT2 (high grade), pT3 or greater, including N1, ccRCC were randomised 1:1 to pazopanib (800mg) or placebo for one year. Due to toxicity attrition, the pazopanib dose was reduced to 600mg and the primary endpoint was changed to DFS for pazopanib 600mg compared to placebo. Despite a trend towards benefit, there was no significant difference in DFS between pazopanib (600mg) and placebo, supporting that there is no benefit of adjuvant TKI treatment, in accordance with ASSURE. However, there was a 31% decrease in recurrence risk in patients receiving pazopanib 800mg, suggesting that higher doses are more effective at prolonging DFS; nonetheless, the pazopanib 800mg group accounted for just one third of the whole study group and this result is therefore less reliable than the pazopanib 600mg group. Deranged liver function was common, and 21% of the patients receiving pazopanib 800mg were unable to continue due to transaminase elevations, making this an unrealistic dose for widespread clinical use.
ATLAS (Adjuvant Axitinib Therapy of Renal Cell Cancer in High Risk Patients) was a phase III double-blind trial comparing adjuvant axitinib versus placebo in 724 patients with locoregional RCC.40 Axitinib is a selective VEGFR1/2/3 inhibitor associated with longer PFS compared to sorafenib in metastatic RCC41 and which is approved as second-line treatment in this context. Patients who had undergone nephrectomy and had no evidence of residual or metastatic disease were randomised 1:1 to receive either axitinib twice a day (5mg) or placebo for a minimum of one year and up to three years. The trial was stopped following interim analysis due to futility as there was no significant difference detected between DFS in treatment and placebo groups. However, in the highest-risk subpopulation (pT3 disease with Fuhrman grade 3 or above, or pT4 and/or lymph node positive, any T and any Fuhrman grade), reduction in risk of a DFS event of 36% and 27% was detected by investigators and independent review committee, respectively. Although there were similar rates of AEs, there were more grade three or four AEs in the axitinib than the placebo group (61% and 30%, respectively). The disappointing results of this study are consistent with ASSURE and PROTECT, reinforcing that there is no strong evidence for adjuvant TKIs in RCC. However, consistent with S-TRAC, axitinib did lead to a significant improvement in DFS in the highest-risk subpopulation, further highlighting the need for future studies to focus on identifying those patients most likely to benefit from adjuvant TKI therapy.
These findings are compounded by the primary efficacy analysis from SORCE (Sorafenib in Treating Patients at Risk of Relapse After Undergoing Surgery to Remove Kidney Cancer) published in October of last year.42 This was a randomised double-blind phase III trial assessing sorafenib versus placebo in 1711 RCC patients at intermediate and high recurrence risk (Leibovich classification). Participants from 147 sites were randomised 2:3:3 to three years of placebo, one year of sorafenib followed by two years of placebo or three years of sorafenib. During the study, sorafenib dose was reduced from 400mg twice a day to 400mg daily, and the primary analysis was revised to compare three years of sorafenib to placebo in order to focus on the question of longer exposure on outcomes. In accordance with previous trials, no differences in DFS or OS were detected between treatment and placebo. Importantly, no difference was detected in a sub-population analysis of high-risk patients, in contrast to S-TRAC and ATLAS. Furthermore, even with dose reductions over half of patients stopped treatment early and grade three hand-foot syndrome occurred in 24% of sorafenib-treated patients, highlighting significant toxicity associated with TKI treatment. The results of this study further support that TKIs are not suitable for adjuvant RCC treatment regardless of treatment duration and suggest that this may be the case regardless of recurrence risk.
The final newer generation study testing TKIs in the adjuvant setting is EVEREST (Everolimus in Treating Patients with Kidney Cancer Who Have Undergone Surgery), which was designed to assess the efficacy of everolimus versus placebo.14,43 This multicentre randomised phase III trial will assess RFS and is ongoing, with completion due in October 2021 (NCT01120249).44 A higher than expected dropout rate and toxicities including rash and hypertriglyceridemia have been reported,43 raising concerns that toxicity could present a barrier to everolimus as an adjuvant therapy regardless of efficacy results. Nonetheless, given the unclear benefit of adjuvant TKI therapy yielded from studies so far, the results of EVEREST are keenly awaited.
Based on the results of S-TRAC, sunitinib has been FDA approved as an adjuvant RCC therapy. However, of the five phase III studies discussed here with results available, four failed to meet their primary endpoint and demonstrated no benefit of TKI therapy on DFS or OS in the adjuvant setting, calling into question the results of S-TRAC. The results of these studies do, however, suggest that higher-risk sub-populations may be more likely to benefit from adjuvant TKIs; this was found in both S-TRAC and ATLAS, however, was not reproduced in SORCE. In a meta-analysis of ASSURE, S-TRAC, PROTECT and ATLAS including 4820 patients, there was no statistically significant OS benefit of adjuvant TKI therapy.45 However, a benefit was seen in DFS, particularly in high-risk populations. While adjuvant TKIs did not significantly affect DFS in a low-risk population, a benefit was seen in high-risk patients with one or more of positive nodes, T3/T4 tumours and Fuhrman grades 3/4. Therefore, improved methods of patient selection may allow more reliable evaluation of adjuvant therapies and more favourable outcomes by targeting patients most at risk. However, results from SORCE were not included in this meta-analysis; had these been included, the findings may have been less favourable and it will be important to incorporate results of both SORCE and EVEREST into a meta-analysis when available in order to make definitive conclusions regarding benefit of adjuvant TKIs in both low- and high-risk populations.
Arising from these studies, there are significant concerns surrounding TKI toxicity and resultant high rates of treatment discontinuation, further calling into question the utility of adjuvant TKIs in routine practice. Indeed, the death of an otherwise healthy patient on the SORCE trial due to an idiosyncratic reaction to sorafenib culminating in hepatotoxicity and associated renal failure is a sobering reminder of potential hazards of adjuvant TKI therapy.46 As MHC associations with susceptibility to other TKIs have been reported,47,48 this case further highlights the need for genetic profiling to identify markers of idiosyncratic drug reactions.
The SORCE trial investigators surveyed 233 patients to explore what survival benefits they judged sufficient to warrant therapy with sorafenib.49 For 1 year of treatment versus no intervention, median increase in survival time required to justify treatment was an extra nine months beyond a hypothetical 5 years or an extra year beyond a hypothetical 15 years. Further, patients randomised to sorafenib judged larger benefits necessary than those allocated to placebo and clinicians also judged larger benefits necessary.50 These studies highlight importance of toxicity and quality of life considerations when weighing up benefits and harms of adjuvant treatment.
The disappointing results yielded thus far from studies of TKIs have prompted a shift in focus to alternatives which might provide more effective adjuvant RCC treatments, in particular immunotherapy approaches.
Novel Approaches to Adjuvant Treatment: Immunotherapy
Novel immunotherapy approaches have been developed based on our understanding of immune checkpoints (Figure 2).16 The genetic heterogeneity that provides a substrate for tumour cell evolution simultaneously results in presentation of a range of neoantigens on cancer cell surfaces, providing a mechanism for the immune system to recognise them as “foreign”. Antigen-specific T-cells are activated upon recognition of tumour antigen (TA) by the T-cell receptor (TCR); this mechanism also requires co-stimulation, in particular between CD80/CD86 on the tumour cell and CD28 on the T-cell. In response, tumour cells have developed mechanisms to evade recognition; in particular, by downregulating the response via Cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1) and programmed cell death 1 ligand 2 (PD-L2). There has therefore been significant interest in the targeting of CTLA-4, PD-1 and PD-L1 with the aim of enhancing the immune response against tumour cells, an approach supported by the observation that PD-L1 expression is associated with poor prognosis in RCC.16,51,52
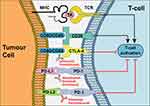 |
Figure 2 Mechanism of action of ICIs. In response to recognition of TA presented by cancer cells, T-cell activation depends on coalescence of a range of signals including co-stimulation by CD80/CD86 which bind to CD28 on the T-cell. Cancer cells evade the immune response via competitive binding of CD80/CD86 to CTLA-4 and via binding of PD-L1/PD-L2 to PD-1, inhibiting T-cell activation. Antibodies targeting CTLA-4, PD-1 and PD-L1 prevent activation of immunosuppressive signals, thus enhancing host immune responses against cancer cells. Adapted by permission from Springer Nature © (2020). Xu W, Atkins MB, McDermott DF. Checkpoint inhibitor immunotherapyin kidney cancer. Nat Rev Urol. 2020;17(3):137–150.16 |
Novel immunotherapies developed based on an understanding of immune checkpoints are now well established in the metastatic setting in RCC.16 In CheckMate 025, 821 patients with advanced RCC received either nivolumab or everolimus. Median OS was 25.0 and 19.6 months in nivolumab and everolimus groups, respectively; a 27% OS benefit was observed in the nivolumab group compared to everolimus and there were fewer grade three or four AEs in the nivolumab group.5 Nivolumab is now approved as second-line treatment in metastatic RCC. Furthermore, in Checkmate 214,9 1096 patients with advanced or metastatic RCC were randomised to nivolumab and ipilimumab or sunitinib; combination immunotherapy was superior in both OS and PFS. Combination trials of TKIs and immune checkpoint inhibitors (ICIs) have also shown promising results in the metastatic setting. In KEYNOTE-426 the combination of axitinib and pembrolizumab was superior to sunitinib alone, extending both OS and PFS in patients with metastatic disease.15 Several trials are ongoing assessing various combinations of TKIs and ICIs in metastatic RCC, including CheckMate 9ER, COSMIC-313 and CLEAR.16
Promising results showing that immunotherapies are more effective, and less toxic, than TKIs in the metastatic setting have provided impetus to assess whether this will translate to adjuvant RCC, particularly as these approaches are already established in adjuvant melanoma treatment.53–55 Five phase III clinical trials are currently ongoing to address this question (Table 6),14,16 including PROSPER, IMmotion010, Keynote-564, CheckMate 914 and RAMPART.
 |
Table 6 Summary of Newer Generation Studies Testing Adjuvant ICIs |
PROSPER (NCT03055013)56 is a randomised phase III trial assessing neoadjuvant and adjuvant nivolumab versus surgery alone in patients with biopsy-proven T2 high-risk disease. Patients in the treatment group will receive neoadjuvant nivolumab every two weeks for two cycles, then surgery followed by adjuvant nivolumab every two weeks for six cycles and then every four weeks for a further six cycles, while control patients will receive surgery followed by observation. The trial is currently recruiting and projected to complete in 2023. The primary outcome is RFS, with secondary outcomes including OS, RFS in ccRCC and incidence of toxicity.
IMmotion010 (NCT03024996)57 is a phase III multicentre randomised double-blind study evaluating efficacy and safety of adjuvant atezolizumab versus placebo in RCC patients at high recurrence risk following nephrectomy. This study is ongoing, with completion projected in 2022. The primary outcome is DFS as per independent review facility (IRF), with secondary outcomes including OS, investigator-assessed DFS, distant metastasis-free survival and AEs.
KEYNOTE-564 (NCT03142334)58 is a phase III randomised double-blind placebo-controlled trial evaluating efficacy and safety of adjuvant pembrolizumab monotherapy in patients with intermediate- or high-risk RCC including those with resectable oligometastases. The study is ongoing, with completion projected in 2022. The primary outcome is investigator-assessed DFS, with secondary outcomes including OS and AEs.
CheckMate 914 is a phase III clinical trial (NCT03138512)59 evaluating adjuvant nivolumab alone or combined with ipilimumab versus placebo. The trial is currently recruiting RCC patients at high-risk of relapse and is projected to complete in 2022. The primary outcome is DFS, with secondary outcomes including OS and AEs.
RAMPART (Renal Adjuvant MultiPle Arm Randomised Trial; NCT03288532)60 is a phase III open-label randomised trial assessing adjuvant durvalumab alone or combined with tremelimumab versus active monitoring. This trial is currently recruiting intermediate- and high-risk RCC patients, and is projected to complete in 2023. Primary outcomes are DFS and OS, with secondary outcomes including metastasis-free survival and RCC-specific survival time. By including both intermediate- and high-risk patients, this study may shed light on the utility of ICIs in patients at varying recurrence risk. As both CheckMate 914 and RAMPART are assessing monotherapy versus combination ICIs, they will also inform on any advantage of combination therapies in the adjuvant setting.
If ICIs are shown to improve PFS and OS in adjuvant RCC patients, there will be further barriers to overcome before they can be routinely deployed in this context. Studies in the metastatic setting have suggested that ICIs may be less toxic than TKIs.16 However, the toxicity profile is broader and potentially life-threatening, with treatment-related deaths occurring in up to 2%.61 The effects of immunotherapy treatment are less predictable as any system can be affected by the activated immune response; common manifestations include fatigue, colitis, hepatitis, pneumonitis, endocrinopathies, hypophysitis, inflammatory arthritis and dermatitis. Cardiovascular, haematological, renal, neurological and ophthalmological side effects are less common but well-established immunotherapy toxicities.61 Furthermore, toxic effects have a prolonged duration compared to chemotherapy and often continue after treatment discontinuation. Moreover, combination immunotherapies, while more effective, further increase toxicity risk.62–64 Clinicians must, therefore, remain alert to manifestations of immunotherapy toxicity and their potential delayed onset; the risk–benefit balance of these therapies, while often favouring treatment in patients with metastatic disease, may be less favourable in the adjuvant setting.
Current Clinical Landscape and Future Directions
The European Association of Urology published guidelines regarding adjuvant RCC treatment in 2017.65 Based on the results of ASSURE and S-TRAC, the guidelines do not recommend adjuvant sunitinib following surgical resection in high-risk RCC patients due to poor benefit-to-harm ratio and absence of evidence of an OS benefit. Furthermore, updated European Association of Urology guidelines published in 2019 do not recommend adjuvant therapy with TKIs in high-risk patients following nephrectomy based on lack of proven benefit, and recommend that no adjuvant therapy is provided outside of clinical trials.66 This is in accordance with the National Comprehensive Cancer Network Kidney Cancer Guidelines, which favour involvement in clinical trials over adjuvant treatment with sunitinib.
To develop effective adjuvant therapies for RCC and address this unmet need in the future, there are several areas of focus for ongoing research. It will be important to develop improved methods of risk stratification, improve matching of patients to appropriate therapies through development of molecular biomarkers, improve prediction of treatment toxicities and continue to invest in development of novel targeted treatment strategies.
As discussed, a consistent finding from S-TRAC and ATLAS was that higher-risk subpopulations may be more likely to benefit from adjuvant TKIs. This was supported by a meta-analysis of ASSURE, S-TRAC, PROTECT and ATLAS which showed benefit of TKI treatment on DFS in a high-risk population but not a low-risk population.45 This suggests that improved methods of patient selection may enable more reliable evaluation of adjuvant therapies and, by targeting those patients most at risk, lead to more favourable outcomes. Therefore, further work to develop unified and robust stratification systems for identifying high-risk patients will be important in the search for effective adjuvant therapies. Indeed, despite existence of a range of stratification systems there remains a lack of consensus between recent studies. A comprehensive analysis was recently performed using the multi-centre CORONA database, with the aim of optimising adjuvant therapy inclusion criteria.67 This study considered trials including ASSURE, SORCE, EVEREST, PROTECT, S-TRAC and ATLAS and concluded that results of contemporary adjuvant trials will not be comparable as their inclusion criteria differ significantly.
Developing our understanding of molecular biomarkers will further inform patient stratification. For example, Rini et al68 developed a prognostic multigene signature to predict recurrence risk in ccRCC and found that a panel of 16 genes could reliably predict clinical outcomes. By incorporating molecular information such as this into existing stratification systems, patients could be more reliably stratified into high- and low-risk populations.
With the advent of novel immunotherapies, there has been considerable interest in biomarker development to predict which patients will benefit from specific treatments, although this has largely been in the metastatic setting.16 The most extensively explored biomarker in this context is PD-L1, which has proved useful for predicting response to PD-1 and PD-L1 blockade in other cancers, including non-small-cell lung carcinoma and melanoma.69–72 While these results are supported by some studies in metastatic RCC, for example, in CheckMate 214 combination immunotherapy with nivolumab and ipilimumab improved PFS relative to sunitinib only in PD-L1 positive tumours, this has not been the case consistently.37 Indeed, in CheckMate 025, OS benefits of nivolumab were similar irrespective of PD-L1 expression.5 Several possible explanations for this discrepancy have been proposed; in particular, PD-L1 expression levels are often discordant between the primary tumour and metastases, are often heterogeneous within the primary tumour itself and can change over time.16 Whether PD-L1 expression would be a reliable predictive biomarker in adjuvant RCC remains to be investigated.
Studies of gene expression profiling have led to the possibility of targeting RCC treatments based on expression profiles. For example, RNA sequencing performed during IMmotion150 demonstrated three distinct gene expression signatures (angiogenic, T-effector cell and myeloid inflammatory signatures).16,73 Accordingly, in IMmotion151, patients with T-effector cell signatures experienced a PFS benefit from combination atezolizumab and bevacizumab while those with angiogenic signatures responded well to sunitinib alone.74
Other approaches currently being investigated as predictive biomarkers for response to ICIs include assessment of tumour mutational burden, PBRM1 mutations, CD8+ T-cell density and IFN-γ expression profiles.16 High tumour mutational and neoantigen burden are markers of genomic instability associated with clinical response to ICIs in a range of tumours.75–77 However, no association was found between tumour mutational or neoantigen burden and PFS in metastatic RCC patients treated with atezolizumab in a randomised study.73 PBRM1 encodes a subunit of the PBAF switch-sucrose nonfermentable (SWI/SNF) chromatin remodelling complex, whose inactivation has been implicated in vulnerability to IFN-γ and T-cell-mediated toxicity as well as effects on various signalling pathways, in particular HIF signalling.78–80 Accordingly, loss-of-function PBRM1 mutations are associated with clinical benefit in metastatic RCC patients receiving nivolumab78 and clinical response to VEGFR inhibitors.73,81 CD8+ T-cell density has been implicated in response to both ICIs and TKIs. Greater CD8+ T-cell density is associated with improved DFS in adjuvant RCC patients treated with sunitinib versus placebo82 and improved PFS in advanced RCC patients treated with axitinib plus avelumab versus sunitinib.83 Finally, a T-cell-inflamed gene expression profile containing IFN-γ-responsive genes has been identified as predicting response to PD-1 blockade84; this profile successfully predicted responses to pembrolizumab in metastatic urothelial cancer.85 While many of these studies were performed in metastatic RCC or in other tumour types, they highlight the importance of matching patients to treatments based on the molecular features of their tumour and it will be important to assess whether these approaches can be translated to the care of adjuvant RCC patients.
Further investment in biomarker development is also urgently needed in efforts to predict which patients are likely to develop severe treatment toxicity. In S-TRAC, 48% of the patients experienced grade three or four AEs and there are significant concerns surrounding TKI toxicity. Furthermore, while ICIs are generally better tolerated, they have a broad toxicity profile with potentially life-threatening AEs. Development of biomarkers able to predict treatment toxicity would enable us to avoid life-threatening side effects in those most at risk.
Finally, although results of ICIs in adjuvant phase III studies are eagerly awaited, these may be equally as disappointing as adjuvant TKI studies and we currently have no effective adjuvant RCC therapies. Efforts must, therefore, continue to be invested in basic research aiming to develop further targeted treatment strategies which may ultimately address this unmet need and improve survival in this patient group.
Conclusions
Despite extensive efforts having been invested in the search for an effective adjuvant RCC therapy, there are currently no adjuvant treatments proven to improve outcomes and no treatments are recommended for routine use outside of clinical trials. Although TKIs have shown clinical benefit in metastatic RCC, these findings have failed to translate to the adjuvant setting, with phase III trials of TKIs in this context yielding conflicting and disappointing results. However, there is hope that ICIs may address this unmet need based on their promising results in metastatic disease, and several phase III trials addressing adjuvant ICIs are currently ongoing. While the results of these studies are eagerly awaited, it will not be possible to draw meaningful conclusions for several years.
Future work should focus on developing improved methods of risk stratification and using them consistently in all forthcoming trials. Developing an improved understanding of molecular biomarkers will inform patient stratification to different risk groups, will enable us to match patients to personalised treatments based on tumour-specific features and may also help predict which patients are most vulnerable to serious treatment-related AEs. In parallel, it will be important to continue to invest in development of further novel targeted treatment strategies if we are to identify an effective adjuvant therapy for RCC patients and improve survival.
Disclosure
The authors report no conflicts of interest for this work.
References
1. CRUK. Kidney cancer statistics. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer#heading-Zero.
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
3. Dizman N, Adashek JJ, Hsu J, Bergerot PG, Bergerot CD, Pal SK. Adjuvant treatment in renal cell carcinoma. Clin Adv Hematol Oncol. 2018;16(8):555–563.
4. Janowitz T, Welsh SJ, Zaki K, Mulders P, Eisen T. Adjuvant therapy in renal cell carcinoma-past, present, and future. Semin Oncol. 2013;40(4):482–491. doi:10.1053/j.seminoncol.2013.05.004
5. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi:10.1056/NEJMoa1510665
6. Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi:10.1016/S0140-6736(08)61039-9
7. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–731. doi:10.1056/NEJMoa1303989
8. Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590. doi:10.1200/JCO.2008.20.1293
9. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, Phase 3 trial. Lancet Oncol. 2019;20(10):1370–1385. doi:10.1016/S1470-2045(19)30413-9
10. Escudier B, Michaelson MD, Motzer RJ, et al. Axitinib versus sorafenib in advanced renal cell carcinoma: subanalyses by prior therapy from a randomised phase III trial. Br J Cancer. 2014;110(12):2821–2828. doi:10.1038/bjc.2014.244
11. Escudier B, Porta C, Bono P, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Clin Oncol. 2014;32(14):1412–1418. doi:10.1200/JCO.2013.50.8267
12. Massari F, Di Nunno V, Ciccarese C, et al. Adjuvant therapy in renal cell carcinoma. Cancer Treat Rev. 2017;60:152–157. doi:10.1016/j.ctrv.2017.09.004
13. Posadas EM, Limvorasak S, Figlin RA. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol. 2017;13(8):496–511. doi:10.1038/nrneph.2017.82
14. Blick C, Ritchie AWS, Eisen T, Stewart GD. Improving outcomes in high-risk, nonmetastatic renal cancer: new data and ongoing trials. Nat Rev Urol. 2017;14(12):753–759. doi:10.1038/nrurol.2017.123
15. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi:10.1056/NEJMoa1816714
16. Xu W, Atkins MB, McDermott DF. Checkpoint inhibitor immunotherapy in kidney cancer. Nat Rev Urol. 2020;17(3):137–150. doi:10.1038/s41585-020-0282-3
17. Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours.
18. Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97(7):1663–1671. doi:10.1002/cncr.11234
19. Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20(23):4559–4566. doi:10.1200/JCO.2002.05.111
20. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):706–720. doi:10.1093/annonc/mdz056
21. Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–696. doi:10.1200/JCO.1995.13.3.688
22. Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–296. doi:10.1200/JCO.2002.20.1.289
23. Clark JI, Atkins MB, Urba WJ, et al. Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma: a cytokine working group randomized trial. J Clin Oncol. 2003;21(16):3133–3140. doi:10.1200/JCO.2003.02.014
24. Messing EM, Manola J, Wilding G, et al. Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: an Eastern Cooperative Oncology Group/Intergroup trial. J Clin Oncol. 2003;21(7):1214–1222. doi:10.1200/JCO.2003.02.005
25. Pizzocaro G, Piva L, Colavita M, et al. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: a multicentric randomized study. J Clin Oncol. 2001;19(2):425–431. doi:10.1200/JCO.2001.19.2.425
26. Passalacqua R, Caminiti C, Buti S, et al. Adjuvant low-dose interleukin-2 (IL-2) plus interferon-alpha (IFN-alpha) in operable renal cell carcinoma (RCC): a Phase III, randomized, multicentre trial of the Italian Oncology Group for Clinical Research (GOIRC). J Immunother. 2014;37(9):440–447. doi:10.1097/CJI.0000000000000055
27. Atzpodien J, Schmitt E, Gertenbach U, et al. Adjuvant treatment with interleukin-2- and interferon-alpha2a-based chemoimmunotherapy in renal cell carcinoma post tumour nephrectomy: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). Br J Cancer. 2005;92(5):843–846.
28. Aitchison M, Bray CA, Van Poppel H, et al. Adjuvant 5-flurouracil, alpha-interferon and interleukin-2 versus observation in patients at high risk of recurrence after nephrectomy for renal cell carcinoma: results of a phase III randomised European Organisation for Research and Treatment of Cancer (Genito-Urinary Cancers Group)/National Cancer Research Institute trial. Eur J Cancer. 2014;50(1):70–77.
29. Adler A, Gillon G, Lurie H, et al. Active specific immunotherapy of renal cell carcinoma patients: a prospective randomized study of hormono-immuno-versus hormonotherapy. Preliminary report of immunological and clinical aspects. J Biol Response Mod. 1987;6(6):610–624.
30. Galligioni E, Quaia M, Merlo A, et al. Adjuvant immunotherapy treatment of renal carcinoma patients with autologous tumor cells and Bacillus Calmette-Guerin - five-year results of a prospective randomized study. Cancer. 1996;77(12):2560–2566. doi:10.1002/(SICI)1097-0142(19960615)77:12<2560::AID-CNCR20>3.0.CO;2-P
31. Jocham D, Richter A, Hoffmann L, et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet. 2004;363(9409):594–599. doi:10.1016/S0140-6736(04)15590-6
32. Wood C, Srivastava P, Bukowski R, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372(9633):145–154. doi:10.1016/S0140-6736(08)60697-2
33. Chamie K, Donin NM, Klopfer P, et al. Adjuvant weekly girentuximab following nephrectomy for high-risk renal cell carcinoma: the ARISER randomized clinical trial. JAMA Oncol. 2017;3(7):913–920. doi:10.1001/jamaoncol.2016.4419
34. Massari F, Bria E, Maines F, et al. Adjuvant treatment for resected renal cell carcinoma: are all strategies equally negative? Potential implications for trial design with targeted agents. Clin Genitourin Cancer. 2013;11(4):471–476. doi:10.1016/j.clgc.2013.04.018
35. Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387(10032):2008–2016. doi:10.1016/S0140-6736(16)00559-6
36. Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375(23):2246–2254. doi:10.1056/NEJMoa1611406
37. Motzer RJ, Ravaud A, Patard -J-J, et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: subgroup analyses and updated overall survival results. Eur Urol. 2018;73(1):62–68. doi:10.1016/j.eururo.2017.09.008
38. Gyawali B, Ando Y. Adjuvant sunitinib for high-risk-resected renal cell carcinoma: a meta-analysis of ASSURE and S-TRAC trials. Ann Oncol. 2017;28(4):898–899. doi:10.1093/annonc/mdw667
39. Motzer RJ, Haas NB, Donskov F, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol. 2017;35(35):3916–3923. doi:10.1200/JCO.2017.73.5324
40. Gross-Goupil M, Kwon TG, Eto M, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol. 2018;29(12):2371–2378. doi:10.1093/annonc/mdy454
41. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–1939. doi:10.1016/S0140-6736(11)61613-9
42. Eisen TQG, Frangou E, Smith B, et al. Primary efficacy analysis results from the SORCE trial (RE05): adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: an international, randomised double-blind phase III trial led by the MRC CTU at UCL. Ann Oncol. 2019;30:
43. Synold TW, Plets M, Tangen CM, et al. Everolimus exposure as a predictor of toxicity in renal cell cancer patients in the adjuvant setting: results of a pharmacokinetic analysis for SWOG S0931 (EVEREST), a Phase III Study (NCT01120249). Kidney Cancer. 2019;3(2):111–118. doi:10.3233/KCA-180049
44. ClinicalTrials.gov. S0931, Everolimus in treating patients with kidney cancer who have undergone surgery. 2010. Available from: https://clinicaltrials.gov/ct2/show/NCT01120249.
45. Massari F, Di Nunno V, Mollica V, Graham J, Gatto L, Heng D. Adjuvant tyrosine kinase inhibitors in treatment of renal cell carcinoma: a meta-analysis of available clinical trials. Clin Genitourin Cancer. 2019;17(2):e339–e344. doi:10.1016/j.clgc.2018.12.011
46. Fairfax BP, Pratap S, Roberts IS, et al. Fatal case of sorafenib-associated idiosyncratic hepatotoxicity in the adjuvant treatment of a patient with renal cell carcinoma. BMC Cancer. 2012;12:590. doi:10.1186/1471-2407-12-590
47. Spraggs CF, Budde LR, Briley LP, et al. HLA-DQA1*02:01 is a major risk factor for lapatinib-induced hepatotoxicity in women with advanced breast cancer. J Clin Oncol. 2011;29(6):667–673. doi:10.1200/JCO.2010.31.3197
48. Xu CF, Reck BH, Goodman VL, et al. Association of the hemochromatosis gene with pazopanib-induced transaminase elevation in renal cell carcinoma. J Hepatol. 2011;54(6):1237–1243. doi:10.1016/j.jhep.2010.09.028
49. Blinman PL, Davis ID, Martin A, et al. Patients’ preferences for adjuvant sorafenib after resection of renal cell carcinoma in the SORCE trial: what makes it worthwhile? Ann Oncol. 2018;29(2):370–376. doi:10.1093/annonc/mdx715
50. Lawrence NJ, Martin A, Davis ID, et al. What survival benefits are needed to make adjuvant sorafenib worthwhile after resection of intermediate- or high-risk renal cell carcinoma? Clinical investigators’ preferences in the SORCE trial. Kidney Cancer. 2018;2(2):123–131. doi:10.3233/KCA-180038
51. Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101(49):17174–17179. doi:10.1073/pnas.0406351101
52. Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. doi:10.1158/0008-5472.CAN-05-4303
53. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–1855. doi:10.1056/NEJMoa1611299
54. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. doi:10.1016/S1470-2045(15)70122-1
55. Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. doi:10.1056/NEJMoa1802357
56. ClinicalTrials.gov. Nivolumab in treating patients with localized kidney cancer undergoing nephrectomy. 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03055013.
57. ClinicalTrials.gov. A study of atezolizumab as adjuvant therapy in participants with Renal Cell Carcinoma (RCC) at high risk of developing metastasis following nephrectomy (IMmotion010). 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03024996. .
58. ClinicalTrials.gov. Safety and efficacy study of pembrolizumab (MK-3475) as monotherapy in the adjuvant treatment of renal cell carcinoma post nephrectomy (MK-3475-564/KEYNOTE-564). 2017. Available from: https://clinicaltrials.gov/ct2/show/study/NCT03142334.
59. ClinicalTrials.gov. A study comparing nivolumab, nivolumab in combination with ipilimumab and placebo in participants with localized kidney cancer who underwent surgery to remove part of a kidney (CheckMate 914). 2017. Available from: https://clinicaltrials.gov/ct2/show/study/NCT03138512.
60. ClinicalTrials.gov. Renal adjuvant multiple arm randomised trial (RAMPART). 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03288532.
61. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5(1):95. doi:10.1186/s40425-017-0300-z
62. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, Phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. doi:10.1016/S1470-2045(16)30098-5
63. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi:10.1056/NEJMoa1709684
64. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi:10.1056/NEJMoa1302369
65. Bex A, Albiges L, Ljungberg B, et al. Updated European Association of urology guidelines regarding adjuvant therapy for renal cell carcinoma. Eur Urol. 2017;71(5):719–722. doi:10.1016/j.eururo.2016.11.034
66. Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75(5):799–810. doi:10.1016/j.eururo.2019.02.011
67. Wolff I, May M, Hoschke B, et al. Do we need new high-risk criteria for surgically treated renal cancer patients to improve the outcome of future clinical trials in the adjuvant setting? Results of a comprehensive analysis based on the multicenter CORONA database. Eur J Surg Oncol. 2016;42(5):744–750. doi:10.1016/j.ejso.2016.01.009
68. Rini B, Goddard A, Knezevic D, et al. A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: development and validation studies. Lancet Oncol. 2015;16(6):676–685. doi:10.1016/S1470-2045(15)70167-1
69. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643
70. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005
71. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi:10.1056/NEJMoa1810865
72. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi:10.1056/NEJMoa1412082
73. McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–757. doi:10.1038/s41591-018-0053-3
74. Rini BI, Huseni M, Atkins MB, et al. Molecular correlates differentiate response to atezolizumab (atezo) + bevacizumab (bev) vs sunitinib (sun): results from a phase III study (IMmotion151) in untreated metastatic renal cell carcinoma (mRCC). Ann Oncol. 2018;29:viii724–viii725. doi:10.1093/annonc/mdy424.037
75. Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, Phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi:10.1016/S0140-6736(16)00561-4
76. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi:10.1126/science.aaa1348
77. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi:10.1056/NEJMoa1613493
78. Miao D, Margolis CA, Gao W, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359(6377):801–806. doi:10.1126/science.aan5951
79. Pan D, Kobayashi A, Jiang P, et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science. 2018;359(6377):770–775. doi:10.1126/science.aao1710
80. Nargund AM, Pham CG, Dong Y, et al. The SWI/SNF protein PBRM1 restrains VHL-loss-driven clear cell renal cell carcinoma. Cell Rep. 2017;18(12):2893–2906. doi:10.1016/j.celrep.2017.02.074
81. Voss MH, Kuo F, Chen D, et al. Integrated biomarker analysis for 412 renal cell cancer (RCC) patients (pts) treated on the phase 3 COMPARZ trial: correlating common mutation events in PBRM1 and BAP1 with angiogenesis expression signatures and outcomes on tyrosine kinase inhibitor (TKI) therapy. J Clin Oncol. 2017;35(15_suppl):4523.
82. George DJ, Martini JF, Staehler M, et al. Immune biomarkers predictive for disease-free survival with adjuvant sunitinib in high-risk locoregional renal cell carcinoma: from randomized phase III S-TRAC Study. Clin Cancer Res. 2018;24(7):1554–1561. doi:10.1158/1078-0432.CCR-17-2822
83. Choueiri TK, Albiges L, Haanen JBAG, et al. Biomarker analyses from JAVELIN renal 101: avelumab + axitinib (A+Ax) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). J Clin Oncol. 2019;37(15_suppl):101. doi:10.1200/JCO.2019.37.15_suppl.101
84. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi:10.1172/JCI91190
85. O’Donnell PH, Grivas P, Balar AV, et al. Biomarker findings and mature clinical results from KEYNOTE-052: first-line pembrolizumab (pembro) in cisplatin-ineligible advanced urothelial cancer (UC). J Clin Oncol. 2017;35(15_suppl):4502. doi:10.1200/JCO.2017.35.15_suppl.4502
86. Kjaer M, Iversen P, Hvidt V, et al. A randomized trial of postoperative radiotherapy versus observation in stage II and III renal adenocarcinoma. A study by the copenhagen renal cancer Study Group. Scand J Urol Nephrol. 1987;21(4):285–289. doi:10.3109/00365598709180784
87. Pizzocaro G, Piva L, Di Fronzo G, et al. Adjuvant medroxyprogesterone acetate to radical nephrectomy in renal cancer: 5-year results of a prospective randomized study. J Urol. 1987;138(6):1379–1381. doi:10.1016/S0022-5347(17)43647-0
88. Naito S, Kumazawa J, Omoto T, et al. Postoperative UFT adjuvant and the risk factors for recurrence in renal cell carcinoma: a long-term follow-up study. Kyushu University Urological Oncology Group. Int J Urol. 1997;4(1):8–12. doi:10.1111/j.1442-2042.1997.tb00130.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
