Back to Journals » OncoTargets and Therapy » Volume 12
Review and perspective on adjuvant and neoadjuvant immunotherapies in NSCLC
Authors Yi C , He Y , Xia H, Zhang H, Zhang P
Received 5 June 2019
Accepted for publication 14 August 2019
Published 6 September 2019 Volume 2019:12 Pages 7329—7336
DOI https://doi.org/10.2147/OTT.S218321
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Chengxiang Yi*,1,2, Yayi He*,3, Haoran Xia*,1, Helin Zhang4, Peng Zhang1
1Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200433, People’s Republic of China; 2Tongji University School of Medicine, Tongji University, Shanghai 200433, People’s Republic of China; 3Department of Medical Oncology, Shanghai Pulmonary Hospital, Tongji University Medical School Cancer Institute, Tongji University School of Medicine, Shanghai 200433, People’s Republic of China; 4Department of Thoracic Surgery, The Second Hospital of Hebei Medical University, Shijiazhuang City, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peng Zhang
Department of Thoracic Surgery, Shanghai Pulmonary Hospital, No. 507, Zhengmin Road, Yangpu District, Shanghai, People’s Republic of China
Tel +86 1 351 218 5932
Email [email protected]
Helin Zhang
Department of Thoracic Surgery, Hebei The Second Hospital of Hebei Medical University, No. 53, Huaxi Road, Xinhua District, Shijiazhuang City, People’s Republic of China
Email [email protected]
Abstract: Postoperative patients have risk recurring, even for completed resected early stage non-small-cell lung cancer (NSCLC). To control the recurrence rate, neoadjuvant and adjuvant therapies have been applied widely in clinical practice; however, neoadjuvant and adjuvant immunotherapy clinical trials on NSCLC are still being explored. In this review, we summarized the research progress and outline the issues need to be solved on adjuvant and neoadjuvant immunotherapies in NSCLC.
Keywords: adjuvant immunotherapies, neoadjuvant immunotherapies, immune checkpoint inhibitor, non-small-cell lung cancer, biomarker, liquid biopsy
Introduction
The data of global cancer statistics 2018 showed that lung cancer was the most commonly diagnosed cancer (11.6% of all cases) and the leading cause of cancer death (18.4% of the total cancer deaths).1 Even for postoperative patients with early-stage lung cancer, the rate of death or recurring varied from 8% to 66%.2,3 Owing to the presence of micro-metastases before surgery, it was tough to control relapses in surgery patients.4
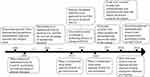 |
Figure 1 Time axis flowchart on research progress about adjuvant and neoadjuvant immunotherapies. |
To improve prognosis, adjuvant and neoadjuvant chemotherapies are proposed and two meta-analyses of randomized trials testified the survival advantage of adjuvant and neoadjuvant chemotherapies.5,6 Moreover, immunotherapy is a hotspot in the treatment of lung cancer. Programmed death-1 (PD‐1)/programmed death-ligand 1 (PD‐L1) monoclonal antibodies have shown promising efficacy in advanced nonsquamous (p=0.002) and squamous (p<0.001) non-small-cell lung cancer (NSCLC).7,8 However, adjuvant and neoadjuvant immunotherapies in lung cancer are still worth to be explored. Here, by reviewing the research progress about adjuvant and neoadjuvant immunotherapies (as Figure 1 shown), we summarized these researches and outline the issues need to be solved on adjuvant and neoadjuvant immunotherapies in NSCLC.
Adjuvant immunotherapies
Adjuvant therapies are aimed to improve prognosis and survival for patients with resected NSCLC. Immunotherapies for NSCLC have developed rapidly in recent years. Adjuvant immunotherapies have attracted the researches’ attention, and strategies of adjuvant therapies are increasingly diverse. Here, we concluded the studies on adjuvant immunotherapies.
- Adjuvant passive immunotherapy
Adjuvant passive immunotherapies have initially focused on the dendritic cell‐cytokine induced killer (DC-CIK) and tumor vaccines. A study including 157 patients with stage III NSCLC showed that the median survival time of the patients in the control and adjuvant DC-CIK cell immunotherapy group was 22 months (95% CI, 16.23–27.77) and 28 months (95% CI, 24.39–31.61).9
The Melanoma-associated antigen 3 (MAGE-A3) gene was presented to specific T cells by human leukocyte antigen (HLA) molecules at the cell surface as a tumor-specific antigen.10 MAGE-A3 antigen was a particular interest target for a vaccination strategy. In a double-blind, randomized, placebo-controlled phase II postoperative study,11 MAGE-A3 immunization did not show significant improvement in disease-free survival (DFS), but the toxicity is controllable. A parallel-group phase I study12 showed that adjuvant MAGE-A3 could induce MAGEA3-specific immune responses no matter with concurrent chemotherapy or not. In a randomized, double-blind, placebo-controlled trial,13 adjuvant treatment with the MAGE-A3 immunotherapeutic did not significantly increase DFS compared with placebo in patients with MAGE-A3-positive surgically resected NSCLC and median DFS was 60.5 months for the MAGE-A3 immunotherapeutic group and 57.9 months for the placebo group. These disappointing results led to the discontinuation of further clinical development of the MAGE-A3 immunotherapies.
Immune checkpoint inhibitors, such as PD‐1/PD‐L1 monoclonal antibodies have been successfully used in advanced lung cancer patients. Immune checkpoint inhibitors anti-PD-1 and PD-L1 antibodies alone,14 or combined with chemotherapy15 showed significant overall survival (OS) advantage in stage IV lung cancer. As to the resectable patients, a meta-analyze showed patients might get benefits from adjuvant checkpoint inhibitors (PD‐1/PD‐L1 inhibitor).16 Given these positive trials, immune checkpoint inhibitors have been used as adjuvant treatment in some on-going clinical trials, including pembrolizumab (NCT02504372), durvalumab (NCT02273375), atezolizumab (NCT02486718), nivolumab (NCT02595944) (as Table 1 shown). Nevertheless, there hasn’t been a standard formulation for adjuvant immune checkpoint inhibitors, neither dosage nor circles of treatment.
 |
Table 1 Clinical trials of adjuvant immunotherapies for NSCLC |
Neoadjuvant immunotherapies
- Advantage of immunotherapies in neoadjuvant strategy
Preoperative chemotherapies combined with surgery had better survival than surgery only.17,18 However, neoadjuvant therapies didn’t show significant longer survival in all studies.19,20 For immunotherapies, preclinical work suggests that neoadjuvant application of checkpoint inhibitors could be superior to neoadjuvant chemotherapy.21 A clinical trial included 20 patients (adjuvant 10: neoadjuvant 10) with stage III melanoma showed that the rate of death was lower in the neoadjuvant group than that in the adjuvant group.22
It has been considered that administration of checkpoint inhibitors before resection maybe induce a stronger and more prolonged antitumor T cell immune response compared to administration of checkpoint inhibitors after surgery, resulting in more effective prevention of tumor relapse.23 Moreover, massive structure of lymphatic system around lung cancer before resection was relatively intact and checkpoint inhibitors could work better.24 Also, a hypothesis that higher tumor burden can assist checkpoint inhibitors to stimulate antitumor T cell immune response better before an operation is considerable.
Pathological complete response (PCR), defined as eradication of all tumors from resected lung and lymph node tissue, was regarded as a surrogate for OS in neoadjuvant research. Depierre et al25 investigated 179 patients with stage IB–IIIA NSCLCs treated with neoadjuvant chemotherapy and shown that 11% of patients got a PCR and had a relative risk of death of 0.42 (p<0.001). In a study combined analysis of two French Cooperative Thoracic Intergroup (the Intergroupe Francophone de Cancérologie Thoracique, IFCT) randomized trials,26 5-year OS was 80.0% in the PCR group, compared with 55.8% in the non-PCR (p=0.0007) and hazard ratios (HR) for death with PCR was 0.34 (95% CI, 0.18–0.64) by multivariate analysis.
However, the rarity of PCR in patients with cisplatin-based chemotherapy restricted was usually less than 10%. It was reported that27 each percentage of viable tumor was associated with a 1% increase in the risk of death (HR, 1.01, p=0.005). In a follow-up study,28 only pathological stage and viable tumor (≤10%) associated with OS (HR 2.39, p=0.05), therefore major pathological response (MPR), defined as 10% or less residual tumor tissue in resected lung and lymph node tissue, were proposed as a surrogate of OS in patients with resectable NSCLC given neoadjuvant chemotherapy.29 As for neoadjuvant immunotherapies, MPR has been used as a primary or second endpoint in some researches.30 Above experience about MPR comes from trials of neoadjuvant chemotherapies mostly, due to the different mechanisms of chemotherapy and immunotherapy, there are differences in pathological assessment between them. Histopathologic features of the regression bed (the area of immune-mediated tumor clearance) were found in the pathological assessment of NSCLC patients with neoadjuvant nivolumab and were proposed to develop “Immune-Related Pathologic Response Criteria” (irPRC) that standardize pathologic assessment of immunotherapeutic efficacy, which add the area of the regression bed to the areas of residual viable tumor and necrosis and detailed terms “stroma,” “fibrosis,” and “inflammation” to include only proliferative fibrosis (vs old, hyalinized fibrosis or any fibrosis), dense (vs mild) tumor infiltrating lymphocytes, and tertiary lymphoid structures (vs non-organized lymphoid aggregates).32 Long-term follow-up is needed to validate MPR assessed by as a surrogate for recurrence-free survival and OS in researches about neoadjuvant immunotherapies.
Neoadjuvant therapies provide opportunities to implement preoperative smoking cessation and reduce tumor burden before surgery. It was reported that neoadjuvant immunotherapy with nivolumab was associated with little side effects and did not postpone the surgery.30 Clinical trial LCMC3, with neoadjuvant atezolizumab (n=77), reported MPR rate was 19% and only 6 grade 3–4 treatment-related adverse reactions occurred in 101 patients. In a clinical trial with nivolumab (n=21),30 MPR occurred amazingly in 9 of 20 completely resected NSCLC and the number of T-cell clones changed after PD-1 inhibitors in 8 of 9 patients. Neoadjuvant immune checkpoint inhibitors might play a key role in activating specific immune killing of tumor cancer before operation.
These excellent results from neoadjuvant mono immunotherapy stimulate interest in neoadjuvant immunotherapies combined with chemotherapy or other checkpoint inhibitors. In the NEOSTAR trial, MPR + PCR rate of group nivolumab plus ipilimumab is only 16% higher than group nivolumab, but combined therapy significantly reduced the chance of subsequent surgical treatment (2 in group nivolumab vs 5 in the combined group). Moreover, trial CheckMate-617 about neoadjuvant combined checkpoint inhibitors was completely terminated in an early stage. Even so, trial NADIM about neoadjuvant nivolumab + chemotherapies showed an excellent result that MPR rate reached 83% and PCR rate reached 71%, but the trial is a small sample (n=46) research, next, more and more trials would be needed for exploring the best dose, circle, and combination for neoadjuvant immune checkpoint inhibitors. Recently, many clinical trials about neoadjuvant immune checkpoint inhibitors are ongoing (Table 2). We can expect them to bring exciting results.
 |
Table 2 Clinical trials of neoadjuvant immune checkpoint inhibitors |
Predictive biomarkers for adjuvant and neoadjuvant immunotherapies
An efficient predictive biomarker will be specific for patients’ election in the clinical trial of neo- and adjuvant immune checkpoint inhibitors. As the proposed detection item by the Food and Drug Administration (FDA), PD-L1 on tumor cell is suggested to be a biomarker for anti-PD-1 inhibitor. PD-L1 protein expression assessed by immunochemistry (IHC) has emerged as a biomarker to select NSCLC patients for pembrolizumab therapy.14,33,34 Moreover, Zaric et al35 reported that PD-1 expression was an independent prognostic factor for recurrence and death, which revealed that PD-1 and PD-L1 expression were associated with favorable OS in patients with completely resected adenocarcinoma of the lung. However, Tsao et al36 held a different opinion and showed that PD-L1 protein expression was not a prognostic factor in early-stage NSCLC patients. PD-L1 expression as an effective predictor to select patients with lung cancer for neo- and adjuvant immunotherapies needs to be further explored.
High tumor mutation burden (TMB), an emerging biomarker for response to immunotherapy, means the total number of mutations present in a tumor specimen.37 TMB was first associated with clinical benefit in melanoma patients treated with anti-cytotoxic T lymphocyte associated antigen-4 (CTLA-4).38 Afterward, Owada-Ozaki et al39 found TMB >62 was associated with shorter OS (HR=12.31, p=0.019) in patients with resected NSCLC, while Roszik et al40 reported that high TMB group treated with ipilimumab were correlated with better prognosis (HR=0.272, p=0.003). Moreover, it was reported that the rate of MPR has no significant difference between PD-L1–positive and PD-L1–negative tumors, but a significantly higher mean mutational burden was observed in tumors with an MPR than in tumors without a major response.30 Therefore, TMB is a potential predictive biomarker for MPR following adjuvant and neoadjuvant immunotherapies.
Liquid biopsy
Liquid biopsy is a promising tool for noninvasive monitoring response in neoadjuvant or adjuvant immunotherapies. Circulating tumor DNA (ctDNA) appears to be present in 50–95% of patients with stages I through III,41–43 suggesting it may be a more broadly applicable biomarker in this setting. Moreover, immunotherapies could cause dramatic activation in blood CD4(+) and CD8(+) T cells in some researches.44 Updated data from trials CA209-159 also suggested that ctDNA clearance and peripheral blood T cell amplification may be potential predictors of therapeutic response and monitoring recurrence. However, it’s still a question whether a change of ctDNA and peripheral blood T cell correlate with MPR, even with OS or DFS. Moreover, blood collection procedures, collection tubes, anticoagulant,45 blood storage condition, blood centrifugation speed for plasma isolation,46 and plasma storage condition47 are also limiting factors associated with the standardization of circulating tumor DNA (ctDNA) to the clinical practice. To explore the clinical utility of these assays in patients receiving adjuvant and neoadjuvant immunotherapy, future trials should include serial sample collection for liquid biopsies.
Challenges and prospects
Lung cancer is the most commonly diagnosed cancer with the highest rate of death. Even for early stage resectable NSCLC, the rate of recurring or death is more than 8%. Recently, immune checkpoint inhibitors are hotspots in the treatment of cancer, but the best timing of immunotherapies use still need to explored. Neoadjuvant and adjuvant immunotherapies attached researchers’ attention for some excellent results. Due to the particularity of treatment, immunotherapies will have bright prospects as neoadjuvant and adjuvant therapies, but some challenges have to be faced.
First of all, compared to chemotherapies, neoadjuvant and adjuvant immunotherapies have significant survival beneficial, but the use of immunotherapies has a risk of causing autoimmune disease, especially for neoadjuvant therapies, which causes patients to be unable to undergo surgery. Moreover, it should be considered that premature use of immunotherapies in early-stage lung cancer would increase immunotherapies drug resistance occurs in advance. Especially for neoadjuvant immunotherapies, it needs more exploration whether neoadjuvant immunotherapy will aggravate the specific problems of clinical practice such as adhesion and hemorrhage during operation, increase the difficulty of surgery and prolong the time of thoracic drainage.
Secondly, exploration about pathological response for neoadjuvant immunotherapies is a very worthwhile challenge. As a surrogate for recurrence-free survival and OS, it can help researchers greatly reduce research time. However, the prerequisite for MPR to be used in clinical practice is to be able to predict the patient’s OS. Long-term follow-up is needed to validate MPR as a surrogate for recurrence-free survival and OS in NSCLC researches about neoadjuvant immunotherapies.
Thirdly, neoadjuvant and adjuvant immunotherapies are still in the start-up stage, the dose and circles are both in the exploration. In current clinical trials, mono immunotherapies, immunotherapies + chemotherapies and immunotherapies + immunotherapies are commonly used programs. The combination of the anti-CTLA-4 antibody ipilimumab with either nivolumab or pembrolizumab has shown to have higher response rates than anti-PD-1 monotherapy, but at the cost of significant toxicity.48,49 More recently, immunotherapies + chemotherapies (the trials NEOSTAR) showed best results, however, more clinical trials with large sample size are needed to verify. Moreover, whether neoadjuvant combined adjuvant immunotherapies or mono neoadjuvant or adjuvant immunotherapies are better, it’s also a challenge that we need to explore.
Last, researchers have long wanted to screen for appropriate patients through molecular markers. To date, there are some promising indicators, however, whether PD-L1, TMB or recently emerging liquid biopsy (ctDNA, peripheral blood T cell and so on), there is not sufficient evidence to prove that they are directly related to MPR or OS. It’s still controversial to screen for appropriate patients through these markers. In future research, according to the characteristics of immunotherapy itself, the most important object is to develop a comprehensive index that can reflect both the oncological response and the immunological response. Only in this way can we truly predict the immunotherapies’ effect in real time.
Acknowledgments
This study was supported in part by a grant from the National Natural Science Foundation of China (81802255), Shanghai Pujiang Program (17PJD036) and a grant from Shanghai Municipal Commission of Health and Family Planning Program (20174Y0131), National Key Research & Development Project (2016YFC0902300), Major Disease Clinical Skills Enhancement Program of three year action plan for promoting clinical skills and clinical innovation in municipal hospitals, Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1001A), “Dream Tutor “ Outstanding Young Talents Program (fkyq1901). The article is also supported by the National Natural Science Foundation of China (Grant No. 81622001, 91642108), Shanghai Municipal Health Commission (Grant No. 2017BR026), Shanghai Science and Technology Committee (Grant No. 19XD1423200), Shanghai Municipal Human Resources and Social Securitiy Bureau (Grant No. 2017114), Shanghai Hospital Development Center (Grant No. SHDC12018122), Fundamental Research Funds for the Central Universities (Grant No. 22120180510), and Shanghai Pulmonary Hospital (Grant No. fkgg1801, fkcx1904).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10(7):990–1003. doi:10.1097/JTO.0000000000000559
3. Asamura H, Chansky K, Crowley J, et al. The international association for the study of lung cancer lung cancer staging project: proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10(12):1675–1684. doi:10.1097/JTO.0000000000000678
4. Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14(9):611–622. doi:10.1038/nrc3793
5. Listed N; Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311(7010):899–909.
6. Sarah B, Lesley S, Anne A, Jean-Pierre P. Chemotherapy in non-small-cell lung cancer: an update of an individual patient data meta-analysis. J Clin Oncol. 2005;23(4):925–926.
7. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643
8. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi:10.1056/NEJMoa1504627
9. Zhao M, Hongbing LI, Lei LI, Zhang Y. Effects of a gemcitabine plus platinum regimen combined with a dendritic cell-cytokine induced killer immunotherapy on recurrence and survival rate of non-small cell lung cancer patients. Exp Ther Med. 2014;7(5):1403–1407. doi:10.3892/etm.2014.1574
10. Plaen ED, Traversari C, Gaforio JJ, et al. Structure, chromosomal localization, and expression of 12 genes of the MAGE family. Immunogenetics. 1994;40(5):360–369.
11. Vansteenkiste J, Zielinski M, Linder A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol. 2013;31(19):2396–2403. doi:10.1200/JCO.2012.43.7103
12. Pujol JL, Vansteenkiste JF, De Pas TM, et al. Safety and immunogenicity of MAGE-A3 cancer immunotherapeutic with or without adjuvant chemotherapy in patients with resected stage IB to III MAGE-A3-positive non-small-cell lung cancer. J Thorac Oncol. 2015;10(10):1458–1467. doi:10.1097/JTO.0000000000000653
13. Vansteenkiste JF, Cho BC, Vanakesa T, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(6):822–835. doi:10.1016/S1470-2045(16)00099-1
14. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
15. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005
16. Zhu J, Li R, Tiselius E, et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database Syst Rev. 2017;12:CD011300.
17. Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst. 1994;86(9):
18. Rosell R, Gómez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330(3):153–158. doi:10.1056/NEJM199401203300301
19. Brandt WS, Yan W, Zhou J, et al. Outcomes after neoadjuvant or adjuvant chemotherapy for cT2-4N0-1 non-small cell lung cancer: A propensity-matched analysis. J Thorac Cardiovasc Surg. 2019;157(2):743–753.e3. doi:10.1016/j.jtcvs.2018.09.098
20. Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol. 2010;28(19):3138–3145. doi:10.1200/JCO.2009.27.6204
21. Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6(12):1382–1399. doi:10.1158/2159-8290.CD-16-0577
22. Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–1661. doi:10.1038/s41591-018-0198-0
23. Robert C. Is earlier better for melanoma checkpoint blockade? Nat Med. 2018;24:1645.
24. Blumenthal GM, Bunn PA
25. Depierre A, Milleron B, Moro-Sibilot D, et al. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J Clin Oncol. 2002;20(1):247–253. doi:10.1200/JCO.2002.20.1.247
26. Mouillet G, Monnet E, Milleron B, et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol. 2012;7(5):841–849. doi:10.1097/JTO.0b013e31824c7d92
27. Apar P, Neda K, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2012;7(5):825–832. doi:10.1097/JTO.0b013e318247504a
28. William WN
29. Hellmann MD, Chaft JE, William WN
30. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. doi:10.1056/NEJMoa1716078
31. Rusch VW, Chaft JE, Johnson B, Wistuba II, Kris MG, Lee JM. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): initial results from a multicenter study (LCMC3). J Clin Oncol. 2018;36:15. doi:10.1200/JCO.2018.36.15_suppl.8541
32. Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol. 2018;29(8):1853–1860. doi:10.1093/annonc/mdy218
33. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi:10.1056/NEJMoa1501824
34. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-7
35. Zaric B, Brcic L, Buder A, et al. PD-1 and PD-L1 protein expression predict survival in completely resected lung adenocarcinoma. Clin Lung Cancer. 2018;19(6):e957–e963. doi:10.1016/j.cllc.2018.08.014
36. Tsao MS, Le Teuff G, Shepherd FA, et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann Oncol. 2017;28(4):882–889. doi:10.1093/annonc/mdx003
37. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi:10.1186/s13073-017-0424-2
38. Alexandra S, Vladimir M, Taha M, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;372(8):2189–2199.
39. Owada-Ozaki Y, Muto S, Takagi H, et al. Prognostic impact of tumor mutation burden in patients with completely resected non-small cell lung cancer: brief report. J Thorac Oncol. 2018;13(8):1217–1221. doi:10.1016/j.jtho.2018.04.003
40. Roszik J, Haydu LE, Hess KR, et al. Novel algorithmic approach predicts tumor mutation load and correlates with immunotherapy clinical outcomes using a defined gene mutation set. BMC Med. 2016;14(1):168. doi:10.1186/s12916-016-0705-4
41. Chaudhuri AA, Chabon JJ, Lovejoy AF, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 2017;7(12):1394–1403. doi:10.1158/2159-8290.CD-17-0716
42. Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545(7655):446–451. doi:10.1038/nature22364
43. Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20(5):548–554. doi:10.1038/nm.3519
44. Yi JS, Ready N, Healy P, et al. Immune activation in early-stage non-small cell lung cancer patients receiving neoadjuvant chemotherapy plus ipilimumab. Clin Cancer Res. 2017;23(24):7474–7482. doi:10.1158/1078-0432.CCR-17-2005
45. Das K, Fernando MR, Basiaga S, Wigginton SM, Williams T. Effects of a novel cell stabilizing reagent on DNA amplification by PCR as compared to traditional stabilizing reagents. Acta Histochem. 2014;116(1):55–60. doi:10.1016/j.acthis.2013.05.002
46. Page K, Guttery DS, Zahra N, et al. Influence of plasma processing on recovery and analysis of circulating nucleic acids. PLoS One. 2013;8(10). doi:10.1371/journal.pone.0077963
47. Barrett AN, Thadani HA, Laureano-Asibal C, Ponnusamy S, Choolani M. Stability of cell-free DNA from maternal plasma isolated following a single centrifugation step. Prenat Diagn. 2014;34(13):1283–1288. doi:10.1002/pd.4468
48. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi:10.1056/NEJMoa1504030
49. Carlino MS, Long GV. Ipilimumab combined with nivolumab: a standard of care for the treatment of advanced melanoma? Clin Cancer Res. 2016;22(16):3992–3998. doi:10.1158/1078-0432.CCR-15-2944
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
