Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Revefenacin: A Once-Daily, Long-Acting Bronchodilator For Nebulized Treatment Of COPD
Authors Donohue JF , Mahler DA , Sethi S
Received 14 May 2019
Accepted for publication 23 September 2019
Published 19 December 2019 Volume 2019:14 Pages 2947—2958
DOI https://doi.org/10.2147/COPD.S157654
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
James F Donohue,1 Donald A Mahler,2,3 Sanjay Sethi4
1Pulmonary Medicine, UNC School of Medicine, Chapel Hill, NC, USA; 2Geisel School of Medicine at Dartmouth, Hanover, NH, USA; 3Director of Respiratory Services, Valley Regional Hospital, Claremont, NH, USA; 4University at Buffalo, State University of New York, Buffalo, NY, USA
Correspondence: James F Donohue
UNC School of Medicine, 4125 Bioinformatics Building CB#7020, Chapel Hill, NC 27599-0001, USA
Tel +1 984-974-5703
Fax +1 984-974-5737
Email [email protected]
Abstract: Bronchodilation with muscarinic antagonists, β2-agonists, and inhaled corticosteroids remains the foundation of pharmaceutical treatment for patients with stable COPD. These drugs are delivered from a variety of devices, including dry powder inhalers, pressurized metered-dose inhalers, soft-mist inhalers, or nebulizers. Nebulized delivery is often preferable in patients who are elderly, are cognitively impaired, are unable to generate sufficient inspiratory force to use their inhaler, have difficulty coordinating hand-breath activity, are too dyspneic to hold their breath for a sufficient time, and/or may be acutely ill. Revefenacin, a once-daily long-acting muscarinic antagonist for nebulization recently approved by the US FDA for the treatment of patients with COPD, was discovered and developed using “duration and lung selectivity-by-design.” This strategy selected a molecule with a high lung-selective index to maximize bronchodilation and limit systemic anti-muscarinic side effects. In early-phase clinical studies, revefenacin for nebulization led to a rapid onset of bronchodilation that was sustained for 24 hrs in patients with moderate to severe COPD. Revefenacin also demonstrated minimal systemic exposure and good tolerability in these studies. Statistically and clinically significant improvements in lung function (ie, peak and/or trough FEV1) relative to placebo were observed with revefenacin in Phase III clinical trials of up to 3 months in patients with moderate to very severe COPD. Revefenacin was well tolerated in Phase III clinical trials with a low incidence of systemic antimuscarinic adverse events, which is consistent with its lung-selective design. There was no evidence of an increased risk of major cardiovascular events. Patient-reported outcome data from clinical trials indicated statistically significant improvements in several disease-specific measures. Revefenacin 175 μg for nebulization provides an effective once-daily treatment option for patients with moderate to very severe COPD who require or prefer nebulized therapy.
Keywords: long-acting muscarinic antagonist, LAMA, bronchodilator, inhaled, once daily, nebulizer
Introduction
Bronchodilation remains the mainstay of treatment for patients with COPD to relieve symptoms of dyspnea. Short- and long-acting muscarinic antagonists (LAMAs) and short- and long-acting β2-agonists (LABAs) administered alone or in combination, with or without inhaled corticosteroids (ICSs), are the foundation of pharmaceutical treatment in patients with stable COPD (Table 11–3). These agents are commonly delivered through a variety of devices, including dry powder inhalers (DPIs), pressurized metered-dose inhalers, soft-mist inhalers, or nebulizers. When used appropriately, these delivery devices offer similar efficacy.4–6 However, adherence to inhaled therapy in patients with COPD is poor, and nonadherence rates range from 50% to 80%.7–9 One of the main reasons for nonadherence is the misuse of inhalers.10 Certain groups of patients – such as patients who are elderly, are cognitively impaired, are unable to generate sufficient inspiratory force against the resistance of their inhaler, have difficulty coordinating hand-breath activity, are too dyspneic to hold their breath for a sufficient time, and/or may be acutely ill – may have difficulty using the handheld inhalers effectively. Recent evidence suggests that certain individuals with severe COPD have difficulty generating optimal peak inspiratory flow rate (PIFR), which is required for effective handheld inhaler use. The 2019 GOLD strategy emphasizes a personalized approach to the treatment of patients with COPD, and therefore, nebulized delivery may be preferable in these groups of patients.1,11 Where appropriate, using nebulizers can improve patient confidence and treatment adherence,12 achieving comparable symptom relief with greater ease of use compared with DPIs, pressurized metered-dose inhalers, and soft-mist inhalers.13–15 According to a survey by Sharafkhaneh et al, 80% of patients with COPD and caregivers reported that using a nebulizer was better than using only an inhaler.14
 |
Table 1 Commonly Used Long-Acting Anticholinergics For Maintenance Treatment Of COPD1 |
Standard jet and vibrating mesh nebulizers are the two most commonly used nebulizing devices. Depending on the model, limitations reported include longer administration time, variability in residual volume and particle size, daily cleaning requirements, limited portability, and need for device assembly.11,16 Older, cheaper models have also shown inefficiencies in drug delivery.16 Additionally, the same dose of bronchodilator administered using the newer models of vibrating mesh nebulizers may vary substantially from the dose administered with jet nebulizers, increasing the potential for overdosing. However, the benefits of nebulizers may outweigh their limitations, specifically for patients who require or prefer the use of nebulizers.11
LAMAs relieve the symptoms of COPD by preventing bronchoconstriction caused by the binding of acetylcholine to M3 muscarinic receptors expressed in airway smooth muscle.17 Revefenacin is a once-daily, lung-selective LAMA, delivered via a standard jet nebulizer that was approved by the FDA in November 2018 for maintenance treatment of patients with COPD who require or prefer nebulized drug delivery.18 This review summarizes the clinical data on once-daily revefenacin for nebulization and examines the role of this new LAMA in the management of patients with COPD.
Duration And Lung Selectivity By Design: The Discovery Of Revefenacin
From a chemical standpoint, revefenacin is structurally distinct from existing once-daily LAMAs (Figure 119–21). First, it has a distinct M3 binding orthostere, a biphenyl carbamate moiety. Second, unlike tiotropium, it lacks an ester substituent. Third, unlike tiotropium and umeclidinium, revefenacin possesses two tertiary amines instead of a quaternary amine. These characteristics make revefenacin the only nonester, nonquaternary ammonium LAMA discovered to date.
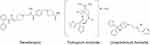 |
Figure 1 Structural formulas of once-daily LAMAs.19–21 Abbreviation: LAMA, long-acting muscarinic antagonist. |
Revefenacin’s lung selectivity, prolonged duration of action, limited antimuscarinic side effects, and formulation for nebulized delivery are characteristics that were sought specifically during the “duration and lung selectivity-by-design” strategy used to identify a LAMA with the ideal structural and pharmacological characteristics to support once-daily nebulized delivery.22 Based on the expectation that a nonester, nonquaternary ammonium-based molecule would enhance tissue residency and maximize chemical stability for aqueous formulation and aerosol delivery, a new orthostere was linked with secondary binding elements to generate novel, chemically stable inhaled antagonists with high-affinity binding and a slow off-rate from the M3 receptor. Drug candidates with high muscarinic receptor affinity, slow off-rate at M3 receptors, slowly reversible antagonism in airway tissues, high functional lung selectivity, ≥24-hr inhibition of agonist-induced bronchoconstriction, metabolic lability (to ensure stability in the lung, with hydrolysis to a less active metabolite in the systemic circulation), and limited gastrointestinal absorption were progressed through the screening funnel. Long-acting bronchodilator efficacy (confirmed in a canine model of bronchoconstriction), rapid plasma clearance via primarily nonrenal mechanisms (also confirmed in animal models), and favorable non-clinical safety were requirements for the final selection of the development candidate.
After identifying a 4-piperidyl biphenyl-2-ylcarbamate (BPC) tertiary amine head group as a chemically stable orthostere starting point, a linker was installed on the BPC head group to preserve M3 potency and extend antagonist binding to distal receptor sites through a secondary allostere, on which an amide was installed to create a metabolic “soft site.” The final candidate was TD-4208 (revefenacin), a competitive antagonist of human muscarinic receptors. It displayed kinetic selectivity at M3 receptors, produced potent and slowly reversible antagonism in animal and human airway tissues with a 24-hr inhibition of bronchoconstriction in rats and dogs after nebulized inhalation,23,24 had low oral bioavailability, and had a favorable lung-selectivity index compared with tiotropium. Revefenacin was chemically stable in the lung but quickly degraded to a metabolite with minimal antimuscarinic activity in the systemic circulation, reducing the potential for side effects.
Exposure–Response Characteristics
The pharmacokinetic and pharmacodynamic properties of revefenacin were evaluated in several clinical trials (Table 225–30). The initial assessment of efficacy and safety of revefenacin in patients with moderate to severe COPD was done in two Phase II trials: Study 0059 (N=32), a single-dose, double-blind, crossover study of revefenacin (350 or 700 μg) versus active-control ipratropium (500 μg) or placebo solution, all administered via standard jet nebulizer,25 and Study 0091 (N=59), a multidose, double-blind, 7-day, 5-way crossover study that evaluated revefenacin (22, 44, 88, 175, 350, or 700 μg once daily) versus placebo.25 In both studies, revefenacin was rapidly absorbed and demonstrated rapid onset (within 1 hr), peak effect 2–3 hrs postdose, and >24 hrs of bronchodilator activity. In the single-dose study, the primary endpoint – mean change in peak FEV1 (0–6 hrs) from baseline – was significantly greater with revefenacin than placebo (least squares [LS] mean treatment difference vs placebo: 177 and 162 mL for 350- and 700-μg doses, respectively, vs 191 mL with ipratropium; all p<0.001; Figure 2A25,31). In the multidose study, the primary endpoint of change from baseline in trough FEV1 24 hrs after the seventh dose showed significant benefit over placebo for all revefenacin doses, with a dose–response effect observed up to 175 μg. Beyond that point, there was no further benefit increasing the dose (LS mean treatment difference vs placebo: 54, 55, 75, 114, 94, and 82 mL for revefenacin doses of 22, 44, 88, 175, 350, and 700 μg, respectively; all p≤0.006). Peak FEV1 difference from placebo also showed dose-dependency (Figure 2B25,31). The minimally clinically important difference for bronchodilation is commonly accepted as a trough FEV1 ≥100 mL.31 This threshold was reached for peak and trough FEV1 after single doses of 350 and 700 μg in the single-dose study, and peak FEV1 exceeded this threshold at doses >88 μg in the multidose study (Figure 2A and B25,31). Other spirometry endpoints were consistent with the primary endpoint in both studies.
 |
Table 2 Summary Of Trials Evaluating Efficacy And Safety Of Revefenacin |
 |
Figure 2 Peak FEV1 treatment difference from placebo in single-dose (A) and multi-dose 7-day (B) trials. Peak FEV1 is the highest value obtained between 0 and 6 hrs after the first dose. *p<0.001. Data are least squares mean±95% confidence interval treatment difference from placebo. Dotted line indicates minimal clinically important difference.31Notes: Reproduced from Quinn D, Barnes CN, Yates W, et al Pharmacodynamics, pharmacokinetics and safety of revefenacin (TD-4208), a long-acting muscarinic antagonist, in patients with chronic obstructive pulmonary disease (COPD): results of two randomized, double-blind, phase 2 studies. Pulm Pharmacol Ther. 2018;48:71–79. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.25 |
The effect of revefenacin on bronchodilation was further confirmed in two multiple-dose Phase II studies in patients with moderate to severe COPD.26,27 In a 7-day randomized, double-blind, placebo-controlled, crossover, Phase II study of 64 patients, twice-daily administration of 44-μg revefenacin and once-daily administration of 175-μg revefenacin produced substantial improvements from baseline in day 7 weighted mean (0–24 hr) FEV1 over placebo (LS mean difference, 105 mL and 113 mL for 44-μg and 175-μg revefenacin, respectively).26 In a longer 28-day, double-blind, dose-ranging study, 355 adults with moderate to severe COPD were randomized to once-daily revefenacin (44, 88, 175, or 350 μg) or matching placebo, administered by standard jet nebulizer for 28 days.27 Revefenacin improved Day 28 trough FEV1 significantly more than placebo at daily doses of 88, 175, and 350 μg (187, 167, and 171 mL, respectively; all p<0.001); the 44-μg once-daily dose was subtherapeutic. At 88 μg and higher doses, >80% of patients randomized to revefenacin (vs 33% of those who received placebo) achieved a ≥100 mL increase from baseline FEV1 in the first 4 hrs postdose, with sustained bronchodilation for 24 hrs, allowing an average decrease in rescue medication of >1 albuterol puff per day. Improvements over placebo in mean FEV1 increase from baseline exceeded 114 mL and were apparent within 1 hr postdose on Day 1 and throughout the 28-day trial. The 350-μg daily dose offered no additional benefit over 175 μg.
Revefenacin was extensively metabolized to its hydrolytic metabolite (THRX-195518) with metabolite-to-parent ratios for the area under the plasma concentration-time curve from time 0 to last detectable time point (AUC0-t) of 4- to 6-fold at doses of 350 and 700 μg in the single-dose study, and 3- to 6-fold on Day 7 at doses ≥175 μg in the multidose study. Elimination was slow, and there was minimal accumulation of either revefenacin or its metabolite on repeat dosing. Across all three studies reported above, revefenacin was well tolerated, with no evidence of sustained systemic exposure.
Exposure–Response Conclusions
In early-phase clinical studies, revefenacin for nebulization led to rapid onset and sustained the duration of bronchodilator effect for 24 hrs in patients with moderate to severe COPD, with minimal systemic exposure and good tolerability. Doses of 88 and 175 μg once daily offered the optimal efficacy-to-exposure ratio for further investigation in Phase III trials.
Efficacy And Safety Of Revefenacin In Moderate To Very Severe COPD
The efficacy and safety of revefenacin in patients with moderate to very severe COPD were evaluated in three randomized, controlled Phase III trials. In this review, we focus mainly on data for the 175-μg dose of revefenacin, which is the FDA-approved daily dose for the maintenance treatment of COPD.18
Efficacy Of Revefenacin
The efficacy of revefenacin administered using a standard jet nebulizer for 12 weeks was examined in patients with moderate to very severe COPD with high risk for exacerbation, in 2 replicate multicenter, randomized, double-blind, placebo-controlled Phase III trials (Study 0126, NCT02459080, and Study 0127, NCT02512510).28 Up to 40% of patients received concomitant LABA or ICS/LABA therapy. FEV1 increased significantly within 2 hrs of revefenacin treatment in both studies, as well as in pooled data analysis (placebo-adjusted LS mean increase in peak FEV1 [0–2 hrs] after the first dose of revefenacin 175 µg was 130 mL in the pooled analysis; p<0.0001). Revefenacin demonstrated statistically significant improvements over placebo in trough FEV1 throughout the treatment period (Figure 328,31) and at 12 weeks (placebo-adjusted LS mean increase in trough FEV1 at Day 85 was ~147 mL in both individual studies, as well as in pooled data analysis [Figure 428,31]). The 175-µg dose also increased overall treatment effect on trough FEV1 by ≥100 mL relative to placebo in both individual studies, and by 142 mL in the pooled analysis. Subgroup analysis of pooled data showed that revefenacin produced greater improvements in Day 85 trough FEV1 than placebo in patients taking concomitant LABA or ICS, in those aged >65 years, in those with very severe airflow limitation, in those with a modified Medical Research Council dyspnea scale ≥2 (severe dyspnea), and in former smokers. Importantly, patient-reported measures of respiratory health (St George’s Respiratory Questionnaire [SGRQ], a secondary endpoint, and COPD Assessment Test, an exploratory endpoint) showed significant improvements in one of the studies.32 In Study 0126, revefenacin produced significant improvements in the SGRQ responders (patients with a score decrease ≥4; the odds ratio for revefenacin 175 μg vs placebo, 2.11; p=0.02) and change from baseline in total score (LS mean placebo-adjusted change from baseline, –4.16; p=0.001). Because of a greater-than-expected placebo response in Study 0127, only change from baseline reached statistical significance versus placebo (LS mean placebo-adjusted change with revefenacin 175 μg, –2.58; p=0.02). The COPD Assessment Test results were consistent with SGRQ.
 |
Figure 3 Sustained increase in trough FEV1 for 85 days in two randomized, double-blind, placebo-controlled Phase III trials in patients with moderate to severe COPD (pooled data from NCT02459080 and NCT02512510; N=1,255). Dotted line indicates minimal clinically important difference.31Notes: Reproduced with permission from Ferguson GT, Feldman G, Pudi KK, et al. Improvements in lung function with nebulized revefenacin in the treatment of patients with moderate to very severe COPD: results from two replicate phase III clinical trials. Chronic Obstr Pulm Dis. 2019;6(2):154–165. doi:10.15326/jcopdf.6.2.2018.0152.28 *p<0.0001 vs placebo. Abbreviations: LS, least squares; REV, revefenacin; SE, standard error. |
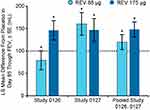 |
Figure 4 Placebo-adjusted changes from baseline at day 85 trough FEV1 in patients with COPD who received once-daily revefenacin (88 and 175 μg) for nebulization.28 *p<0.001 vs placebo. Day 85 trough FEV1 was the average of values obtained at 23.25 and 23.75 hrs following the 84th dose. Dotted line indicates minimal clinically important difference.31 Notes: Reproduced with permission from Ferguson GT, Feldman G, Pudi KK, et al. Improvements in lung function with nebulized revefenacin in the treatment of patients with moderate to very severe COPD: results from two replicate phase III clinical trials. Chronic Obstr Pulm Dis. 2019; 6(2):154–165. doi:10.15326/jcopdf.6.2.2018.0152.28 Abbreviations: LS, least squares; OTE, overall treatment effect; REV, revefenacin; SE, standard error. |
Efficacy In Patients With Markers Of Severe Disease And Suboptimal PIFR
In the 12-week studies described above (Study 0126 and Study 0127), subgroup analyses were conducted among COPD patients with markers of severe disease (age ≥65 years, classified as GOLD class D,33 or concurrent ICS or LABA use).34 Revefenacin 175 μg produced significant improvement in trough FEV1 in the intention-to-treat population and the key subgroups of patients with markers of more severe disease.
Patients with COPD and suboptimal PIFR (<60 L/min) against the resistance of a DPI may have difficulty generating sufficient force during inhalation to overcome the internal resistance of DPI devices, deaggregate the powdered medication, and receive the correct dose.35 In observational studies, up to 78% of stable outpatients with COPD and 32%–52% of inpatients after treatment for COPD exacerbation had suboptimal PIFR.36–40 These patients, in particular, may benefit from nebulized therapy.
In a 28-day, double-blind, parallel-group Phase IIIb trial (NCT03095456), 207 patients with moderate to very severe COPD and PIFR <60 L/min measured using the In-CheckTM DIAL device against simulated resistance of the Diskus® DPI (GlaxoSmithKline, Brentford, UK) were randomized to receive revefenacin 175 μg once daily or tiotropium 18 μg once daily.30 In the intention-to-treat population, revefenacin produced numerically greater improvements in the trough FEV1 and trough forced vital capacity than tiotropium, but the difference was not significant (LS mean difference±standard error [SE], 17±22 mL; p=0.45). Patients with severe airflow obstruction (ie, FEV1 <50% predicted; ~80% of the population) experienced significantly greater improvements with revefenacin than tiotropium in trough FEV1 (between-treatment difference in LS mean change from baseline±SE: 49±22 mL; p=0.03) and trough forced vital capacity (104±49 mL; p=0.03). Revefenacin also led to greater improvement than tiotropium in trough FEV1 among patients with lower PIFR cut points (33–56 L/min).
Safety Of Revefenacin Up To 1 Year
General Safety And Tolerability
A summary of adverse events (AEs) associated with revefenacin 175 μg once daily in Phase II and Phase III trials is presented in Table 3.25,27–29 In the two 12-week randomized controlled trials (Study 0126 and Study 0127), the incidence of treatment-emergent AEs and serious adverse events (SAEs) was similar in all treatment groups in both studies.28 Of the 15 patients (7.6%) taking revefenacin 175 μg who reported SAEs, only two were considered treatment-related (COPD exacerbation and pneumonia). Antimuscarinic AEs (≤1 per patient, most commonly constipation and dry mouth) were evenly distributed across treatment and placebo groups.
 |
Table 3 Incidence (n, %) Of Treatment-Emergent AEs Reported In ≥2% Patientsa Receiving Revefenacin 175 μg Once Daily In Phase II And III Clinical Trials |
In the 52-week tiotropium-controlled safety trial (Study 0128, NCT02518139), the incidence of treatment-emergent AEs was similar in all groups and numerically higher in the tiotropium group (72% for revefenacin 175 μg vs 77% for tiotropium).29 The incidence of AEs was not affected by concomitant ICS/LABA use for patients taking revefenacin 175 μg but was higher for patients taking tiotropium and ICS/LABA than those taking tiotropium alone. The most frequently reported AE was COPD exacerbation, which occurred less frequently with revefenacin 175 μg (97 events in 73 patients [22%]; moderate/severe in 17% of patients) than tiotropium (137 events in 100 patients [28%]; moderate/severe in 26% of patients). The incidence of SAEs was lower for revefenacin 175 μg than tiotropium (12.8% vs 16.3%, respectively); the most frequently reported SAEs were COPD exacerbation and pneumonia. Two treatment-related SAEs were reported during the trial (1 each with revefenacin 175 μg and tiotropium). Antimuscarinic events were less frequent with revefenacin than tiotropium. More patients in the revefenacin 175 μg than the tiotropium group discontinued treatment because of AEs (12% vs 9%, respectively).
Cardiovascular Safety
Cardiovascular disease, including potentially life-threatening arrhythmias, is a well-recognized comorbidity in patients with COPD.41 Revefenacin shows limited inhibition of human ether-à-go-go (hERG) gene-encoded ion channels and is therefore predicted to have a low potential for major adverse cardiovascular events (MACEs) (Theravance Biopharma US, Inc., data on file). This was demonstrated in clinical trials of up to 52 weeks, which showed no increase in the risk of MACE with revefenacin treatment.42
The clinical events committee adjudicated only 1 MACE in the revefenacin 175-μg group in the replicate 12-week studies, and this was considered unrelated to treatment.42 In the 52-week study, only 1 of the 10 MACEs reported in the revefenacin 175-μg group (atrial fibrillation) was appraised as possibly or probably related to treatment.42
Efficacy And Safety Conclusions
Once-daily revefenacin, delivered via standard jet nebulizer at a dose of 175 µg to patients with moderate to very severe COPD, led to statistically and clinically significant improvements in bronchodilation (trough FEV1) from baseline. The benefit was also observed in patients with suboptimal PIFR against the resistance of Diskus DPI and those who had markers of more severe disease. Revefenacin was well tolerated and, consistent with its lung-selective design, led to a low (placebo-level) incidence of systemic antimuscarinic AEs. Revefenacin treatment was not associated with an increased risk for MACEs. Additionally, patient-reported outcome data suggest that revefenacin may improve patients’ subjective respiratory health, but further studies are needed to clarify whether these improvements are clinically significant.
Discussion
Nebulized bronchodilator treatment offers patients with COPD and cognitive or physical limitations, and those experiencing COPD exacerbations, a simpler medication delivery that avoids the need for manual dexterity, strength, or complex hand-breath coordination. As technology advances, quieter, more portable, and increasingly efficient nebulizer models are becoming available. The advent of adaptive aerosol delivery, which works in concert with the patient’s breathing pattern, continues to improve the reliability and precision of dosing, improving dyspnea and fatigue.16,43
The structural and pharmacological design of revefenacin supports once-daily dosing and is associated with limited systemic exposure, thereby reducing the potential for AEs associated with systemic drug distribution.22,25,27 Data generated in 2 replicate 12-week Phase III studies indicate that revefenacin 175 μg once daily, the dose approved by the FDA in 2018 for maintenance COPD treatment,18 has a rapid onset of action and sustained efficacy, measured using peak and/or trough FEV1 versus placebo.28 The data presented provide evidence that the bronchodilator effects associated with this dose meet the minimally clinically important difference threshold of FEV1 ≥100 mL, including in patients with markers of more severe disease, and may reduce the need for rescue medication in some patients.28,34 These data are consistent with studies that evaluated the efficacy and rescue medication use in patients with COPD who received tiotropium via HandiHaler. The results demonstrated significant improvements in FEV144 and a significant decrease in rescue medication usage in those who received tiotropium versus placebo.44,45
The once-daily LAMA tiotropium and twice-daily LABAs salmeterol and formoterol are the most commonly prescribed single bronchodilators for maintenance therapy in patients with COPD and are delivered using handheld inhalers.46–48 Until recently, two LABAs (arformoterol and formoterol) and the anticholinergic agent glycopyrrolate were the only long-acting bronchodilators available for nebulized delivery, and all require twice-daily administration.49–51 Furthermore, a number of clinical trials have been completed to assess a new nebulized bronchodilator, RPL554, for the maintenance treatment of patients with COPD (NCT02307162, NCT03673670, NCT04027439, NCT03443414, NCT02542254, NCT03028142). RPL554 works by inhibiting the phosphodiesterase 3 and phosphodiesterase 4 enzymes. A nebulized ICS (budesonide) is available for the treatment of patients with asthma.52 Other nebulized ICSs (beclomethasone dipropionate, flunisolide) have been used for the treatment of patients with COPD exacerbations.53,54 The FDA approved the first commercially available nebulized LAMA, a glycopyrrolate (SUN-101/eFlow®; Sunovion, Marlborough, MA, USA), in 2017 for twice-daily maintenance treatment of patients with COPD, based on the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) Phase III trial program.55,56 Glycopyrrolate can only be administered by the eFlow® closed system vibrating membrane nebulizer, whereas revefenacin can be used with a standard jet nebulizer with a mouthpiece connected to an air compressor.18,49 As the only LAMA approved for once-daily nebulized delivery, revefenacin may have benefits in patients who need or prefer nebulized treatment, and in those for whom reduced dosing frequency is important. The ability to administer revefenacin using a standard jet nebulizer instead of a proprietary mesh nebulizer may be an additional benefit.
Revefenacin for nebulization may play an important role during recovery after COPD exacerbations when patients commonly have reduced PIFR and/or limited ability to use a handheld device, although further studies are needed to test this hypothesis. Evidence from studies in patients with moderate to very severe COPD and/or suboptimal PIFR suggests that revefenacin may be as effective as tiotropium in patients with suboptimal PIFR and significantly more effective than tiotropium in patients with suboptimal PIFR and severe airflow limitation.30 The design of revefenacin also results in fewer antimuscarinic AEs than with tiotropium.22 Furthermore, nebulized drug delivery is generally preferred by patients discharged after a hospitalization, who have shown consistent difficulty using handheld inhalers and who have impaired manual dexterity, impaired cognition, or chronic muscle weakness.5 A higher percentage of patients have previously reported that they generally prefer nebulizers versus inhalers.14
The safety and efficacy of revefenacin are also being tested sequentially and in combination with formoterol via a standard jet nebulizer in patients with moderate to very severe COPD (NCT0353817). This is of importance to patients who continue to have exacerbations on bronchodilator monotherapy. Additional patient-reported outcomes data will also be important in establishing the value of this once-daily nebulized treatment in patients with moderate to very severe COPD.
Conclusion
Once-daily revefenacin 175 μg for nebulization was well tolerated and improved bronchodilation in patients with moderate to very severe COPD, including those with the markers of more severe disease, without evidence of cardiovascular toxicity. This novel bronchoselective LAMA offers patients with COPD who require or prefer nebulized therapy an effective, convenient once-daily treatment option with low risk of adverse effects.
Abbreviations
AE, adverse event; BPC, 4-piperidyl biphenyl-2-ylcarbamate; DPI, dry powder inhaler; GOLDEN, Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer; ICS, inhaled corticosteroid; hERG, human ether-à-go-go; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LS, least squares; MACE, major adverse cardiovascular event; MDI, metered-dose inhaler; OTE, overall treatment effect; PIFR, peak inspiratory flow rate; REV, revefenacin; SAE, serious adverse event; SGRQ, St George’s Respiratory Questionnaire; SMI, soft-mist inhaler; TIO, tiotropium.
Data Sharing Statement
Theravance Biopharma (and its affiliates) will not be sharing individual deidentified participant data or other relevant study documents.
Acknowledgments
The medical writing support was funded by Theravance Biopharma US, Inc. (South San Francisco, CA, USA) and Mylan Inc. (Canonsburg, PA, USA). The authors acknowledge Esther Berkowitz, MBChB, and Ritu Pathak, PhD, for medical writing and Frederique H. Evans, MBS, for editorial assistance in the preparation of the manuscript (Ashfield Healthcare Communications, Middletown, CT, USA).
Disclosure
JFD is a consultant and advisory committee member for Theravance Biopharma US, Inc., AstraZeneca, GSK, Novartis Pharmaceuticals, Mylan Inc., and Sunovion Pharmaceuticals.
DAM has served on advisory boards for AstraZeneca, Boehringer Ingelheim, GSK, Grifols, Sunovion Pharmaceuticals, Theravance Biopharma US, Inc., and Trevi, and is on the speaker’s bureau for AstraZeneca, Boehringer Ingelheim, and Sunovion Pharmaceuticals.
SS is a consultant and advisory committee member for Theravance Biopharma US, Inc., and received research support from Mylan Inc.
The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2019 report. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.6-FINAL-08Nov2018-wms.pdf.
2. Seebri® Breezhaler® [summary of product characteristics]. Seebri breezhaler inhalation powder, hard capsules 44 mcg. Available from: https://www.medicines.org.uk/emc/medicine/27138.
3. SEEBRI™ NEOHALER® [prescribing information]. Marlborough, MA: Sunovion Pharmaceuticals Inc. Available from: https://www.seebri.us/Seebri-Prescribing-Information.pdf.
4. Dahl R, Kaplan A. A systematic review of comparative studies of tiotropium Respimat® and tiotropium HandiHaler® in patients with chronic obstructive pulmonary disease: does inhaler choice matter? BMC Pulm Med. 2016;16(1):135. doi:10.1186/s12890-016-0291-4
5. Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrar JR. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9(1):58–72. doi:10.3109/15412555.2011.630047
6. Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–371. doi:10.1378/chest.127.1.335
7. George M. Adherence in asthma and COPD: new strategies for an old problem. Respir Care. 2018;63(6):818–831. doi:10.4187/respcare.05905
8. Ari A. Patient education and adherence to aerosol therapy. Respir Care. 2015;60(6):941–955. doi:10.5152/ejp.2014.00087
9. Toy E, Beaulieu N, McHale J, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105(3):435–441. doi:10.1016/j.rmed.2010.09.006
10. Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):1601794. doi:10.1183/13993003.01794-2016
11. Tashkin DP. A review of nebulized drug delivery in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2585–2596. doi:10.2147/COPD.S114034
12. Amin AN, Ganapathy V, Roughley A, Small M. Confidence in correct inhaler technique and its association with treatment adherence and health status among US patients with chronic obstructive pulmonary disease. Patient Prefer Adherence. 2017;11:1205–1212. doi:10.2147/PPA.S140139
13. Kaplan A, Price D. Matching inhaler devices with patients: the role of the primary care physician. Can Respir J. 2018;2018:1–9. doi:10.1155/2018/9473051
14. Sharafkhaneh A, Wolf RA, Goodnight S, Hanania NA, Make BJ, Tashkin DP. Perceptions and attitudes toward the use of nebulized therapy for COPD: patient and caregiver perspectives. COPD. 2013;10(4):482–492. doi:10.3109/15412555.2013.773302
15. Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23–30. doi:10.2147/CIA.S52999
16. Ari A. Jet, ultrasonic, and mesh nebulizers: an evaluation of nebulizers for better clinical outcomes. Eurasian J Pulmonol. 2014;16:1–7. doi:10.5152/ejp.2014.00087
17. Melani AS. Long-acting muscarinic antagonists. Expert Rev Clin Pharmacol. 2015;8(4):479–501. doi:10.1586/17512433.2015.1058154
18. YUPELRI® [prescribing information]. Morgantown, WV: Mylan Specialty LP. Available from: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?type=display&setid=6dfebf04-7c90-436a-9b16-750d3c1ee0a6.
19. National Center for Biotechnology Information. PubChem Database. Revefenacin, CID=11753673, Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Revefenacin.
20. National Center for Biotechnology Information. PubChem Database. Tiotropium bromide, CID=5487426, Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Tiotropium-bromide.
21. National Center for Biotechnology Information. PubChem Database. Umeclidinium bromide, CID=11519069, Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Umeclidinium-bromide.
22. Ji Y, Husfeld C, Pulido-Rios MT, et al. Duration by design: discovery of revefenacin, the first-in-class nebulized once-daily bronchodilator for the treatment of patients with COPD [abstract]. Chest. 2016;150(4 Suppl):970A. doi:10.1016/j.chest.2016.08.1074
23. Hegde SS, Pulido-Rios MT, Luttmann MA, et al. Pharmacological properties of revefenacin (TD-4208), a novel, nebulized long-acting, and lung selective muscarinic antagonist, at human recombinant muscarinic receptors and in rat, guinea pig, and human isolated airway tissues. Pharmacol Res Perspect. 2018;6(3):e00400. doi:10.1002/prp2.400
24. Pulido-Rios MT, McNamara A, Obedencio GP, et al. In vivo pharmacological characterization of TD-4208, a novel lung-selective Inhaled muscarinic antagonist with sustained bronchoprotective effect in experimental animal models. J Pharmacol Exp Ther. 2013;346(2):241–250. doi:10.1124/jpet.113.203554
25. Quinn D, Barnes CN, Yates W, et al. Pharmacodynamics, pharmacokinetics and safety of revefenacin (TD-4208), a long-acting muscarinic antagonist, in patients with chronic obstructive pulmonary disease (COPD): results of two randomized, double-blind, phase 2 studies. Pulm Pharmacol Ther. 2018;48:71–79. doi:10.1016/j.pupt.2017.10.003
26. www.clinicaltrials.gov. A 7-day cross-over study of QD (once daily) and BID (twice daily) TD-4208 in chronic obstructive pulmonary disease (COPD). Available from: https://www.clinicaltrials.gov/ct2/show/study/NCT02109172?term=Revefenacin&rank=9.
27. Pudi KK, Barnes CN, Moran EJ, Haumann B, Kerwin E. A 28-day, randomized, double-blind, placebo-controlled, parallel group study of nebulized revefenacin in patients with chronic obstructive pulmonary disease. Respir Res. 2017;18(1):182. doi:10.1186/s12931-017-0647-1
28. Ferguson GT, Feldman G, Pudi KK, et al. Improvements in lung function with nebulized revefenacin in the treatment of patients with moderate to very severe COPD: results from two replicate phase III clinical trials. Chronic Obstr Pulm Dis. 2019;6(2). doi:10.15326/jcopdf.6.2.2018.0152
29. Donohue J, Kerwin E, Sethi S, et al. Revefenacin, a once-daily, lung-selective, long-acting muscarinic antagonist for nebulized therapy: safety and tolerability results of a 52-week phase 3 trial in moderate to very severe chronic obstructive pulmonary disease. Respir Med. 2019;153:38–43. doi:10.1016/j.rmed.2019.05.010
30. Mahler DA, Ohar JA, Barnes CN, Moran EJ, Pendyala S, Crater GD. Nebulized versus dry powder long-acting muscarinic antagonist bronchodilators in patients with COPD and suboptimal peak inspiratory flow rate. Chronic Obstr Pulm Dis. 2019;6(4):321–331. doi:10.15326/jcopdf.6.4.2019.0137
31. Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189(3):250–255. doi:10.1164/rccm.201310-1863PP
32. Donohue J, Pendyala S, Barnes C, Moran E, Crater G. Improvements in health status with revefenacin, a once-daily long-acting muscarinic antagonist for nebulization: changes in St George’s Respiratory Questionnaire and COPD Assessment Test in replicate 3-Month Studies [abstract]. Chest. 2017;152(4):A819. doi:10.1016/j.chest.2017.08.850
33. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
34. Donohue J, Kerwin E, Barnes C, et al. Efficacy of revefenacin, a long-acting muscarinic antagonist for nebulized therapy, in COPD patients with markers of more severe disease [abstract]. CHEST. 2018;154(4):736A–737A. doi:10.1016/j.chest.2018.08.665
35. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30(6):381–387. doi:10.1089/jamp.2017.1416
36. Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(7):1103–1107. doi:10.1513/AnnalsATS.201702-156PS
37. Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174–179. doi:10.1089/jamp.2012.0987
38. Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217–224. doi:10.15326/jcopdf.4.3.2017.0183
39. Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78–83. doi:10.1183/09031936.00024807
40. Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305–1311. doi:10.1513/AnnalsATS.201611-903OC
41. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
42. Donohue JF, Feldman G, Sethi S, et al. Cardiovascular safety of revefenacin, a once-daily, lung-selective, long-acting muscarinic antagonist for nebulized therapy of chronic obstructive pulmonary disease: Evaluation in phase 3 clinical trials. Pulm Pharmacol Ther. 2019;57:101808. doi:10.1016/j.pupt.2019.101808.
43. Goodman N, Morgan M, Nikander K, Hinch S, Coughlin S. Evaluation of patient-reported outcomes and quality of life with the I-neb AAD system in patients with chronic obstructive pulmonary disease. J Aerosol Med Pulmo Drug Deliv. 2010;23(Suppl 1):S61–S70. doi:10.1089/jamp.2009.0767
44. Freeman D, Lee A, Price D. Efficacy and safety of tiotropium in COPD patients in primary care–the SPiRiva Usual CarE (SPRUCE) study. Respir Res. 2007;8(45). doi:10.1186/1465-9921-8-91
45. Calverley P, Rennard S, Clerisme-Beaty E, Metzdorf N, Zubek V, ZuWallack R. Effect of tiotropium on night-time awakening and daily rescue medication use in patients with COPD. Respir Res. 2016;17:27. doi:10.1186/s12931-016-0340-9
46. Barjaktarevic IZ, Arredondo AF, Cooper CB. Positioning new pharmacotherapies for COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1427–1442. doi:10.2147/COPD.S83758
47. Montuschi P, Macagno F, Valente S, Fuso L. Inhaled muscarinic acetylcholine receptor antagonists for treatment of COPD. Curr Med Chem. 2013;20(12):1464–1476. doi:10.2174/0929867311320120002
48. Fuso L, Mores N, Valente S, Malerba M, Montuschi P. Long-acting beta-agonists and their association with inhaled corticosteroids in COPD. Curr Med Chem. 2013;20(12):1477–1495. doi:10.2174/0929867311320120003
49. LONHALA® MAGNAIR® [prescribing information]. Marlborough, MA: Sunovion Pharmaceuticals. Available from: https://www.lonhalamagnair.com/LonhalaMagnair-Prescribing-Information.pdf.
50. Brovana® [prescribing information]. Marlborough, MA: Sunovion Pharmaceuticals Inc. Available from: http://www.brovana.com/brovana-approved-labeling-text.pdf.
51. PERFOROMIST® [prescribing information]. Morgantown, WV: Mylan Specialty L.P. Available from: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=fb2fe258-fe2e-47f6-8adf-ca75bf6f90af.
52. PULMICORT RESPULES® [highlights of prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP. Available from: https://www.azpicentral.com/pulmicortrespules/pulmicortrespules.pdf#page=1.
53. Melani A, Di Gregorio A. Four-week nebulized beclomethasone dipropionate in stable COPD patients with exertional dyspnoea. Monaldi Arch Chest Dis. 1999;54(3):224–227.
54. Paggiaro P, Vagaggini B, Di Franco A, Zingoni M, Fano M, Biraghi M. Efficacy of nebulized flunisolide combined with salbutamol and ipratropium bromide in stable patients with moderate-to-severe chronic obstructive pulmonary disease. Respiration. 2006;73(5):603–609. doi:10.1159/000089816
55. Kerwin E, Donohue JF, Goodin T, Tosiello R, Wheeler A, Ferguson GT. Efficacy and safety of glycopyrrolate/eFlow® CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trials. Respir Med. 2017;132:238–250. doi:10.1016/j.rmed.2017.07.011
56. Ferguson GT, Goodin T, Tosiello R, Wheeler A, Kerwin E. Long-term safety of glycopyrrolate/eFlow® CS in moderate-to-very-severe COPD: results from the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 5 randomized study. Respir Med. 2017;132:251–260. doi:10.1016/j.rmed.2017.08.020
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
