Back to Journals » Journal of Pain Research » Volume 15
Retrospective Efficacy and Cost-Containment Assessment of 10 kHz Spinal Cord Stimulation (SCS) in Non-Surgical Refractory Back Pain Patients
Authors Kapural L , Calodney A
Received 25 May 2022
Accepted for publication 2 September 2022
Published 16 November 2022 Volume 2022:15 Pages 3589—3595
DOI https://doi.org/10.2147/JPR.S373873
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Krishnan Chakravarthy
Leonardo Kapural,1 Aaron Calodney2
1Carolinas Pain Institute, Winston-Salem, NC, USA; 2Precision Spine Care, Tyler, TX, USA
Correspondence: Leonardo Kapural, Email [email protected]
Background: Non-surgical refractory back pain (NSRBP) is persistent, severe back pain that is not considered surgically correctable. Published studies have demonstrated clinically important long-term improvement in pain and functional capacity when 10kHz spinal cord stimulation (SCS) is used to treat NSRBP. This study examines if real-world patients in interventional pain practice obtain the same outcomes, and have any reduction in health care utilization (HCU) following 10kHz SCS implant.
Methods: We conducted a retrospective chart review of 105 patients from two clinical sites who underwent implantation of 10kHz SCS for NSRBP. The three most frequent diagnoses were lumbosacral radiculopathy, degenerative disc disease (DDD)/discogenic back pain and foraminal stenosis. The complete set of patient-level electronic data, including clinical outcomes, HCU, and at least 12 months (12M) follow-up were available in 90 subjects.
Results: The 90 analyzed patients were 63.9 years old (median 67) with an average of 10.2 years since back pain diagnosis. Reported pain on the Visual Analog Scale (VAS) decreased from 7.78± 1.3 cm to 3.4± 2.4 cm at 12M after SCS implant (p< 0.001). Opioid usage (n = 65) decreased from 57.9± 89.9 mg to 34.3± 66.4 mg MSO4 equivalents (p = 0.004) at 12M. There were 46 patients on various doses of anticonvulsants, mostly gabapentin. The average dose decreased from 1847.91± 973.6 mg at baseline to 1297.9± 1184.6 mg at 12M after implant (p = 0.016). HCU was analyzed comparing the 12M before to the 12M after implant. Number of office visits decreased from 10.83± 8.0 per year to 8.86± 7.64 (p = 0.036), number of procedures to control chronic pain decreased from 2.2± 1.9 to 0.6± 1.2 (p< 0.001). There was no significant change in number of imaging procedures, hospital admissions, or days spent in the hospital.
Conclusion: 10kHz SCS warrants consideration as a therapeutic option for NSRBP patients and appears to provide a substantial reduction in HCU in the year following implant.
Keywords: spinal cord stimulation, high-frequency 10 kHz spinal cord stimulation, neuromodulation, lower back pain, lumbosacral radiculopathy, non-surgical refractory back pain, discogenic back pain, SCS, NSRBP
Introduction
Spinal cord stimulation (SCS) has been used primarily to treat patients with persistent severe back pain after spinal surgery1–5 and has been found to be less costly than long term medical management or reoperation for failed back surgery syndrome.2,3,6 High frequency SCS at 10 kHz is known to produce no paresthesias, and provide superior pain relief with favorable safety and efficacy profile.4,5,7,8 Additionally, patients who failed conventional (40–1200 Hz) SCS may experience a significant improvement in pain scores after the SCS system was changed to a 10 kHz SCS device.9–12 More significant recruitment of dorsal horn pain pathways by 10 kHz SCS, has been postulated as the likely mechanism behind such profound documented clinical pain relief.13
The non-surgical refractory back pain (NSRBP) patient population historically was not treated using SCS, even after conservative approaches failed to provide satisfactory pain relief. SCS was not part of the algorithm for treatment of lower back pain, unless the patient had previously undergone spinal surgery, and in the United States it is not widely supported by commercial payors.14–16 Furthermore, when it comes to various analgesics that include anticonvulsants, antidepressants for pain, anti-inflammatory agents or opioids, long-term positive therapeutic effect may be diminished by lack of efficacy and adverse events.17 Repeated injections, blocks and radiofrequency ablations may provide partial, but not long-lasting pain relief.18 Therefore, there is an unmet need for better long-term pain management of NSRBP. Initial studies using 10kHz SCS provided evidence of long-term pain relief and lasting improvements in functional capacity19–21 which is further supported by recently published data from a large randomized controlled study (RCT).22 The study design was pragmatic23 comparing conventional medical management (CMM) alone to CMM with 10 kHz SCS with primary and secondary endpoints at 3 and 6 months respectively, crossover at 6 months, and follow-up to 12 months. The primary outcome (% of patients with >50% pain relief at 3 months) and all secondary outcomes were met. There were 81% of the patients who received more than 50% of pain relief at 3 months in the 10kHz SCS arm, and the improvement was maintained through 12 months. In addition, improvements in functional capacity were deemed clinically significant (Oswestry Disability Index [ODI] >10 points change) in 78.1% of patients, opioid usage decreased by almost 50% and patient satisfaction was high in patients treated with 10 kHz SCS.22
This study examines if such data are consistent with outcomes in a real-world cohort of patients in interventional pain practices and if such an approach provides savings in healthcare utilization (HCU) following the SCS implant. This retrospective databased analysis was designed to determine the degree of improvement in pain scores, opioids and gabapentin usage, as well as HCU change after implant.
Methods
One hundred five patients underwent high frequency, 10 kHz SCS implantation from June 2016 to July of 2020 at the Carolinas Pain Institute, Winston-Salem, NC and, Precision Spine Care, Tyler, TX. Patients were selected for the procedure based on CMM failure that included medications, spinal injections, nerve blocks and radiofrequency ablations. Sixty out of 90 received spine surgery/neurosurgery consultation and were deemed not an acceptable candidate for spine surgery based on indications (54 patients), comorbidities (3), refusal (1) and age (2). The other 30 patients were deemed to be poor surgical candidates by the investigator physician. Following the Western Institutional Review Board (IRB) (Puyallup, WA) approval, patient data were collected from our electronic medical records (EMRs). Individual sites do not have their institutional Review Board, so Western IRB was used. Study complies with Declaration of Helsinki. The data collected from these records included patients identifier, date of birth/age, sex, age, years that patient suffered chronic pain, chronic pain diagnosis for which patient received SCS system, number of other chronic pain sources, number of contact leads implanted, duration of SCS therapy in months, baseline anticonvulsant usage, 1, 6 and 12 months anticonvulsant usage, baseline daily opioid usage (MSO4 equivalents) 1, 6 and 12 months after SCS implantation, and pain scores at baseline and 1, 6 and 12 months after implant of the 10 kHz SCS system. Health care utilization (HCU) data included number of office visits over 12 months before SCS trial and over the 12 months following implant of a permanent SCS system. For the same time periods we collected number of nerve blocks, imaging procedures, emergency room visits, physical therapy sessions, number of days admitted to hospital, and number of laboratory tests related to chronic pain. Patient identifiers during data collection were removed and not used again. The nature of this retrospective study did not require further reclaim of patient’s identifiers. As such, Western IRB waived the need for obtaining informed consent from the patients.
There were 105 consecutive NSRBP patients implanted and 90 patients’ records contained a full set of data, as detailed above. Flow chart on patient disposition is shown in Figure 1.
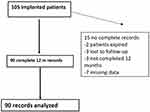 |
Figure 1 Disposition of 105 consecutive patients from two clinical sites who underwent 10 kHz SCS for NSRBP. |
Statistical Analysis
Data were summarized using descriptive statistics for continuous variables. We used Student’s t-tests to determine whether usage of 10 kHz SCS system improved the pain scores significantly. Similar analysis was carried out to determine changes in daily opioid use before and after initiation of 10 kHz SCS. One way ANOVA for repeated measures and Tukey’s test for pairwise mean comparisons between baseline, and 1, 6, and 12 months post implant were also completed. The rates of HCU (for each category of care) were compared pre- and post-implant with a paired t-test, or Wilcoxon Signed-Rank test if the differences have a non-normal population distribution. All analysis was completed using the program SPSS for Windows (version 9.0, Cary, New York, USA).
Procedures Completed
All patients received an implant of two octapolar leads and 10 kHz SCS system for NSRBP. Implants were completed under sterile conditions and fluoroscopic guidance while patients were under deep sedation as per International Neuromodulation Society Guidelines and manufacturers recommendations.24 Anterior-posterior and lateral views were used to confirm a final placement of the leads. All procedures/replacements were completed as a part of our regular clinical practice.
Results
There were 53 women and 37 men with an average age of 63.8 years (median of 67 years) who underwent SCS implant following a successful trial. They averaged 10.2 years of chronic back and/or leg pain and had a median of 2 (range 0–6, average of 2.4±1.5) other sources of chronic pain in addition to the one being treated using SCS. The majority of patients received their SCS implant for a diagnosis of lumbosacral radiculopathy, followed by degenerative disc disease (DDD). Other diagnosis included foraminal stenosis, discogenic low back pain, lumbar spondylosis, spinal stenosis, lower back pain. There was no correlation between the increased age, gender, or baseline of opioid (morphine milligram equivalents [MME]) or other analgesic use, baseline pain on the Visual Analog Scale (VAS) with the degree of pain relief achieved from 10 kHz SCS therapy. The average VAS score at the last clinical visit just before 10 kHz SCS trial was 7.8 ±1.3 cm and decreased to 3.5 ± 2.1 cm at 1 month after the implant, and 3.5±2.4 and 3.4±2.4 at 6- and 12-months following implant respectively (Figure 2). At all of the follow up visits the VAS pain score was significantly lower than before 10 kHz SCS (p<0.001). VAS pain scores were stable with no significant difference at 1.6 and 12 months post implantation, suggesting that such profound pain score improvements were maintained throughout the year (p<0.001)).
There were 65 out of 90 patients receiving opioid therapy ranging from 5–480 mg of MSO4 equivalents (median 30 mg). Opioid usage decreased from 57.9± 89.9 mg to 34.3 ± 66.4 mg MME (p = 0.004; median 20 mg) at 12 months (Figure 3).
There were 46 patients on various doses of anticonvulsants, and most subjects on various doses of gabapentin. We calculated their therapeutic daily usage equivalent to gabapentin in mg. At the baseline their average usage was 1847.91±973.6 mg and significantly decreased to 1297.9 ±1184.6 mg at 12 months after an implant (p = 0.016; Figure 4).
We also calculated available data in EMRs related to HCU. Number of office visits decreased significantly within 12 months after SCS implant compared to 12 months before (Figure 5). From an average of 10.8 ± 8.0 office visits per year to 8.7 ±7.6 (p = 0.036) and the number of procedures to control chronic pain decreased from 2.2 ±1.9 to 0.6 ± 1.2 (p = <0.001). The number of physical therapy appointments decreased significantly (p = 0.010). There was no significant change in the number of imaging procedures per year, number of hospital admissions or days spent in the hospital. However, there was a trend toward decrease in number of emergency room visits that did not reach significance (p = 0.413). There was no significant difference in frequency of laboratory tests conducted in the year before or after an implant (p = 0.763).
Discussion
Clinical evidence from a large RCT and a previously conducted single arm study supported the use of 10 kHz SCS in patients with NSRBP.19–23 Here, in this retrospective database analysis, we have documented significant improvement in pain scores at 6 and 12 months after implant of a 10kHz SCS system for NSRBP that was accompanied by a significant decrease in usage of opioids and anticonvulsants. Longevity of the pain relief and opioid-sparing effect was observed here in a general, real-world population of patients recruited from two practices in the United States. Our patients, in addition to an average of >10 years of chronic back pain, suffered from, on average, an additional two sources of chronic pain including diagnoses of chronic knee, hip, or shoulder pain, migraine headaches, and fibromyalgia. Even with this more complex population, comparable results were achieved to previously published studies where stricter patient selection criteria were used.22,23 Interestingly, in addition to significant decrease in opioid usage, a significant decrease in membrane stabilizers daily dosage, expressed in milligrams of gabapentin, was found at 6 and 12 months after the initiation of SCS therapy. Membrane stabilizers, which are minimally effective for back pain17 also carry a multitude of mild to more severe side effects that include sleepiness, depression, weight gain, dizziness and excessive forgetfulness.17
In this patient population, when treated with 10kHz SCS, the majority of patients significantly decreased or discontinued entirely previously prescribed anticonvulsant medication (Figure 4). In this study, there was no correlation between the age, gender, amount of baseline opioid, membrane stabilizers usage, or amount of baseline pain with the degree of pain relief achieved from 10 kHz SCS therapy. Such lack of correlation may be influenced by heterogeneity of the patient population studied including the variety of additional chronic pain diagnoses.
We found that this difficult to treat patient population with the majority using opioid medications (n = 65/90), did significantly decrease their long-term their opioid usage (Figure 3). Our results add to published wealth of the literature suggesting that 10kHz and other SCS therapies can provide a long-lasting opioid-sparing effect.22,25–27
Most of our patients were deemed not to be surgical candidates based on spine surgeon evaluation. We recommend surgical consultation before SCS trial/implant completed. Surgical assessment helps to rule out impeding instability, weakness related to the patient’s spinal pathophysiology and adds to our assessment of proper candidate for SCS.23 As we continue to gather data on the long-term therapeutic benefits of SCS in NSRBP patients with different pain etiologies, it is prudent to remain conservative in requiring these surgical assessments as part of our clinical decision making.
General outcomes of 10 kHz SCS in patients who underwent previous back surgery (failed back and/or post-laminectomy syndrome patients) compared to those subjects who had no previous spinal surgeries are similar in long-term pain relief, functional improvements and patients’ satisfaction.4,5,7,19,20,22 Our data published here contributes to the evidence supporting the robustness of 10 kHz therapy when treating NSRBP patients and are in line with data from previously published RCTs.4,5,22 Despite the fact that our patients on average carried active chronic pain diagnoses of 2 other body locations, in addition to their back pain location, similar outcomes are documented over 12 months of follow-up.
We collected detailed HCU measures extracted from EMRs. This simplified approach involving retrospective analysis of frequency of HCU events should not be compared to a full cost-effectiveness analysis, but one can conclude some initial savings would likely be accrued within the first year after 10 kHz SCS system was implanted compared to the year before implant. We observed fewer office visits (mainly for pain management issues) and significantly fewer number of interventional pain procedures our patients received during the 12 months after an implant compared to the year before (Figure 5). The number of physical therapy sessions decreased significantly, which was unexpected. It may be explained by a significant increase in patient’s mobility and overall pain decrease resulting in less need for physical therapy. We did not see differences in the number of hospital admissions, days spent in hospital, or emergency room admissions in this patient population with multiple co-morbidities.
Obvious limitation to this is the non-blinded, retrospective nature of the study and missing data as detailed in Figure 1. We tried to offset unintended bias by enrollment of a large cohort of consecutive patients.
To conclude, most of our patients received >50% of pain relief when 10 kHz SCS was used for NSRBP. Such improvement in chronic pain management resulted in a significant decrease of their membrane stabilizer and opioid usage. In addition, a significant decrease in office visits and pain management procedures were observed 12 months after SCS implantation compared to 12 months prior. SCS at 10 kHz provided consistent improvements in chronic back pain in our real-world study, consistent with previously published prospective single arm and randomized prospective studies.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
Study was funded by an Investigator initiated grant from Nevro to Drs Kapural and Calodney.
Disclosure
Drs Kapural and Calodney are paid consultants for Nevro Corp. Dr Leonardo Kapural also reports grants and/or personal fees from Medtronic, Biotronik, Nalu Medical, Saluda, and Gimer Medical, during the conduct of the study. Dr Aaron Calodney is consultant for Medtronic, Boston Scientific, Nevro, PainTeq, Stimwave, Stryker, and Vertos, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Kapural L, Peterson E, Provenzano D, Staats P. Clinical evidence for spinal cord stimulation for Failed Back Surgery Syndrome (FBSS): systematic review. Spine. 2017;42:S61–S66. doi:10.1097/BRS.0000000000002213
2. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98–106; discussion−7. doi:10.1227/01.NEU.0000144839.65524.E0
3. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1–2):179–188. doi:10.1016/j.pain.2007.07.028
4. Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZARCT randomized controlled trial. Anesthesiology. 2015;123:851–860. doi:10.1097/ALN.0000000000000774
5. Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz highfrequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016;79:667. doi:10.1227/NEU.0000000000001418
6. North RB, Kidd D, Shipley J, Taylor RS. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery. 2007;61(2):361–8; discussion 368–9. doi:10.1227/01.NEU.0000255522.42579.EA
7. Van Buyten JP, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation. 2013;16(1):59–65. doi:10.1111/ner.12006
8. Stauss T, El Majdoub F, Surges E, et al. A multicenter real-world review of 10 kHz SCS outcomes for treatment of chronic trunk and/or limb pain. Ann Clin Transl Neurol. 2019;6(3):496–507. doi:10.1002/acn3.720
9. Youn Y, Pilitsis JG, Pilitsis JG. Successful use of high-frequency spinal cord stimulation following traditional treatment failure. Stereotact Funct Neurosurg. 2015;93:190–193. doi:10.1159/000380825
10. Ghosh PE, Gill JS, Simopoulos T. The evolving role of high-frequency SCS as salvage therapy in neuromodulation. Pain Pract. 2020;20:706–713. doi:10.1111/papr.12898
11. Kapural L, Sayed D, Kim B, Harstroem C, Deering J. Retrospective assessment of salvage to 10 kHz Spinal Cord Stimulation (SCS) in patients who failed traditional SCS therapy: RESCUE study. J Pain Res. 2020;13:2861–2867. doi:10.2147/JPR.S281749
12. Gill JS, Asgerally A, Simopoulos T. High-frequency spinal cord stimulation at 10 kHz for the treatment of complex regional pain syndrome “A case series of patients with or without previous SCS implant”. Pain Pract. 2019;19(3):289–294. doi:10.1111/papr.12739
13. Lee KY, Bae C, Lee D, et al. Low-intensity, kilohertz frequency spinal cord stimulation differently affects excitatory and inhibitory neurons in the rodent superficial dorsal horn. Neuroscience. 2020;428:132–139. doi:10.1016/j.neuroscience.2019.12.031
14. Spinal cord stimulators for chronic pain. UnitedHealthcare medicare advantage policy guideline approved; 2021. Available from: www.uhcprovider.com.
15. Spinal cord stimulation. Aetna clinical policy bulletin; 2021. Available from: www.aetna.com.
16. Spinal cord and dorsal root stimulation. Blue cross and blue shield medical policy; 2021. Available from: www.bluecross.com.
17. Finnerup N, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
18. Manchikanti L, Abdi S, Atluri S, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16(2 Suppl):S49–283.
19. Al-Kaisy A, Van Buyten JP, Kapural L, et al. Rotte A 10 kHz spinal cord stimulation for the treatment of non-surgical refractory back pain: subanalysis of pooled data from two prospective studies. Anaesthesia. 2020;75(6):775–784. doi:10.1111/anae.15036
20. Al-Kaisy A, Palmisani S, Smith TE, et al. Long-term improvements in chronic axial low back pain patients without previous spinal surgery: a cohort analysis of 10-kHz high-frequency spinal cord stimulation over 36 months. Pain Med. 2018;19(6):1219–1226. doi:10.1093/pm/pnx237
21. Eckermann JM, Pilitsis JG, Vannaboutathong C, Wagner BJ, Province-Azalde R, Bendel MA. Systematic literature review of spinal cord stimulation in patients with chronic back pain without prior spine surgery. Neuromodulation. 2021. doi:10.1111/ner.13519
22. Kapural L, Jameson J, Johnson C, et al. Treatment of nonsurgical refractory back pain with high-frequency spinal cord stimulation at 10kHz: 12-month results of a pragmatic, multicenter, randomized controlled trial. J Neurosurg. 2022;1:1–2.
23. Patel N, Calodney A, Kapural L, et al. High-frequency spinal cord stimulation at 10 kHz for the treatment of nonsurgical refractory back pain: design of a pragmatic, multicenter, randomized controlled trial. Pain Pract. 2021;21(2):171–183. doi:10.1111/papr.12945
24. Deer TR, Russo MA, Grider JS, et al. The Neurostimulation Appropriateness Consensus Committee (NACC): recommendations for surgical technique for spinal cord stimulation. Neuromodulation. 2022;25(1):1–34. doi:10.1016/j.neurom.2021.10.015
25. Al-Kaisy A, Van Buyten JP, Carganillo R, et al. 10 kHz SCS therapy for chronic pain, effects on opioid usage: post hoc analysis data from two prospective studies. Sci Rep. 2019;9:11441. doi:10.1038/s41598-019-47792-3
26. Al-Kaisy A, Van Buyten JP, Amirdelfan K, et al. Opioid-sparing effects of 10 kHz spinal cord stimulation: a review of clinical evidence. Ann N Y Acad Sci. 2020;1462(1):53–64. doi:10.1111/nyas.14236
27. Deer TR, Grider JS, Lamer TJ, et al. Systematic literature review of spine neurostimulation therapies for the treatment of pain. Pain Med. 2020;21(7):1421–1432. doi:10.1093/pm/pnz353
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




