Back to Journals » Infection and Drug Resistance » Volume 11
Retrospective comparative analysis of risk factors and outcomes in patients with carbapenem resistant Acinetobacter baumannii bloodstream infections: cefoperazone–sulbactam associated with resistance and tigecycline increased the mortality
Authors Niu T, Xiao TT, Guo L, Yu W, Chen Y , Zheng B, Huang C, Yu X, Xiao Y
Received 27 March 2018
Accepted for publication 18 July 2018
Published 26 October 2018 Volume 2018:11 Pages 2021—2030
DOI https://doi.org/10.2147/IDR.S169432
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Tianshui Niu,1 TingTing Xiao,1 Lihua Guo,1 Wei Yu,2 Yunbo Chen,1 Beiwen Zheng,1 Chen Huang,1 Xiao Yu,1 Yonghong Xiao1
1Collaborative Initiative Center for Diagnosis and Treatment of Infectious Diseases, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, 310003, China; 2Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, 310003, China
Background: Carbapenem-resistant Acinetobacter baumannii (CRAB) is a rapidly emerging, life-threatening nosocomial infection. This study aimed to explore the risk factors, clinical features, antimicrobial therapy, and outcomes of CRAB bloodstream infections (BSIs).
Methods: This is a retrospective, comparative analysis of data from patients with A. baumannii BSI, treated from 2012 to 2015 at a tertiary teaching hospital. Risk factors associated with CRAB BSI and factors associated with the 28-day mortality were evaluated using logistic analyses.
Results: Data from 293 patients with confirmed A. baumannii BSI were included; 242 (82.6%) patients had CRAB BSI and 51 (17.4%) patients had non-CRAB BSI. Risk factors significantly associated with CRAB BSI were a previous intensive care unit (ICU) stay (P=0.029), cefoperazone–sulbactam use (P=0.030), and carbapenem use (P=0.004). Among 236 patients with A. baumannii BSI who were evaluable at 28 days after receiving antibacterial therapy, there were 86 deaths. Factors associated with the 28-day mortality were ICU stay after BSI (P=0.040), readmission within 90 days (P=0.029), Acute Physiology and Chronic Health Evaluation II (APACHE II) score at diagnosis >19 (P=0.012), tigecycline therapy (P=0.021), presence of septic shock (P=0.029), and multiple organ failure (P=0.016). Death rates in patients treated with tigecycline were 53.5% vs 24.1% in patients treated with other agents. Among 186 patients with CRAB BSI evaluable at 28 days, 84 patients died. The associated risk factors were an ICU stay after BSI (P=0.036), APACHE II score >19 at diagnosis (P=0.002), presence of septic shock (P=0.030), and multiple organ failure (P=0.007).
Conclusion: This study demonstrated that an ICU stay and cefoperazone–sulbactam or carbapenem use were seen to be the risk factors associated with the development of CRAB BSI. Critical illness and tigecycline therapy were significantly associated with higher mortality of patients with A. baumannii BSI.
Keywords: Acinetobacter baumannii, carbapenem-resistant, bloodstream infection, cefoperazone–sulbactam, tigecycline
Introduction
Acinetobacter baumannii is one of the most important nosocomial pathogens associated with hospital-acquired infections worldwide and is the cause of serious infections including pneumonia, meningitis, bacteremia, and catheter-related urinary tract infections.1,2 A. baumannii is inherently difficult to eliminate by desiccation and disinfection, and resistance is emerging to many antibiotics.3 Acinetobacter species are the fifth most common pathogens in intensive care units (ICUs) in 75 countries.4 A. baumannii bloodstream infection (BSI) significantly extend hospital stay and increase mortality, with studies reporting an overall mortality rate of 29%–63%.5–7 Overuse of invasive procedures, antibiotics, and steroids may have triggered the increase in A. baumannii BSI in recent years.8
Carbapenem antibiotics are the principal agents used in the treatment of A. baumannii infections.9 Extensive use has, however, resulted in a rapid increase in the incidence of carbapenem-resistant A. baumannii (CRAB) especially in critical care. One study conducted in the USA indicated that carbapenem resistance increased from 20.6% in 2002 to 49.2% in 2008.10 In China, isolates of A. baumannii increased from 9.2% in 2005 to 11.1% in 2014 and resistance of A. baumannii strains to carbapenems increased from 31% to 66.7% during this period.11,12 Higher bacterial resistance is associated with an increased patient morbidity and health care costs.13,14 Sulbactam, tigecycline, polymyxin, aminoglycosides, and rifampicin have been recommended for the treatment of CRAB infections, but these recommendations lack systematic and comprehensive research-based clinical evidence, and no large-scale clinical trials have been conducted to evaluate treatment approaches in patients with A. baumannii BSI.15,16
In this retrospective analysis, we analyzed data from patients with confirmed A. baumannii BSI to identify risk factors associated with carbapenem resistance. We also aimed to improve the understanding of clinical manifestations, antimicrobial therapy, and patient mortality in order to identify potential approaches to improve infection control and clinical treatment.
Methods
Inclusion criteria and study objectives
This study was conducted at the First Affiliated Hospital, College of Medicine, Zhejiang University, after receiving approval from the research ethics committee (Reference Number: 2017-699). This study did not directly interfere with the patients or show the medical record number, patients’ name, or other personal information. There was no adverse effect on the rights of patients. Moreover, we keep patient’ data confidentiality. Therefore, consent to review their medical records was not required by the Institutional Review Board. Patients evaluated in this study included those with A. baumannii BSI hospitalized from January 2012 to December 2015. BSIs were assessed by following the criteria proposed by the American agency, the Centers for Disease Control and Prevention (CDC). If patients had more than one episode of A. baumannii-BSI, only data from the first episode were included.
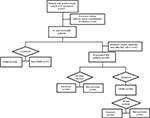
In this study, a three-part analysis was conducted (Figure 1), which aimed to 1) identify risk factors associated with CRAB BSI, 2) explore the prognosis of A. baumannii BSI, and 3) assess the risk factors associated with the 28-day mortality. Patients were categorized as “survivors” if they were alive after 28 days of infection; otherwise, they were included in the nonsurvivor group. Patients whose treatment time was <48 hours after BSI were excluded.
Data collection
Clinical data and laboratory results were collected from the hospital information system (HIS). The following data were collected: demographics, disease diagnosis, vital signs, laboratory reports at admission and infection, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, invasive procedures prior to BSI, bacteriological examination, and use of antibiotics, steroids, and immunosuppressive agents before and after BSI.
Microbiological assessment and definition of terms
The identification and antimicrobial susceptibility of A. baumannii were determined using the VITEK 2 system (bioMérieux, Marcy-l’ Etoile, France). Carbapenem resistance was defined as a minimum inhibitory concentration (MIC) of ≥8 µg/mL for imipenem and meropenem resistance by the breakpoints of the Clinical and Laboratory Standards Institute (CLSI) standards (2015).17 Cefoperazone–sulbactam susceptibility was based on the breakpoints of ampicillin–sulbactam (MIC 16/8 µg/mL).18 Tigecycline susceptibility was determined using the US Food and Drug Administration (FDA) breakpoints.19 Methylprednisolone therapy was defined as >20 mg/day administered for ≥7 days. Invasive procedure within 30 days prior to BSI was defined as invasive procedure used for ≥7 days within 30 days prior to BSI. Antimicrobial drug exposure was defined as the use of antibiotics for >72 hours within the 14 days prior to A. baumannii BSI. Appropriate therapy was defined as the treatment of a systemic antibacterial agent that demonstrated in vitro activity against A. baumannii within the initial 72 hours after A. baumannii BSI diagnosis.
Statistical analysis
SPSS Version 22.0 was used for the statistical analysis. The chi-squared and Fisher exact tests were used to analyze categorical variables. The Student’s t-test and Wilcoxon rank sum test were used to analyze continuous variables. Variables with a P-value of <0.05 in the univariate analysis were entered into a multivariate analysis using the multiple logistic regression method to determine the independent variables that were associated with carbapenem resistance and mortality. Variables with a P-value of <0.05 were considered independent risk factors, and the results are presented as the ORs and 95% CIs. The Kaplan–Meier method was used to estimate the survival distribution function. All tests were two tailed, and a P-value of <0.05 was considered statistically significant.
Results
Patient’s characteristics
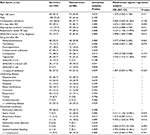
Data from 293 patients with A. baumannii BSI were included, 201 (68.6%) of whom were male (Table 1). The mean age of the patients was 59.2±16.2 years; 99 (33.8%) patients were aged >60 years (Tables 1 and S1). Most patients (n=193, 65.9%) were diagnosed in the ICU (Table S2). Most patients (n=213, 72.7%) had underlying disease, the most common being hypertension, hepatitis/cirrhosis, and diabetes. Comorbid conditions occurred in 168 (57.3%) patients, the most common being pulmonary infection and septic shock. The mean APACHE II scores were 14.5±4.5 on admission and 18.8±5.8 at infection.
Bacterial isolates and drug susceptibility
Drug sensitivity testing showed that the resistance rates of A. baumannii to tigecycline, amikacin, and levofloxacin were low (10.0%, 28.6%, and 34.8% respectively), while the resistance rates of A. baumannii to cefepime, ciprofloxacin, imipenem, and piperacillin–tazobactam were high (82.9%, 81.9%, 82.6%, and 82.9% respectively; Table S3).
Risk factors associated with the development of carbapenem resistance
Univariate analysis showed that invasive procedures, a previous ICU stay, in-hospital complications, and the use of methylprednisolone, antifungal drugs, and antibiotics were associated with CRAB. The results of multivariate logistic regression analysis showed that a previous ICU stay before BSI (OR =6.884, P=0.029), use of cefoperazone–sulbactam (OR =8.136, P=0.030), and use of carbapenem antibiotics (OR =10.432, P=0.004) within 30 days prior to BSI diagnosis were independent risk factors associated with carbapenem resistance (Table 1).
Risk factors for 28-day mortality in patients with A. baumannii BSI
Of the 293 patients, 150 patients were classified as survivors and 86 patients were classified as nonsurvivors; 57 patients were excluded as their treatment time was <48 hours. Multivariate logistic regression analysis showed that independent risk factors included ICU stay after BSI (OR =2.676, P=0.040), readmission within 90 days (OR =4.167, P=0.029), APACHE II score >19 at infection (OR =2.998, P=0.012), use of tigecycline after BSI (OR =3.750, P=0.021), septic shock (OR =4.151, P=0.029), and multiple organ failure (OR =9.705, P=0.016). Appropriate treatment within 72 hours of BSI diagnosis was seen to be a protective factor (OR =0.254, P=0.011; Table 2).
As shown in Table 2, use of tigecycline after BSI was an independent risk factor for the 28-day mortality. When the 236 patients with A. baumannii BSI were divided into two groups based on treatment (nontigecycline therapy group [n=137] and tigecycline therapy [n=99]), the 28-day mortality of the nontigecycline group was lower than that of the tigecycline group (24.1% vs 53.5%, P=0.000) (Table 2 and Figure 2A). The APACHE II scores of the tigecycline group were higher than those of the nontigecycline group (Table S4), suggesting that, for a more serious infection, tigecycline was used more frequently. Patients were also divided into three subgroups based on the APACHE II score at infection (APACHE II scores ≤15, 16–25, and ≥26) to clarify the impact of infection severity on the outcome of those patients. Patients receiving nontigecycline therapy had lower mortality rates than those receiving tigecycline therapy in all the three APACHE score groups, with this difference being statistically significant in the subgroup with an APACHE II score of 16–25 (P=0.031; Table 2). Assessment of differences in daily dosage of tigecycline (>0.1 and ≤0.1 g/day) did not change the mortality findings (Figure 2B).
Risk factors for 28-day mortality in patients with CRAB BSI
A total of 186 patients (excluding 56 patients with CRAB BSI who died within 48 hours of diagnosis) were included in this analysis; 69.4% of patients stayed in the ICU after infection, and the 28-day mortality rate among these patients was 45.2%. Multivariate logistic regression analysis showed that the independent risk factors for the 28-day mortality included ICU stay after BSI (OR =2.649, P=0.036), APACHE II score >19 at infection (OR =3.738, P=0.002), septic shock (OR =3.907, P=0.030), and multiple organ failure (OR =22.751, P=0.007) (Table S5).
Discussion
A. baumannii can cause a variety of serious infections and has emerged as an important nosocomial pathogen globally. The detection rate of CRAB and the incidence of multidrug-resistant A. baumannii (MDRAB) and pan-drug-resistant A. baumannii (PDRAB) are increasing especially in critical patients, as are the associated patient mortality and medical care costs. Therefore, identifying approaches to limiting the development of resistance, as well as defining effective treatment regimens that can reduce patient mortality, are an urgent requirement.
In this study, an ICU stay and antimicrobial therapy before BSI were shown to be independent risk factors for CRAB. ICU patients are typically in a critical condition, have low immunity and high APACHE II scores, receive extensive antibiotic therapy, and undergo invasive procedures, making them susceptible to infection with CRAB.20,21
Regarding the association between antibiotic use and A. baumannii resistance, one study found that the use of fluoroquinolones, broad-spectrum cephalosporins, and carbapenems increased the risk of CRAB.22 The present study showed that the use of cefoperazone–sulbactam and carbapenem antibiotics within the 30 days prior to BSI was independent risk factor for carbapenem resistance, that is, the first study to identify cefoperazone–sulbactam use as an independent risk factor for carbapenem resistance in A. baumannii. Sulbactam has activity against A. baumannii by binding to penicillin-binding protein 2 and has been recommended as a therapeutic agent to treat patients with A. baumannii-BSI,23 while cefoperazone–sulbactam has been widely used to treat extended spectrum β-lactamase-producing enterobacterial and A. baumannii infections in China;24 its use is not supported by any systematic evaluation of efficacy. However, due to the extensive use of this drug, the rate of A. baumannii resistance to cefoperazone–sulbactam has increased from 25.0% in 2004 to 37.7% in 2007 in China.25 The association between cefoperazone–sulbactam use and bacterial resistance has not previously been analyzed, and our findings suggest that more attention should be paid to the resistance incurred from the use of cefoperazone–sulbactam and further evaluation is urgently required.
In this study, an ICU stay after BSI, septic shock, multiple organ failure, and APACHE II score >19 at infection were shown to be risk factors for mortality, possibly indicating that the severity of the patient’s disease was the main cause of death. Prognostic analysis showed that appropriate treatment within 72 hours was an independent protective factor for outcome. Previous reports show that effective drug therapy in the early stages is a key factor in survival.8,26 Erbay et al27 showed that the mortality in patients receiving appropriate vs inappropriate antibiotic therapy within 48 hours is 39.5% vs 65%, respectively. We believed that appropriate antimicrobial treatment in the early stages is essential for A. baumannii-BSI patients.
CRAB BSI patients had more complications and more often died of critical illness. This study found that an ICU stay after BSI, APACHE II score >19 at infection, septic shock, and multiple organ failure were risk factors for the 28-day mortality in these patients.
As the incidence of CRAB has increased in recent years, tigecycline has been widely used for the management of this infection. However, the use of tigecycline regimens to treat patients with A. baumannii-BSI is controversial.28,29 Some small studies report the clinical cure rate to be 80%,30 while a range of other studies31–33 show tigecycline regimens to be less effective in the treatment of patients with A. baumannii BSI. Drug safety guidelines published by the US FDA refer to an increased mortality risk associated with intravenous tigecycline compared with other drugs used to treat serious infections.31 A meta-analysis of 14 randomized trials with ~7,400 patients showed no benefit associated with tigecycline over standard antimicrobial agents for the treatment of serious infections, and the treatment success rate was lower with tigecycline than with control antibiotic agents.32 Prasad et al33 also found that tigecycline was associated with increased mortality (P=0.01) and noncure rates (P=0.01). In the present study, we found the use of tigecycline to be an independent risk factor for the 28-day mortality. By stratifying patients on the basis of APACHE II score, the mortality of the nontigecycline group was shown to be lower than that shown in patients treated with tigecycline in all APACHE II score subgroups, reaching statistical significance in patients with an APACHE II score of 16–25. We also demonstrated that a higher dose of tigecycline did not change the 28-day mortality trend. This result, in addition to data from previous studies, indicates that the use of tigecycline for the treatment of A. baumannii BSI should be considered with greater caution, despite this treatment approach being recommended by a Chinese consensus on the treatment of multidrug resistant (MDR) Gram-negative bacilli.34 Although the underlying cause of higher mortality in trials of tigecycline is uncertain, inadequate antimicrobial activity in serum maybe an important reason. The standard dosing tigecycline regimen (100 mg load, followed by 50 mg every 12 hours) produces a maximum steady-state serum concentration of only 0.6 µg/mL.35 In severe infections with high bacteremia risk, low serum levels combined with bacteriostatic rather than bactericidal activity may lead to an unfavorable microbiological response. Direct drug toxicity or other mechanisms cannot be completely eliminated also as possible contributors to the higher mortality observed with tigecycline.33
We acknowledge some limitations to this study. This was a retrospective study, and all patients with AB complex bacteremia treated at a single tertiary medical center during the study period were included in this study. Because the rate of CRAB, A. baumannii BSI treatment programs, and the use of tigecycline will differ in other hospitals, the study sample may not be broadly representative. However, to our knowledge, this is the largest cohort of patients with BSI caused by A. baumannii and is also the largest cohort of patients with BSI caused by CRAB. As such, the results can be used to facilitate the pre-emptive identification and targeting of patients who are at increased risk of CRAB and to evaluate optimal therapeutic approaches.
Conclusion
This study showed that CRAB BSI was associated with higher levels of mortality, and an ICU stay and cefoperazone–sulbactam or carbapenem use were identified as risk factors associated with its emergence. Critical illness and tigecycline use significantly increased the mortality of patients with A. baumannii BSI. With a higher observed mortality rate, we suggest that tigecycline should be used with caution for the treatment of A. baumannii BSI.
Acknowledgments
The authors acknowledge the role of all support staff and participating patients in the study. This work was partially supported by a grant from the Key Research and Development Program of Zhejiang Province (2015C03032).
Disclosure
The authors report no conflicts of interest in this work.
References
Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65(2):233–238. | ||
Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12. | ||
Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis. 2008;8(12):751–762. | ||
Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. | ||
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. | ||
Magret M, Lisboa T, Martin-Loeches I, et al. Bacteremia is an independent risk factor for mortality in nosocomial pneumonia: a prospective and observational multicenter study. Crit Care. 2011;15(1):R62. | ||
Nutman A, Glick R, Temkin E, et al. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin Microbiol Infect. 2014;20(12):O1028–O1034. | ||
Chopra T, Marchaim D, Awali RA, et al. Epidemiology of bloodstream infections caused by Acinetobacter baumannii and impact of drug resistance to both carbapenems and ampicillin-sulbactam on clinical outcomes. Antimicrob Agents Chemother. 2013;57(12):6270–6275. | ||
Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46(8):1254–1263. | ||
Mera RM, Miller LA, Amrine-Madsen H, Sahm DF. Acinetobacter baumannii 2002-2008: increase of carbapenem-associated multiclass resistance in the United States. Microb Drug Resist. 2010;16(3):209–215. | ||
Xiao YH, Wang J, Zhao CY, et al. Mohnarin bacterial resistance surveillance 2006-2007. Chin J Nosocomiol. 2008;18:1051–1056. | ||
Hu FP, Zhu DM, Wang F, Jiang XF, Yc X, Zhang XJ. Chinet 2014 surveillance of bacterial resistance in China. Chin J Infect Chemother. 2015;15:401–410. | ||
Falagas ME, Bliziotis IA, Siempos II. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care. 2006;10(2):R48. | ||
Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. Daily cost of delay to adequate antibiotic treatment among patients surviving a hospitalization with community-onset Acinetobacter baumannii pneumonia or sepsis. Crit Care. 2017;21(1):130. | ||
Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis. 2010;51(1):79–84. | ||
Viehman JA, Nguyen MH, Doi Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs. 2014;74(12):1315–1333. | ||
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 25th informational supplement. In: CLSI Document M100-S25. Wayne, PA; 2015. | ||
Liu X, Zheng H, Zhang W, et al. Tracking cefoperazone/sulbactam resistance development in vivo in A. baumannii isolated from a patient with hospital-acquired pneumonia by whole-genome sequencing. Front Microbiol. 2016;7:1268. | ||
TYGACIL (tigecycline) [Package Insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2005. | ||
García-Garmendia JL, Ortiz-Leyba C, Garnacho-Montero J, et al. Risk factors for Acinetobacter baumannii nosocomial bacteremia in critically ill patients: a cohort study. Clin Infect Dis. 2001;33(7):939–946. | ||
Ye JJ, Huang CT, Shie SS, et al. Multidrug resistant Acinetobacter baumannii: risk factors for appearance of imipenem resistant strains on patients formerly with susceptible strains. PLoS One. 2010;5(4):e9947. | ||
Chusri S, Silpapojakul K, Mcneil E, Singkhamanan K, Chongsuvivatwong V. Impact of antibiotic exposure on occurrence of nosocomial carbapenem-resistant Acinetobacter baumannii infection: a case control study. J Infect Chemother. 2015;21(2):90–95. | ||
Choi JY, Kim CO, Park YS, et al. Comparison of efficacy of cefoperazone/sulbactam and imipenem/cilastatin for treatment of Acinetobacter bacteremia. Yonsei Med J. 2006;47(1):63–69. | ||
Gao C, Tong J, Yu K, Sun Z, An R, du Z. Pharmacokinetics of cefoperazone/sulbactam in critically ill patients receiving continuous venovenous hemofiltration. Eur J Clin Pharmacol. 2016;72(7):823–830. | ||
Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect. 2016;22(Suppl 1):S9–14. | ||
Ogutlu A, Guclu E, Karabay O, Utku AC, Tuna N, Yahyaoglu M. Effects of Carbapenem consumption on the prevalence of Acinetobacter infection in intensive care unit patients. Ann Clin Microbiol Antimicrob. 2014;13:7. | ||
Erbay A, Idil A, Gözel MG, Mumcuoğlu I, Balaban N. Impact of early appropriate antimicrobial therapy on survival in Acinetobacter baumannii bloodstream infections. Int J Antimicrob Agents. 2009;34(6):575–579. | ||
Schafer JJ, Goff DA, Stevenson KB, Mangino JE. Early experience with tigecycline for ventilator-associated pneumonia and bacteremia caused by multidrug-resistant Acinetobacter baumannii. Pharmacotherapy. 2007;27(7):980–987. | ||
Curcio D. Off-label use of antibiotics in hospitalized patients: focus on tigecycline. J Antimicrob Chemother. 2009;64(6):1344–1346. | ||
Curcio D, Fernández F, Cané A, Barcelona L, Stamboulian D. Indications of a new antibiotic in clinical practice: results of the tigecycline initial use registry. Braz J Infect Dis. 2008;12(3):198–201. | ||
US Food and Drug Administration. FDA drug safety communication. Increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections; 2010 [September 1]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm224370.htm. Accessed April 18, 2011. | ||
Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis. 2011;11(11):834–844. | ||
Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis. 2012;54(12):1699–1709. | ||
Chinese XDR Consensus Working Group, Guan X, He L, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect. 2016;22(Suppl 1):S15–S25. | ||
Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin Infect Dis. 2005;41(suppl 5):S333–S340. |
Supplementary materials
  | Table S1 Patients age distribution Abbreviation: CRAB, carbapenem-resistant Acinetobacter baumannii. |
  | Table S2 Location of diagnosis department Abbreviation: ICU, intensive care unit. |
  | Table S3 Drug susceptibility of A. baumannii Abbreviation: A. baumannii, Acinetobacter baumannii. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.