Back to Journals » Infection and Drug Resistance » Volume 11
Retrospective analysis of relationships among the dose regimen, trough concentration, efficacy, and safety of teicoplanin in Chinese patients with moderate–severe Gram-positive infections
Authors Zhou L, Gao Y, Cao W , Liu J, Guan H, Zhang H, Shi Y, Lv W, Cheng L
Received 30 July 2017
Accepted for publication 21 November 2017
Published 5 January 2018 Volume 2018:11 Pages 29—36
DOI https://doi.org/10.2147/IDR.S146961
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Lijuan Zhou,1,* Yanqiu Gao,2 Wei Cao,1 Jia Liu,1 Hongya Guan,1 Hua Zhang,2 Yun Shi,3 Wenying Lv,4 Long Cheng5,*
1Translational Medicine Center, 2Department of Respiratory Medicine, Affiliated Zhengzhou Central Hospital of Zhengzhou University, Zhengzhou, 3Department of Gynecology, Dongzhimen Hospital, Beijing University of Chinese Medicine, 4Chaoyangmen Community Health Service Center, 5Key Laboratory of Bioactive Substances and Resources Utilization of Chinese Herbal Medicine, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China
*These authors contributed equally to this work
Objectives: Teicoplanin, an antibiotic, has poor clinical efficacy when using the current drug label’s recommended regimen, which is approved by the China Food and Drug Administration. This study explores the appropriate loading and maintenance doses of teicoplanin and evaluates the therapeutic target of teicoplanin trough concentration (minimum concentration [Cmin]).
Subjects and methods: All patients treated with teicoplanin from February 2015 to August 2016 at Zhengzhou Central Hospital were screened for enrollment. A total of 113 subjects were included and then divided into four groups: A (received three to six doses at a loading dose of 400 mg at 12-hour intervals, followed by maintenance dosing of 400 mg/day), B (received three doses at a loading dose of 400 mg at 12-hour intervals, followed by maintenance dosing of 400 mg/day), C (received two doses at a loading dose of 400 mg at 12-hour intervals, followed by maintenance dosing of 200 mg/day), and D (received one to three doses at a loading dose of 400 mg at 12-hour intervals, followed by maintenance dosing of 200 mg/day). Cmin values of teicoplanin were detected with high-performance liquid chromatography on day 4, 30 minutes before maintenance-dose administration. Teicoplanin Cmin, efficacy, and safety were compared among the four groups.
Results: Mean Cmin differed significantly among the four groups (A, 18.11±6.37 mg/L; B, 15.91±4.94 mg/L; C, 17.06±5.66 mg/L; D, 11.97±3.76 mg/L) (P<0.001), with creatinine clearance of 89.62 (53.72–162.48), 49.66 (40.69–59.64), 27.17 (9.7–39.45), and 96.6 (17.63–394.73) mL/min, respectively. The ratio of loading dose for 3 days to creatinine clearance and serum Cmin were significantly correlated (R=0.59, P<0.001). The correlation between the estimated probability of success and teicoplanin Cmin was assessed using binary logistic regression (OR 2.049, P<0.001). Hepatotoxicity- and nephrotoxicity-incidence rates did not significantly differ among the four groups (P=0.859 and P=0.949, respectively).
Conclusion: A loading dose of 400 mg at 12-hour intervals three to six times is needed to achieve the early target range (15–20 mg/L) and improve the clinical efficacy rate for normal-renal-function patients. It is urgently necessary to amend the drug label for the recommended regimen.
Keywords: teicoplanin, loading dose, serum trough concentration, efficacy, safety, Chinese drug label
Corrigendum for this paper has been published
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a major pathogen in health care-associated infections, leading to high morbidity and mortality.1–5 As per current guidelines, teicoplanin and vancomycin are the first-line antibiotics for clinical infections caused by Gram-positive bacteria, especially MRSA.3,6–10 Teicoplanin is a semisynthetic glycopeptide antibiotic that kills Gram-positive bacteria by inhibiting the synthesis of cell-wall peptidoglycans. Meta-analysis has indicated that teicoplanin is equally effective as vancomycin, with less risk of nephrotoxicity and red man syndrome.7,11–14 Teicoplanin has a long elimination half-life of 30–180 hours, due to its high plasma protein-binding rate (over 90%); therefore, teicoplanin is slow to reach a steady-state concentration. To achieve target serum trough concentration (minimum concentration [Cmin]), a sufficient loading dose must be administered.15–17
Recent research found that teicoplanin Cmin was strongly influenced by loading dosage and creatinine clearance (Clcr) rate.16,18–20 A teicoplanin Cmin range of 10–30 mg/L was identified as the therapeutic target with optimal clinical efficacy and safety.15–20 An individualized initial loading dose followed by therapeutic drug monitoring was recommended to achieve the target Cmin. The Cmin must be maintained at a minimum of 10–20 mg/L for moderate Gram-positive infections and 20–60 mg/L for all severe staphylococcal infections, including endocarditis. Optimal Cmin has been associated with treatment success. Several reports have indicated that teicoplanin Cmin correlates with loading dose and clinical efficacy.7,14–22
The drug label approved by the China Food and Drug Administration recommends a load dosage of 200 mg or 400 mg one to three times at 12-hour intervals, followed by maintenance dosing of 200 mg or 400 mg/day; however, this treatment regimen results in poor clinical efficacy.23 In clinical practice, doctors must increase the loading dose to achieve the desired effect, but this is illegal in the view of the drug administration. Therefore, it is urgently necessary to determine the optimal load and maintenance doses to ensure clinical efficacy and safety in Chinese patients. We will push to revise the drug label and clinical guidelines by collecting sufficient data and clinical evidence. In the present work, we conducted a retrospective study to analyze the relationship between the revised regimen (an increased load and maintenance dose), teicoplanin Cmin, and clinical efficacy and safety (adverse events, nephrotoxicity, and hepatotoxicity) in Chinese patients with Gram-positive infections.
Subjects and methods
Patients and protocol
This was a retrospective study of all teicoplanin-treated adult patients with Gram-positive infections admitted to Zhengzhou Central Hospital, affiliated with Zhengzhou University, from February 2015 to August 2016. Hospitalized patients who met the following criteria were included: age ≥18 years; met the criteria of community-acquired pneumonia, confirmed by bacterial culture of drug-resistant Gram-positive bacterial infections;1,24 duration of teicoplanin therapy ≥5 days, received teicoplanin as initial therapy for ≥3 days, with maintenance therapy ≥2 days; during teicoplanin therapy, therapeutic drug-monitoring data were accurate, complete, and available; efficacy and safety data were accurate, complete, and available; and written informed consent to participate in the study had been provided. Exclusion criteria were patients allergic to teicoplanin, pregnant patients, patients with abnormal hematopoietic function, and those for whom efficacy and safety could not be evaluated. A standardized case-report form was designed to collect enrolled patients’ demographic, clinical, and routine laboratory data. Laboratory data from serum or blood collected 24 hours before and/or after the hospital stay included levels of albumin, CRP, creatinine, ALT, hemoglobin, leukocytes, and platelets. The end point was the clinical response evaluated at the end of the teicoplanin therapy. This study was conducted in accordance with the Declaration of Helsinki, approved by the institutional review boards at Zhengzhou Central Hospital, and registered at ClinicalTrials.gov (NCT03229135).
Treatment regimen and groups
As per the decision issued by the European Medicines Agency on September 12, 2013 (http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Targocid_30/WC500143827.pdf) and the teicoplanin drug label approved by the China Food and Drug Administration in 2009, all enrolled patients were categorized into four groups. Group A (Clcr ≥60 mL/min) received teicoplanin intravenously three to six times for moderate–severe infections at a loading dose of 400 mg at 12-hour intervals, followed by maintenance dosing of 400 mg/day. Group B (40–60 mL/min) received teicoplanin intravenously three times at a loading dose of 400 mg at 12-hour intervals, followed by maintenance dosing of 400 mg/day. Group C (Clcr <40 mL/min) received teicoplanin intravenously twice at a loading dose of 400 mg at 12-hour intervals, followed by maintenance dosing of 200 mg/day. Group D (standard regimen, according to the drug label approved by the China Food and Drug Administration) received teicoplanin intravenously one to three times at a loading dose of 400 mg at 12-hour intervals, followed by maintenance dosing of 200 mg/day. Maintenance doses were adjusted as per drug level (Cmin) on day 4. The target Cmin was set to 15–30 mg/L.15,18 Clcr values for males and females were calculated as follows:
([140 – age {years}] × body weight [kg])/(0.818 × serum creatinine [Scr; µmol/L]) (male)
([140 – age {years}] × body weight [kg])/(0.818 × Scr [µmol/L]) ×0.85 (female)
Renal function was assessed by Clcr levels as normal (> 60 mL/min), mildly impaired (40–60 mL/min), or impaired (<40 mL/min).
Serum teicoplanin trough concentrations
Serum teicoplanin Cmin of enrolled patients was monitored on day 4, 30 minutes before maintenance-dose administration. As per the technical standard of the hospital, blood samples (2–3 mL per subject) were collected in blood-collection tubes without any additives and centrifuged at 3,500 rpm for 10 minutes. Serum teicoplanin Cmin was determined by high-performance liquid chromatography, as previously described.16,21 Therapeutic drug monitoring was implemented at the Translational Medicine Center of Zhengzhou Central Hospital.
Data collection
The hospital medical records of all patients treated with teicoplanin from February 2015 to August 2016 at Zhengzhou Central Hospital were screened, reviewed, and analyzed by trained reviewers (Figure 1). Clinical data were collected: demographics, comorbidities, concomitant medications, source of infection, clinical information regarding teicoplanin dosing, duration of teicoplanin therapy, patients’ symptoms, body temperature, chest X-rays, and adverse events. Laboratory data included routine blood work, bacteriology, liver function, renal function, and a blood coagulation index. This included white blood cell counts, CRP, AST, ALT, ALP, total bilirubin, albumin, Scr, platelet counts, and Clcr.
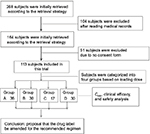  | Figure 1 Research process flowchart. Abbreviation: Cmin, minimum concentration. |
Efficacy assessment of teicoplanin
Efficacy was assessed as per the guidance for clinical trials of antibacterial drugs published by the Centre for Drug Evaluation of the China Food and Drug Administration.25 Clinical response to teicoplanin was evaluated based on patients’ clinical symptoms, laboratory data, and bacteriological findings on the day after the last dose of maintenance administration. The final clinical response to teicoplanin was evaluated and confirmed based on patients’ clinical symptoms, laboratory data, and bacteriological findings on days 5–7 after the last dose. The final confirmed response was classified as clinical cure or clinical failure. A clinical cure was defined as the disappearance of clinical symptoms, obvious improved laboratory data, and eradicated bacteria. A clinical failure was defined as no disappearance or worsening of clinical symptoms and/or laboratory data, requiring other antibacterial therapy. In other words, patients without clinical cure were included as clinical failures.
Safety assessment
Nephrotoxicity was defined as increased Scr of 0.5 mg/dL or 50% increase from the pretreatment value.3,12,25–27 Hepatotoxicity was defined as ALT or AST more than three times the upper limit of the institution’s normal reference ranges (AST 13–33 U/L, ALT 6–30 U/L). For patients with abnormal values at baseline, hepatotoxicity was defined as ALT or AST more than three times the baseline value. All teicoplanin-related adverse events were recorded throughout teicoplanin treatment.
Statistical analysis
All statistical analyses were conducted using SPSS statistics for Windows, version 17.0. Data are described as number (percentage) for categorical variables and mean ± SD, and median values (range/IQR) for continuous variables. Fisher’s exact or c2 tests was used to compare groups for categorical variables. Comparisons were conducted using Student’s t-test or Mann–Whitney U test for continuous variables and one-way analysis of variance or Kruskal–Wallis test for continuous variables. Relationships among the teicoplanin loading-regimen ratio and Clcr and Cmin were analyzed using Pearson’s correlation analysis. Relationships between clinical response and Cmin and between safety assessment and Cmin were analyzed using binary logistic regression. P<0.05 was considered statistically significant.
Results
Patient demographic data
Demographic data and clinical characteristics for all included patients are displayed in Table 1. No differences were found for age, sex, body weight, serum albumin, ALT, or AST among the four groups. No significant differences were found in the ratio of coadministered antibiotics (β-lactams, carbapenems, quinolones, or aminoglycosides) with teicoplanin or in the basic diseases (hypertension, diabetes, coronary heart disease, cerebral infarction, hyperlipidemia, or bone disease) among the four groups (all P>0.05; data not shown). Teicoplanin-therapy duration differed significantly among the four groups (F=3.415, P=0.02), but did not differ significantly between groups A, B, and C (A vs B, P=0.805; B vs C, P=0.749; A vs C, P=0.903).
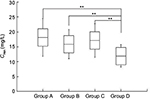
Teicoplanin trough concentration
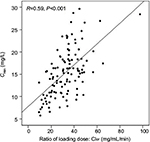
Teicoplanin Cmin values in the four groups are shown in Table 2 and Figure 2. Cmin was 18.11±6.37 mg/L, 15.91±4.94 mg/L, 17.06±5.66 mg/L, and 11.97±3.76 mg/L in groups A, B, C, and D, respectively. The proportion of patients achieving the target range (15–30 mg/L) was 69.44% (25 of 36), 66.67% (20 of 30), 70.59% (12 of 17), and 23.33% (seven of 30) in groups A, B, C, and D (c2=18.529, P<0.001) on day 4, respectively. Compared with group D, Cmin was higher in groups A, B, and C (11.97 vs 18.11, 15.91, and 17.06). The proportion of Cmin 15–30 mg/L and 20–30 mg/L was higher in groups A, B, and C than in group D (Figure 3). As shown in Figure 4, there was a significant correlation between the Cmin and the ratio of loading dose for the 3 days to Clcr (R=0.59, P<0.001).
  | Table 2 Teicoplanin trough concentration Notes: Data presented as mean ± SD and n (%). aOne-way analysis of variance; bc2 test for categorical variables. Abbreviation: Cmin, minimum concentration. |
Clinical efficacy of teicoplanin
Efficacy among the four groups differed significantly (Table 3). Compared with patients in group D, patients in groups A, B, and C had significantly higher clinical response rates (c2=11.438, P=0.001; c2=5.455, P=0.020; c2=5.797, P=0.016). No obvious differences were seen in clinical efficacy for patients in group A (80.56%, 29 of 36), group B (70%, 21 of 30), or group C (76.47%, 13 of 17) (c2=1.001, P=0.606). Body temperature did not significantly differ among the four groups after treatment. White blood cell counts and CRP were lower for patients in groups A, B, and C than group D. However, no significant differences were observed among groups A, B, and C posttreatment. Differences in bacteriological efficacy rates were significant among the four groups (c2=11.999, P=0.007). As a result, the mean teicoplanin Cmin for a successful response (18.92±5.12 mg/L) was significantly higher than for a failure (10.01±2.37 mg/L) (t=12.451, P<0.001). The correlation between the estimated probability of a successful response and teicoplanin Cmin was assessed using binary logistic regression (OR 2.038, 95% CI 1.553–2.676; P<0.001), which demonstrated that the Cmin was significantly associated with an improved clinical response to teicoplanin.
  | Table 3 Comparisons of teicoplanin clinical efficacy, n (%) |
Assessment of hepatotoxicity and nephrotoxicity
For all 113 included patients, 10.62% and 4.42% had hepatotoxicity and nephrotoxicity, respectively. Incidence rates for hepatotoxicity and nephrotoxicity are shown in Table 4, and they did not significantly differ among the four groups (P=0.859, P=0.949). Compared with baseline levels, AST, ALT, and Scr did not significantly differ after teicoplanin therapy (AST, group A, P=0.12; group B, P=0.471; group C, P=0.256; group D, P=0.323; ALT, group A, P=0.251; group B, P=0.367; group C, P=0.189; group D, P=0.403; Scr, group A, P=0.546; group B, P=0.352; group C, P=0.238; group D, P=0.437). The correlation between hepatotoxicity or nephrotoxicity and covariates was assessed using binary logistic regression. As shown in Table 5, no covariates significantly affected hepatotoxicity or nephrotoxicity.
  | Table 4 Adverse-effect rates, n (%) Note: Comparisons with c2 test. |
Discussion
Microorganisms resistant to antibiotics increase the mortality, morbidity, and cost of infections. MRSA is the most frequently identified antimicrobial drug-resistant pathogen in US and Chinese hospitals.1,3,10,24 Appropriate antibiotic use can help preserve the efficacy of current antibiotics, extend their life span, and protect the public from antibiotic-resistant infections.2,3,6,7,10,28
For severe infections, loading dose should be increased to maintain the Cmin >20 mg/L according to the recommended guide.20 Quickly achieving the target Cmin helps to reduce drug resistance; however, methods for adopting the optimal individualized loading regimen should be explored for pneumonia patients with Gram-positive infections to achieve the target Cmin. In this study, we also explored the relationships among teicoplanin-loading regimens, serum Cmin, efficacy, and patient safety.
In our study, mean Cmin on the fourth day differed significantly among the four groups (F=7.849, P<0.001; Table 2); however, it did not differ between group A and group B (P=0.095), group B and group C (P=0.477), or group A and group C (P=0.499). The proportion of patients who achieved the target range (15–30 mg/L) on day 4 was higher in groups A, B, and C than in group D (Table 2). Therefore, a high-loading-dose regimen is necessary to achieve the target Cmin range. This is consistent with previous research.9,14,15,17,20,21,23,27,29 All patients were divided into groups based on Clcr and loading regimens. As a result, ratios of the 3-day loading dose to Clcr and Cmin were significantly correlated (R=0.59, P<0.001; Figure 4). This illustrated that a high loading regimen helped to achieve a high Cmin with normal renal function.
Previous findings implied that a high Cmin might be associated with better outcomes.15,16,18,21,30 In this study, the mean teicoplanin Cmin of the 75 patients who had a successful response (clinical cure; 18.92±5.12 mg/L) was significantly higher than that of the 38 patients who got a failed response (10.01±2.37mg/L; P<0.001). Cmin was significantly associated with an improved clinical response to teicoplanin using binary logistic regression (P<0.001).
In general, patients with teicoplanin Cmin >60 mg/L had high incidence of hepatotoxicity and nephrotoxicity.12,14,17,21,23,31 In this study, hepatotoxicity and nephrotoxicity incidence rates did not differ significantly among the four groups (group A, 11.11%, 5.56%; group B, 6.67%, 3.33%; group C, 11.76%, 5.88%; group D, 13.33%, 3.33%; P=0.859, P=0.949) (Table 4). Correlations between hepatotoxicity/nephrotoxicity and covariates were assessed using binary logistic regression. None of the covariates significantly affected hepatotoxicity or nephrotoxicity (Table 5). In other words, the Cmin was not correlated with hepatotoxicity or nephrotoxicity. In our study, no patients had Cmin >40 mg/L. Therefore, it could be inferred that the Cmin range in the loading regimen was not associated with hepatotoxicity or nephrotoxicity based on the Clcr. Therefore, teicoplanin is suggested as safe for patients infected with Gram-positive bacteria if Cmin <30 mg/L.
Conclusion
This study evaluated the relationships among teicoplanin-loading regimen, Cmin, clinical efficacy, and patient safety. The Cmin on day 4 was significantly associated with an improved clinical response to teicoplanin. A loading dose of 400 mg at 12-hour intervals three to six times is needed to achieve the early target range (15–20 mg/L) and improve clinical efficacy. Therefore, therapeutic drug monitoring is recommended to ensure teicoplanin Cmin is maintained within the target therapeutic range. Based on the day 4 Cmin, the maintenance dose was adjusted to achieve the optimal Cmin and clinical efficacy and to avoid hepatotoxicity or nephrotoxicity. It is urgently necessary to revise the drug label on the recommended regimen to achieve satisfactory clinical efficacy.
Acknowledgments
The authors are grateful to all members of the Translational Medicine Center and the Department of Respiratory Medicine at Zhengzhou Central Hospital for assistance with therapeutic drug monitoring, blood samples, and clinical data collection. This work was funded by the Health and Family Planning Commission of Henan Province (201602333) as part of our routine clinical work.
Disclosure
The authors report no conflicts of interest in this work.
References
Qu JM, Cao B. [Guidelines for the diagnosis and treatment of adult community acquired pneumonia in China (2016 edition)]. Zhonghua Jie He He Hu Xi Za Zhi. 2016;39:241–242. Chinese. | ||
Maudsley J, Stone SP, Kibbler CC, et al. The community prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in older people living in their own homes: implications for treatment, screening and surveillance in the UK. J Hosp Infect. 2004;57:258–262. | ||
Nathwani D, Morgan M, Masterton RG, et al. Guidelines for UK practice for the diagnosis and management of methicillin-resistant Staphylococcus aureus (MRSA) infections presenting in the community. J Antimicrob Chemother. 2008;61:976–994. | ||
Wilke M, Petrik C, Weber B, Kloss S. Treatment of MRSA pneumonia: economical and clinical comparison of linezolid verse vancomycin. Value Health. 2014;17:A669. | ||
Bettiol E, Wetherington JD, Schmitt N, Harbarth S. Challenges and solutions for clinical development of new antibacterial agents: results of a survey among pharmaceutical industry professionals. Antimicrob Agents Chemother. 2015;59:3695–3699. | ||
Welte T, Pletz MW. Antimicrobial treatment of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) pneumonia: current and future options. Int J Antimicrob Agents. 2010;36:391–400. | ||
Lin SH, Lai CC, Tan CK, Liao WH, Hsueh PR. Comparative efficacy of vancomycin and teicoplanin in the treatment of hospitalised elderly patients with persistent methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia. Int J Antimicrob Agents. 2011;37:179–181. | ||
Kuehn BM. IDSA creates MRSA treatment guideline. JAMA. 2011;305:768–769. | ||
Thiessen K, Lloyd AE, Miller MJ, Homco J, Gildon B, O’Neal KS. Assessing guideline-concordant prescribing for community-acquired pneumonia. Int J Clin Pharm. 2017;39:674–678. | ||
Ekren PK, Ranzani OT, Ceccato A, et al. Evaluation of the 2016 Infectious Diseases Society of America/American Thoracic Society Guideline Criteria for Risk of Multi-drug Resistant Pathogens in Hospital-acquired and Ventilator-associated Pneumonia Patients in the Intensive Care Unit. Am J Respir Crit Care Med. Epub 2017 Sep 13. | ||
Vas KE, Török A, Cordoş B, Vancea S, Brassai A, Székely E. Effect of teicoplanin on Staphylococcus aureus with heterointermediate susceptibility to glycopeptides in experimental infective endocarditis model. J Chemother. 2016;28:446–449. | ||
Yoon YK, Park DW, Sohn JW, et al. Multicenter prospective observational study of the comparative efficacy and safety of vancomycin versus teicoplanin in patients with health care-associated methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2014;58:317–324. | ||
Peng Y, Ye X, Li Y, et al. Teicoplanin as an effective alternative to vancomycin for treatment of MRSA infection in Chinese population: a meta-analysis of randomized controlled trials. PloS One. 2013;8:e79782. | ||
Bugano DD, Cavalcanti AB, Goncalves AR, Almeida CS, Silva E. Cochrane meta-analysis: teicoplanin versus vancomycin for proven or suspected infection. Einstein (Sao Paulo). 2011;9:265–282. | ||
Kato H, Hamada Y, Hagihara M, et al. Retrospective study of teicoplanin loading regimen that rapidly achieves target 15-30 μg/mL serum trough concentration. J Infect Chemother. 2016;22:308–313. | ||
Hiraki Y, Yasumori N, Nagano M, et al. Optimal loading regimen and achievement of trough concentrations for teicoplanin using Japanese population parameters. Int J Antimicrob Agents. 2015;45:87–88. | ||
Seki M, Yabuno K, Miyawaki K, Miwa Y, Tomono K. Loading regimen required to rapidly achieve therapeutic trough plasma concentration of teicoplanin and evaluation of clinical features. Clin Pharmacol. 2012;4:71–75. | ||
Ueda T, Takesue Y, Nakajima K, et al. Enhanced loading regimen of teicoplanin is necessary to achieve therapeutic pharmacokinetics levels for the improvement of clinical outcomes in patients with renal dysfunction. Eur J Clin Microbiol Infect Dis. 2016;35:1501–1509. | ||
Kobayashi R, Otomo S, Shiba Y, Ebinuma K, Sudoh T. Improvement of predictivity of teicoplanin serum trough concentrations at steady state calculated by vancomycin pharmacokinetic parameter. Yakugaku Zasshi. 2016;136:1557–1562. | ||
Nakamura A, Takasu O, Sakai Y, et al. Development of a teicoplanin loading regimen that rapidly achieves target serum concentrations in critically ill patients with severe infections. J Infect Chemother. 2015;21:449–455. | ||
Byrne CJ, Egan S, Fennell JP, et al. Teicoplanin use in adult patients with haematological malignancy: exploring relationships between dose, trough concentrations, efficacy and nephrotoxicity. Int J Antimicrob Agents. 2015;46:406–412. | ||
Sipahi OR, Arda B, Yurtseven T, et al. Vancomycin versus teicoplanin in the therapy of experimental methicillin-resistant Staphylococcus aureus (MRSA) meningitis. Int J Antimicrob Agents. 2005;26:412–415. | ||
Dong YL, Dong HY, Hu SS, et al. An assessment of teicoplanin use and monitoring serum levels in a Chinese teaching hospital. Int J Clin Pharmacol Ther. 2011;49:14–22. | ||
Cao B, Huang Y, She DY, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Respir J. Epub 2017 Jul 29. | ||
Guidance for Clinical Trials of Anti-bacterial Drugs released by the Centre for Drug Evaluation China Food and Drug Administration.Zhongguo Yao Xue Hui. 2014;13. Available from: http://www.cde.org.cn/zdyz.do?method=largePage&id=244. Accessed December 21, 2017. | ||
Huang Y, Huang LF. [Implementation of a new guideline to minimize mistakes in the diagnosis and treatment of severe community acquired pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2016;39:243–245. Chinese. | ||
Mimoz O, Rolland D, Adoun M, et al. Steady-state trough serum and epithelial lining fluid concentrations of teicoplanin 12 mg/kg per day in patients with ventilator-associated pneumonia. Intensive Care Med. 2006;32:775–779. | ||
Chastre J, Blasi F, Masterton RG, Rello J, Torres A, Welte T. European perspective and update on the management of nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin Microbiol Infect. 2014;20:19–36. | ||
Byrne CJ, Roberts JA, McWhinney B, et al. Variability in trough total and unbound teicoplanin concentrations and achievement of therapeutic drug monitoring targets in adult patients with hematological malignancy. Antimicrob Agents Chemother. 2017;61:e02466. | ||
Bassetti M, Baguneid M, Bouza E, Dryden M, Nathwani D, Wilcox M. European perspective and update on the management of complicated skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin Microbiol Infect. 2014;20:3–18. | ||
He LX, Pan J, Chen SY, Wang AX, Xie CM, Shen ZY. [Clinical study of teicoplanin in the treatment of patients with Gram-positive cocci: the Chinese experience]. Zhonghua Nei Ke Za Zhi. 2005;44:337–341. Chinese. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.