Back to Journals » International Journal of General Medicine » Volume 17
Retinoblastoma Incidence in Taiwan Over a Recent 20-Year Period: A Comprehensive Nationwide Study
Authors Lin HY , Ho CH , Lin YS, Kuo SC, Chen YC , Cheng YJ
Received 14 December 2023
Accepted for publication 23 February 2024
Published 8 March 2024 Volume 2024:17 Pages 909—917
DOI https://doi.org/10.2147/IJGM.S452277
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hsin-Ying Lin,1 Chung-Han Ho,2,3 Yu-Shiuan Lin,1 Shu-Chun Kuo,1,4 Yi-Chen Chen,2 Yung-Jen Cheng5
1Department of Ophthalmology, Chi Mei Medical Center, Tainan, Taiwan; 2Department of Medical Research, Chi Mei Medical Center, Tainan, Taiwan; 3Department of Information Management, Southern Taiwan University of Science and Technology, Tainan, Taiwan; 4Department of Optometry, Chung Hwa University of Medical Technology, Tainan, Taiwan; 5Department of Radiation Oncology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
Correspondence: Yung-Jen Cheng, Department of Radiation Oncology, National Cheng Kung University Hospital, No. 138, Sheng Li Road, Tainan, 704, Taiwan, Email [email protected]
Purpose: Continuous advancements in medical diagnostic technology and the growing availability of resources suggest a potential for fluctuations in the incidence rate of retinoblastoma (Rb). This study aimed to analyze incidence data of Rb patients in Taiwan from 1999 to 2018, utilizing the nationwide Taiwan Cancer Registry (TCR) database. Additionally, we investigated the treatment modalities used for these Rb patients and compared them with those observed in other countries.
Patients and Methods: We conducted a retrospective cohort study utilizing data from the TCR database. The study cohort comprised individuals who were newly diagnosed with Rb between 1999 and 2018. The incidence of Rb was calculated as the number of patients with Rb per million live births, both for the entire population and for different gender groups and time periods. The trends in Rb incidence from 1999 to 2018 across various age groups and sexes were presented with the linear trend test.
Results: From 1999 to 2018, a total of 248 cases of Rb were identified. The overall incidence rate over this 20-year period was 60.20 cases per million live births, corresponding to 1 case per 16,611 live births. Incidence rates for each 5-year period between 1999 and 2018 exhibited no significant differences. The study cohort was predominantly male, with 134 cases (54.03%) being males and 114 cases (45.97%) being females, resulting in an overall male-to-female sex ratio of 1.18. Females had lower relative risk than males (RR: 0.92, 95% CI: 0.72– 1.19). Primary surgical intervention was the preferred treatment modality for over 75% of the cases.
Conclusion: This retrospective epidemiology study, using TCR from 1999 to 2018, indicated that no discernible trend of retinoblastoma incidence in Taiwan. Nevertheless, continuous monitoring of incidence rates and exploration of treatment strategies for retinoblastoma within the Taiwanese population are important to address potential changes in developing medical practices.
Keywords: retinoblastoma, incidence rate, treatment outcome, Taiwan cancer registry
Introduction
Retinoblastoma (Rb), the most prevalent intraocular cancer in children, has the potential to affect one or both eyes. Failure to treat or delay in diagnosing retinoblastoma can result in blindness or even pose a life-threatening risk. The incidence rate of Rb, ranging from 1 per 13,000 to 1 per 24,000 live births, has been reported in various countries worldwide.1–17 In Taiwan, a retrospective study revealed an incidence rate of 1 per 21,691 live births for Rb during the era of 1980s to 2000s.18 With the continuous advancements in medical diagnostic technology and the availability of more resources, the incidence rate of Rb may potentially undergo fluctuations.
The nationwide Taiwan Cancer Registry database (TCR), which has been organized by the Health Promotion Administration, Ministry of Health and Welfare in Taiwan for over 30 years, recruits over 97% of newly diagnosed cancer and has significantly contributed to the foundation support for academic cancer research and most of the cancer control policies in Taiwan.19 This database, in comparison to the National Health Insurance Research Database (NHIRD), provides a more comprehensive array of information regarding cancer-specific data, including cancer staging and detailed information on the initial treatment modalities.
Utilizing the TCR, this study aimed to analyze the most current incidence data for Rb patients in Taiwan from 1999 to 2018. Furthermore, we sought to investigate the treatment modalities utilized for Rb patients and compare these treatment decisions with those made in other countries.
Materials and Methods
Data Source
Taiwan Cancer Registry (TCR) from the Health and Welfare Data Science Center (HWDC) were used in this study. TCR is a population-based cancer registry database that has systematically collected cancer-related information since 1979. The TCR presents high-quality data in terms of completeness and timeliness.20 The diagnosis codes in the TCR were categorized according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) before 2001 and the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) after 2002. While the TCR maintained high-quality data, the accuracy rates of specific treatment-related information may be affected by the variations in cancer types and the expertise of registrars.20,21 For research purposes, HWDC, an integrated data center, supplied relevant information from various health databases, including TCR. For safeguarding personal data protection, HWDC plays a crucial role in effectively managing de-identified patient data. This study was conducted in compliance with the Declaration of Helsinki and received an exemption from review by the Institutional Review Board of the Chi-Mei Medical Center due to the utilization of de-identification data (IRB number: 11103-E01).
Selection of Patients
Using the TCR database, patients diagnosed with ICD-9-CM code (190.5) or ICD-10-CM code (C6920, C6921, C6922) between 1999 and 2018 were screened. A total of 620 patients were identified through coding based on the histology type of retinoblastoma (9510 Retinoblastoma, NOS; 9511 Retinoblastoma, differentiated; 9512 Retinoblastoma, undifferentiated & 9513 Retinoblastoma, diffuse). The exclusion criteria for this study included individuals with birth dates prior to 1999 or any missing data during the study period. The cohort selection process is illustrated in a flow diagram for the selection of study subjects in Figure 1.
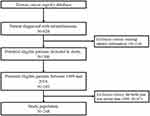 |
Figure 1 The patients’ selection of the study. |
Statistical Analysis
In this study, descriptive statistics were used to summarize the baseline characteristics of the patients, including age, sex, and treatment types. The documentation of treatment types includes the initial treatments, which could be a combination, recorded when multiple treatment methods were simultaneously employed. The incidence of retinoblastoma was calculated as the number of patients with retinoblastoma per million live births, both for the overall population and within different sexes and time periods. Relative risks with 95% confidence intervals were estimated using Poisson regression, a statistical method particularly well suited for modeling rare events, such as the occurrence of retinoblastoma in our study. This approach provides a robust framework for analyzing incidence rates and estimating relative risks, especially when dealing with time-to-event or count data. Therefore, Poisson regression could be used to estimate the association between independent variables and the incidence of retinoblastoma with suitable interpretability. In addition, the trends in retinoblastoma incidence from 1999 to 2018 across various age groups and sexes were presented using the Joinpoint regression model to estimate the critical point and the trend changes.22 The average annual percentage change (APC) reflects the increasing or decreasing rates over a defined time period. Consistent with a previous study,23 the terms “increase” or “decrease” signify that the APC is significantly different from zero. All statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC, USA), and statistical significance was set as a p-value of less than 0.05.
Results
Diagnostic Age Distribution
The demographic information for the study group was outlined in Table 1. From 1999 to 2018, a total of 248 cases of Rb were identified, with none exceeding a diagnostic age of 10 years. Among these cases, 183 (73.79%) patients had a diagnostic age of less than 2, while 65 (26.21%) had a diagnostic age of 2 or older. The majority of cases were diagnosed at the age of 0–1. As age increased, the number of diagnoses decreased (Figure 2).
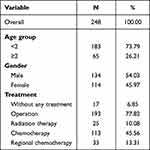 |
Table 1 Demographic Information of Retinoblastoma in Taiwan During 1999 to 2018, Total Candidate Participants |
 |
Figure 2 Number of cases and ratio between sex by diagnostic age in Taiwan during 1999 to 2018, total candidate participants. |
Trend of Incidences During 1999–2018
The overall incidence rate over this 20 years period was 60.20 per million live births (Table 2), which corresponded to 1 per 16,611 live births. The incidence rates among males and females were 62.44 and 57.75 per million live births, respectively. Although females presented a slightly lower relative risk of 0.92 (95% CI: 0.72–1.19) compared to males, the difference did not reach statistical significance. The incidence rates for each 5-year period between 1999–2018 exhibited no significant differences, ranging from 51.49 to 72.04 per million live births. While no statistically significant disparities were observed in comparisons with the reference birth year of 1999–2003, it is worth noting that individuals born during 2009–2013 displayed a higher relative risk (RR: 1.36; 95% CI: 0.97–1.19), and those born in 2014–2018 exhibited a reduced relative risk of retinoblastoma (RR: 0.97; 95% CI: 0.87–1.40). Furthermore, Figure 3A indicated that the significant increasing APC were observed in the trends of retinoblastoma (Rb) incidence over the 20-year period (APC: 5.56; 95% CI: 2.54–8.67, p < 0.05). The incidence trend within specific age groups also demonstrated significant increases, with an APC of 3.27 (95% CI = 0.52–6.09, p < 0.05) for the age group less than 2 years old, and an APC of 6.11 (95% CI = 0.91–11.68, p < 0.05) for the age group larger and equal 2 years old (Figure 3B). However, although the APC indicated increasing trends in different sexes, the significance was observed only in females (APC: 7.34; 95% CI: 4.23–10.61, p < 0.05), with no significant APC noted in males (APC: 2.59; 95% CI: −3.52–9.02, p > 0.05) (Figure 3C).
 |
Table 2 Incidence of Retinoblastoma in Taiwan During 1999 to 2018, Total Candidate Participants (N = 248) |
 |
Figure 3 Incidence Trend of Retinoblastoma from 1999 to 2018. (A). Overall; (B). different age population; (C). different sex population; *p<0.05. |
Dynamic Diagnostic Age-Specific Sex Ratio Across Ages
The population was predominantly male, comprising 134 (54.03%) males and 114 (45.97%) females. The overall male-to-female sex ratio was 1.18 (Table 1). Aside from the age-specific rates in the age 3 group being the same for male and female, the age-specific sex ratio increased from 0.94 for ages younger than 1 year to 0.67 for ages above 4 years (Figure 2).
Treatment Selection
Over 75% of cases underwent surgical intervention as the primary treatment modality. Around 10% of cases received radiation therapy, while approximately 46% and 13% opted for chemotherapy and regional chemotherapy, respectively. Approximately 6.9% of cases received no treatment (Table 1).
Discussion
While Rb constitutes less than 2% of all pediatric cancers, its potential for devastating outcomes underscores the necessity of early detection and management. Our research aimed to provide a reliable estimation of the Rb incidence rate by conducting a nationwide population-based analysis to minimize possible selection bias.
The present study revealed an Rb incidence rate in Taiwan from 1999 to 2018 of 1 per 16,611 live births. This rate is consistent with data from older publications in various countries, such as the Netherlands (1 per 17,000 from 1862 to 1995),2 Northern Europe (1 per 16,642 from 1958 to 1998),4 Kenya (1 per 17,030 from 2006 to 2007),6 and Korea (1 per 16,938 from 1993 to 2010).9 Recent data also aligns, including Poland (1 per 20,561 from 2010 to 2017),12 Finland (1 per 16,130 from 1964 to 2014),13 and a range of 1 per 18,000–24,000 live births in the USA.10,24 However, it is lower than a study from 40 European countries during only the 2017 timeframe (1 per 13,844), where potential bias might be introduced due to its method of data collection and short duration.14 Furthermore, the 40-year analysis study conducted in the USA revealed no statistically significant trend in incidence rates across all racial and gender groups.10 This stability has likewise been observed in other countries.1,14,25 Similarly, our study demonstrated no discernible trend in incidence over the 20-year period, including subgroups based on gender and age.
Over 70% of Rb cases in this study were diagnosed before the age of two, with a male predominance observed across nearly all age groups. The majority of Rb cases diagnosed at such a young age aligns with data from other countries (80% before three years of age).5,11 However, disparities in sex ratios were noted among different countries. In most studies, the incidence rate of Rb was similar between the sexes,9,12,25 while a study in Lebanon showed the incidence was nearly twice as high in girls than in boys.11 A study conducted in Latin America reported no sex difference in retinoblastoma incidence among patients under 15 years old. However, in the age group over 14, females exhibited a higher incidence rate.23 Consistent with our study, a higher rate was also observed in males in the US Surveillance, Epidemiology, and End Results (SEER) Program, despite no clear explanation for this finding.24 The age-dependent increase in the male-to-female rate ratio indicated the necessity for further investigation into potential genetic or cultural factors influencing retinoblastoma occurrence.
Given that the only known cause of Rb is a mutation in the RB1 tumor suppressor gene on chromosome 13, initially caused by either a hereditary or sporadic mutation, there exists a 50% likelihood of transmitting this mutation to the subsequent generation in the hereditary form. Conversely, in the sporadic form, the mutation cannot be passed on to the next generation. Therefore, Rb genetics should theoretically results in an even distribution between males and females. The gender disparities observed could possibly be attributed to a relatively small population size and potentially influenced by cultural behaviors within patrilineal societies, which prioritize sons, particularly evident in Taiwan.26
A previous population-based study on Rb incidence in Taiwan utilized the Taiwan NHIRD.27 The NHIRD comprehensively covers inpatient and outpatient claims of various diseases based on ICD coding, providing extensive information on demographic data, clinical visit dates, prescription details and associated costs. However, this study utilized the TCR database, a more cancer-focused database established for surveying the incidence of cancer, capturing over 97% of newly diagnosed cancer cases in Taiwan. This database offers a wealth of cancer-specific data, including cancer staging and detailed insights into initial treatment modalities. The overall Rb incidence in the NHIRD study was 1 in 17,373 live births without a notable trend over the 1998–2011 period. With a more contemporary period analysis of the cohort, our data were found to be consistent with the findings of this previously published study.27 In their study, the incidence rates based on gender were 65.81 and 48.53 per million live births for males and females, respectively. The other Taiwan’s study with study periods from 1978 to 2015 also indicated that males had higher incidence rate than females.28 In our study, the corresponding rates were 62.44 and 57.75 per million live births for males and females.
Notably, our study revealed that 77.8% of newly diagnosed Rb cases underwent surgical intervention as their first treatment choice during the study period. Approximately 10% received radiation therapy, 45.6% underwent chemotherapy, while 13.3% opted for regional chemotherapy as their initial treatment preference. In TCR, regional chemotherapy was typically coded when a patient received localized administration of chemotherapeutic agents, such as Intra-vitreal chemotherapy or intra-arterial chemotherapy, which is distinct from conventional intravenous chemotherapy. The observed surgical intervention rate was relatively higher compared to other studies.3,12 This could be attributed to the convenience of seeking medical care in Taiwan and the comprehensive coverage of national health insurance implemented by the Taiwan’s government to improve population healthcare. Particularly, surgery was deemed the most effective curative method. The ease of accessing medical care and the overall cost-effectiveness associated with surgery interventions may contribute to the observed higher rates of surgeries in Taiwan. A report from a practically large, heterogeneous, real-world population with RB, including a total of 2854 eyes of 2097 patients from 18 ophthalmic oncology centers across 13 countries over 6 continents, demonstrated a primary enucleation rate of 41.3%.29
However, comparing surgical rates among different studies can be challenging due to the complex decision-making process regarding performing enucleation or not. This complexity is usually influenced by various factors, including diverse clinical situations, institutional resources, and considerations related to patients’ psychosocial factors and cultural beliefs. Furthermore, the limited consensus on how to classify and treat advanced tumors, with at least three different versions of the commonly used International Intraocular Retinoblastoma Classification (IIRC) system, has posed significant challenges.30 A recently published international survey highlighted the lack of consensus in classification schemes, which can confound comparisons. The survey results demonstrated substantial variation in enucleation rates among centers worldwide. Additionally, when considering geographic regions, primary enucleation rates were significantly higher in the Latin America (57%) and Asia (40%) compared to Europe (36%), Africa (10%) and the US (8%) regions.31
Seventeen cases in our study group were recorded as receiving no treatment. However, it was plausible that some of these cases sought medical care abroad or even informal treatment, rendering the ultimate treatment method unknown. In the constantly evolving landscape of Rb treatment, various alternative treatment modalities have been developing, such as intravenous chemotherapy, intra-arterial chemotherapy, intravitreal chemotherapy, intracameral chemotherapy, among others. Additionally, less commonly used treatments like cryotherapy, transpupillary thermotherapy, and radiation-based therapies still remain viable options in certain circumstances.32 The choice of treatment modality continues to vary, underscoring the need for further collaboration and the development of a more unified treatment protocol based on a consensus classification in the future.
The consistent incidence trend observed over this 20-year period and the predominant occurrence of Rb cases at a young age in our country align with data reported in other developed countries.23 This indicates the effectiveness of the current screening policy and healthcare accessibility in Taiwan, especially given the ease of medical care access and the overall affordability of treatment, as mentioned earlier. However, there is an ongoing need to continually optimize treatment policies. As previously mentioned, the emergence of reliable treatments, such as intravenous chemotherapy and intra-arterial chemotherapy, has the potential to simultaneously address organ preservation and control rates. Further studies are warranted to explore and address this question.
There are some limitations to our present study. Firstly, essential information such as family history and genetic data related to Rb could not be assessed using our database. To address this challenge, the future research should collect more data about family history and genetic data to explore the effects of Rb incidence. Additionally, the inconsistency in the classification system led to ambiguous cancer staging. Other potential risk factors, such as the extent of tumor invasion and laterality, were also unavailable for study analysis. Lastly, the relatively small sample size in this study may be due to the relatively small total population in Taiwan, despite the inclusion of data spanning nearly 20 years. Therefore, future research should consider the implementation of a multi-region database to collect more sample size for increasing the statistical power and the generalizability of findings. Nevertheless, this national recruitment data does hold clinical importance.
Conclusion
This nationwide population-based study presents updated epidemiological insights into Rb in Taiwan, revealing an incidence rate of 1 per 16,611 live births during the recent era (1999–2018). No discernible trend in incidence was observed over this 20-year period. These results show a consistent pattern and align closely with studies from various countries. Continued vigilance through nationwide population-based studies is essential for deepening the understanding and propelling the treatment of this rare Rb disease forward. Understanding the epidemiology of Rb can support ongoing monitoring. It also establishes the foundation knowledge for future research, significantly impacting disease understanding, clinical management, and patient outcomes.
Data Sharing Statement
The data sources was Taiwan Cancer Registry. The data are available with permission from the Taiwan Health and Welfare Data Science Centre (https://dep.mohw.gov.tw/DOS/cp-5119-59201-113.html, accessed on 14 Dec 2023). Restrictions apply to the availability of these data, which were used under license for this study.
Ethics Approval
This study was conducted in compliance with the Declaration of Helsinki and received an exemption from review by the Institutional Review Board of the Chi-Mei Medical Center due to the utilization of de-identification data (IRB number: 11103-E01).
Acknowledgments
The authors thank Health Data Science Center, National Cheng Kung University Hospital for providing administrative and technical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants CMFHR11119 from the Chi-Mei Medical Center and by grants NCKUH-11103011 from the National Cheng Kung University Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sanders BM, Draper GJ, Kingston JE. Retinoblastoma in Great Britain 1969-1980: incidence, treatment, and survival. Br J Ophthalmol. 1988;72(8):576–583. doi:10.1136/bjo.72.8.576
2. Moll AC, Kuik DJ, Bouter LM, et al. Incidence and survival of retinoblastoma in The Netherlands: a register based study 1862-1995. Br J Ophthalmol. 1997;81(7):559–562. doi:10.1136/bjo.81.7.559
3. Saw SM, Tan N, Lee SB, Au Eong KG, Chia KS. Incidence and survival characteristics of retinoblastoma in Singapore from 1968-1995. J Pediatr Ophthalmol Strabismus. 2000;37(2):87–93. doi:10.3928/0191-3913-20000301-07
4. Seregard S, Lundell G, Svedberg H, Kivela T. Incidence of retinoblastoma from 1958 to 1998 in Northern Europe: advantages of birth cohort analysis. Ophthalmology. 2004;111(6):1228–1232. doi:10.1016/j.ophtha.2003.10.023
5. Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975-2004. Br J Ophthalmol. 2009;93(1):21–23. doi:10.1136/bjo.2008.138750
6. Nyamori JM, Kimani K, Njuguna MW, Dimaras H. The incidence and distribution of retinoblastoma in Kenya. Br J Ophthalmol. 2012;96(1):141–143. doi:10.1136/bjophthalmol-2011-300739
7. Dean M, Bendfeldt G, Lou H, et al. Increased incidence and disparity of diagnosis of retinoblastoma patients in Guatemala. Cancer Lett. 2014;351(1):59–63. doi:10.1016/j.canlet.2014.04.023
8. Moreno F, Sinaki B, Fandino A, Dussel V, Orellana L, Chantada G. A population-based study of retinoblastoma incidence and survival in Argentine children. Pediatr Blood Cancer. 2014;61(9):1610–1615. doi:10.1002/pbc.25048
9. Park SJ, Woo SJ, Park KH. Incidence of retinoblastoma and survival rate of retinoblastoma patients in Korea using the Korean National Cancer Registry database (1993-2010). Invest Ophthalmol Vis Sci. 2014;55(5):2816–2821. doi:10.1167/iovs.14-14078
10. Fernandes AG, Pollock BD, Rabito FA. Retinoblastoma in the United States: a 40-Year Incidence and Survival Analysis. J Pediatr Ophthalmol Strabismus. 2018;55(3):182–188. doi:10.3928/01913913-20171116-03
11. El Hage S, Wakim E, Daou L, El Masri J, Salameh P. Epidemiology and Incidence of Retinoblastoma in the Middle East: a Nationwide Study in Lebanon. Cureus. 2021;13(10).
12. Nowak MS, Romanowska-Dixon B, Grabska-Liberek I, Zurek M. Incidence and Characteristics of Retinoblastoma in Poland: the First Nationwide Study 2010-2017. Int J Environ Res Public Health. 2021;18(12):6539.
13. Nummi K, Kivela TT. Retinoblastoma in Finland, 1964-2014: incidence and survival. Br J Ophthalmol. 2021;105(1):63–69. doi:10.1136/bjophthalmol-2019-315744
14. Stacey AW, Bowman R, Foster A, et al. Incidence of Retinoblastoma Has Increased: results from 40 European Countries. Ophthalmology. 2021;128(9):1369–1371. doi:10.1016/j.ophtha.2021.01.024
15. Yousef YA, Mohammad M, Al-Nawaiseh I, et al. Retinoblastoma and uveal melanoma in Jordan: incidence, demographics, and survival (2011-2020). Ophthalmic Genet. 2022;1–8.
16. Stuart KV, Shepherd DJ, Kruger M, Singh E. The Incidence of Retinoblastoma in South Africa: findings from the South African National Cancer Registry (2004-2018). Ophthalmic Epidemiol. 2022;29(6):681–687. doi:10.1080/09286586.2021.2013900
17. Darwich R, Ghazawi FM, Rahme E, et al. Retinoblastoma Incidence Trends in Canada: a National Comprehensive Population-Based Study. J Pediatr Ophthalmol Strabismus. 2019;56(2):124–130. doi:10.3928/01913913-20190128-02
18. Chen YH, Lin HY, Hsu WM, Lee SM, Cheng CY. Retinoblastoma in Taiwan: incidence and survival characteristics from 1979 to 2003. Eye. 2010;24(2):318–322. doi:10.1038/eye.2009.80
19. Chiang C-J, You S-L, Chen C-J, Yang Y-W, W-C L, Lai M-S. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Japanese J Clin Oncol. 2015;45(3):291–296.
20. Chiang C-J, Wang Y-W, Lee W-C. Taiwan’s Nationwide Cancer Registry System of 40 years: past, present, and future. J Formos Med Assoc. 2019;118(5):856–858. doi:10.1016/j.jfma.2019.01.012
21. Cheng C-Y, Chiang C-J, Hsieh C-H, Chang Y-K, Lai M-S. Is quality of registry treatment data related to registrar experience and workload? A study of Taiwan cancer registry data. JFormos Med Assoc. 2018;117(12):1093–1100. doi:10.1016/j.jfma.2017.12.012
22. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi:10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z
23. Vega-Escobar K, Bonilla-Escobar FJ, Salamanca O, et al. Epidemiology of Eye Cancer in Cali, Colombia: a 55-Year Study. Ophthalmic Epidemiol;2023. 1–11. doi:10.1080/09286586.2023.2269253
24. Wong JR, Tucker MA, Kleinerman RA, Devesa SS. Retinoblastoma incidence patterns in the US Surveillance, Epidemiology, and End Results program. JAMA Ophthalmol. 2014;132(4):478–483. doi:10.1001/jamaophthalmol.2013.8001
25. MacCarthy A, Birch JM, Draper GJ, et al. Retinoblastoma: treatment and survival in Great Britain 1963 to 2002. Br J Ophthalmol. 2009;93(1):38–39. doi:10.1136/bjo.2008.139626
26. Chu CC, Xie Y, R-r Y. Effects of sibship structure revisited: evidence from intrafamily resource transfer in Taiwan. Sociol Educ. 2007;80(2):91–113. doi:10.1177/003804070708000201
27. Li SY, Chen SC, Tsai CF, Sheu SM, Yeh JJ, Tsai CB. Incidence and survival of retinoblastoma in Taiwan: a nationwide population-based study 1998-2011. Br J Ophthalmol. 2016;100(6):839–842. doi:10.1136/bjophthalmol-2015-307211
28. Chen P-Y, Kao L-Y, Chao A-N, et al. Retinoblastoma in Taiwan: survival and clinical characteristics. Japanese J Ophthalmol. 2021;65(4):546–553. doi:10.1007/s10384-021-00836-6
29. Tomar AS, Finger PT, Gallie B, et al. A Multicenter, International Collaborative Study for American Joint Committee on Cancer Staging of Retinoblastoma: part II: treatment Success and Globe Salvage. Ophthalmology. 2020;127(12):1733–1746. doi:10.1016/j.ophtha.2020.05.051
30. Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18(1):41–53, viii. doi:10.1016/j.ohc.2004.11.003
31. Scelfo C, Francis JH, Khetan V, et al. An international survey of classification and treatment choices for group D retinoblastoma. Int j Ophthalmol. 2017;10(6):961–967. doi:10.18240/ijo.2017.06.20
32. Ancona-Lezama D, Dalvin LA, Shields CL. Modern treatment of retinoblastoma: a 2020 review. Indian J Ophthalmol. 2020;68(11):2356–2365. doi:10.4103/ijo.IJO_721_20
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
