Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Resveratrol Regulates Glucose and Lipid Metabolism in Diabetic Rats by Inhibition of PDK1/AKT Phosphorylation and HIF-1α Expression
Received 6 January 2023
Accepted for publication 7 April 2023
Published 15 April 2023 Volume 2023:16 Pages 1063—1074
DOI https://doi.org/10.2147/DMSO.S403893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Gian Paolo Fadini
Siyun Li,1 Fuzhen Feng,2 Yanhui Deng1
1Department of Pharmacy, The Third Affiliated Hospital of Southern Medical University, Guangzhou, People’s Republic of China; 2Department of Pharmacy, The Sixth Affiliated Hospital, School of Medicine, South China University of Technology, Foshan, People’s Republic of China
Correspondence: Yanhui Deng, Department of Pharmacy, The Third Affiliated Hospital of Southern Medical University, 183 West Zhongshan Road, Tianhe District, Guangzhou, 510630, People’s Republic of China, Tel +86 020 62784810, Email [email protected]
Purpose: To explore the underlying mechanism of the anti-diabetic effect of resveratrol (RSV) on regulating glycolipid metabolism in diabetic rats induced by streptozotocin (STZ) and a high-fat diet (HFD).
Methods: Male Wistar rats were randomized into three groups. Two groups were fed a high-fat diet and intraperitoneally injected with STZ (35 mg/kg), with one group also treated with RSV (30 mg/kg/d), and the third, control group was fed a normal diet. After 12 weeks, blood lipid levels and fasting blood glucose (FBG) were assessed. Histopathological changes were evaluated by hematoxylin-eosin (HE) staining and periodic acid-Schiff (PAS) staining. The protein expression of hypoxia-inducible factor 1α (HIF-1α) was assessed by Western blotting and immunofluorescence, and the proteins level of 3-phosphoinositide-dependent protein kinase 1 (PDK1), phosphorylated-PDK1 (p-PDK1), phosphorylated-protein kinase B (p-AKT), glucose transporter 1 (GLUT1) and low-density lipoprotein receptor (LDLR) in the liver were analyzed by Western blotting. The mRNA levels of Hif-1α, Glut1 and Ldlr in the liver were determined by RT-qPCR.
Results: RSV treatment significantly reduced liver/body weight ratio (L/W, P < 0.05), FBG (P < 0.01) and serum concentrations of total cholesterol (TC, P < 0.05), triglycerides (TG, P < 0.01) and low-density lipoprotein-cholesterol (LDL-C, P < 0.05) in diabetic rats. RSV also improved diabetic symptoms, attenuated liver steatosis and increased liver glycogen accumulation. RSV treatment significantly downregulated the proteins expression of p-PDK1 and p-AKT (P < 0.01) and the levels of HIF-1α (P < 0.05) and GLUT1 (P < 0.01), while significantly upregulating the level of LDLR (P < 0.05).
Conclusion: RSV was effective in improving glycolipid metabolism in diabetic rats, probably by inhibiting the PDK1/AKT/HIF-1α pathway and regulation of its downstream target levels. These findings may provide new insight into the mechanism of action of RSV in the treatment of diabetes.
Keywords: resveratrol, diabetes, glycolipid metabolism, PDK1, AKT, HIF-1α
Introduction
Type 2 diabetes mellitus (T2DM), a chronic endocrine and metabolic disease influenced by genetic and environmental factors, has become a severe issue worldwide because of its rapidly increasing rates and accompanying economic and social burdens.1 The pathogenesis of this disease is complicated, including autophagy, oxidative stress, metabolic disorders with sustained insulin resistance, inflammation, hypoxia and other factors. Metabolic disorders are regarded as risk factors for the occurrence and development of T2DM, as metabolic disorders can exacerbate inflammatory reactions, obstruct the interaction of insulin receptors with glucose transporters, and compromise β-cell function. Additionally, insulin resistance, a characteristic of irregular glucose metabolism, can increase the concentrations of free fatty acids (FFA) and TG in serum lipids, resulting in dyslipidemia.2 Although these findings suggest interactions between glucose metabolism and lipid metabolism, the underlying mechanisms remain unclear.3
The AKT pathway has been found to regulate glycolipid metabolism, suggesting that this pathway may be a target in the treatment of diabetes.4 This pathway is initiated by the phosphorylation of AKT by PDK1 which acts upstream of AKT, thereby enhancing glucose uptake and utilization.5 Hypoxia-inducible factors (HIFs), which are composed of an oxygen-sensitive α-subunit and a constitutively expressed β-subunit, are critical to regulating adaptive responses to hypoxia. Additionally, the α-subunit, which is rapidly metabolized by the proteasome under normoxic conditions, is stabilized under hypoxic conditions.6 HIF-1α is a downstream target of the AKT pathway that is involved in cancer, including in cancer-associated inflammation, mitochondrial metabolism and angiogenesis.7 Moreover, activation of AKT signaling was found to increase the rate of HIF-1α synthesis.8
In addition to being involved in cancer, the HIF-1α pathway has been shown to be important in metabolic diseases.9–11 HIF-1α overexpression in adipose tissue was found to increase weight and liver lipid content while impairing glucose tolerance in mice fed a high-fat diet (HFD).12 In addition, hyperglycemia prevents the stabilization of HIF-1α, with HIF-1α in hepatocytes having a protective function during the development of T2DM.13 However, the underlying mechanism that HIF-1α influences diabetes is still unclear.
In addition, the hypoglycemic agents used to treat diabetes such as metformin, glucagon-like peptide-1 receptor agonists, sodium-glucose cotransport protein 2 inhibitors and so on, have been limited in clinical use by some deficiencies and side effects. Furthermore, most hypoglycemic agents generally have no lipid-regulating effects. Resveratrol (RSV), an essential active ingredient in many medicinal plants, is a bioactive compound with anti-inflammatory, anti-oxidative, anti-cancer and anti-diabetic properties.14 A clinical trial on breast cancer encouragingly showed that RSV was safe and well tolerated and another clinical trial on T2DM showed that oral supplementation of 250mg/d RSV for 3 months was effective in improving glycemic control, TC and LDL-C.15 Additionally, RSV has been shown to protect mice with HFD-induced obesity from aberrant glucose metabolism and inflammatory responses in visceral white adipose tissue by increasing the expression of p-AKT,16 suggesting that RSV might activate AKT in the liver to regulate metabolism. However, to our knowledge, no study to date has assessed whether the ability of RSV to regulate glycolipid metabolism in T2DM is related to PDK1/AKT phosphorylation, and none has evaluated the association between RSV and HIF-1α in T2DM. The present study therefore evaluated the ability of RSV to improve glycolipid metabolism in diabetes through the PDK1/AKT/HIF-1α pathway and its downstream genes. The result of this study may help design new strategies involving RSV for the treatment of T2DM.
Materials and Methods
Chemicals and Reagents
Streptozotocin (STZ) was acquired from Sigma Corporation (St Louis, MO, USA). Resveratrol (purity>99%, HPLC) was procured from Shanghai Winherb Medical Technology Co., Ltd. (Shanghai, China) and dissolved at a concentration of 3mg/mL in 0.5% carboxymethyl cellulose sodium (CMC-Na). And the rats in the DR group were administered at a dose of 30 mg/kg/d.17,18 The primary antibodies against PDK1 (3062S), p-PDK1 (S241, 3438T), AKT1 (C73H10), p-AKT (Ser473, 4058S) and GLUT1 (D3J3A) were from Cell Signaling Technology (Beverly, MA, USA). Antibodies against HIF-1α (YT2133), LDLR (ab30532) and β-actin (GB12001) were purchased from Immunoway (Plano, TX, USA), Abcam (Cambridge, United Kingdom) and Servicebio (Wuhan, China), respectively. Alexa Fluor 594 goat anti-Rabbit IgG secondary antibody (A-11012) was from Invitrogen (Waltham, MA, USA). DAPI (F6057) was from Sigma-Aldrich (Darmstadt, Germany). Horseradish peroxidase-conjugated secondary antibodies, RIPA lysis buffer, protease inhibitor, phosphatase inhibitor, loading buffer, primary antibody dilution buffer and Dura ECL kit were supplied by FDbio science (Hangzhou, China). TRIzol reagent (9108) and PrimeScriptTM RT Master Mix (Perfect Real Time) Kit (RR036A) were from Takara (Shiga, Japan), and ChamQ SYBR qPCR Master Mix (High ROX Premixed, Q341-02) was from Vazyme (Nanjing, China).
Animal Models and Experimental Design
All animal procedures were conducted in accordance with the China Animal Welfare Legislation and were approved by the Southern Medical University Animal Ethics Committee (Guangzhou, China, No. L2020066). The five-week-old male Wistar rats, weighing 110–140g, were obtained from the Laboratory Animal Centre of Southern Medical University. The rats were housed in a specific pathogen-free (SPF) animal laboratory at a temperature of 22–26 °C and a relative humidity of 45±5% with light/dark alternating every 12 h. After 3 days of adaptive feeding, one group of 12 rats (normal control [NC]) was fed a standard chow diet containing 13% fat, 63% carbohydrate and 24% protein (Beijing KAXL FEED, No. 1016706476803973120), and the other 24 rats were fed an HFD containing 45% fat, 35% carbohydrate and 20% protein (Beijing HFK, No.1236463) for 4 weeks. After 12-h fasting, HFD-fed rats were intraperitoneally injected with STZ (35 mg/kg),19 freshly prepared in 0.1 M cold citrate buffer (pH 4.5), whereas rats in the NC group were administered an equal volume of sodium citrate buffer. Rats with FBG concentrations > 16.7 mmol/L within 72 h after STZ injection were considered diabetic. FBG was measured after tail-tip blood sampling using a blood glucose meter (Accu-Chek Performa, Roche, GER).
Then diabetic rats were divided into 2 groups. Both groups were fed an HFD for 12 weeks, with one group of 12 rats receiving RSV (30 mg/kg/d) dissolved in 0.5% CMC-Na via oral gavage for 6 d every week for 12 weeks (DR group) and the other group of 12 rats (DM group), as well the 12 rats in the NC group, receiving an equal volume of 0.5% CMC-Na for 12 weeks. The dosage of RSV in our study was converted according to the effective dosage in the clinical practice (250mg/d)15,20,21 and also further determined by some references about animal studies.17,18 Body weight (BW) was measured weekly and FBG was measured twice weekly. After 12 weeks, the rats were fasted overnight, weighed and sacrificed under anesthesia to harvest liver tissues and collect blood samples from the abdominal aorta. The detailed schematic representation of the study design was shown in Figure 1.
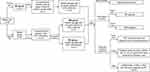 |
Figure 1 Flow chart of study design. |
Biochemical Analysis of Blood Samples
Blood samples were sent to the Department of Laboratory Medicine of the Third Affiliated Hospital of Southern Medical University for analysis. Serum concentrations of TC, TG, LDL-C and high-density lipoprotein-cholesterol (HDL-C) were measured by an automatic biochemical analyzer.
Pathological Analysis
Isolated liver tissues were weighed, cut into parts, rapidly fixed in 4% paraformaldehyde and embedded in paraffin at 4-μm thickness-embedded slices for hematoxylin-eosin (HE) and periodic acid-Schiff (PAS) staining using standard protocols. Images of stained tissue samples were taken under a microscope (Model BX43, Olympus, Japan). Other tissue samples were flash-frozen in liquid nitrogen and stored at −80 °C.
Immunofluorescence Assays
After deparaffinization and rehydration, sections of liver tissue were microwaved in Tris-EDTA buffer (pH 9.0) for antigen retrieval, blocked with 5% goat serum, permeabilized with Triton X-100 and incubated overnight at 4°C with primary anti-HIF-1α antibody (1:200). The slides were washed three times for 10-min each in TBST and incubated in the dark at 37 °C for 1 h with Alexa Fluor 594 secondary antibody (1:500). The samples were stained with DAPI and visualized at ×400 magnification using a fluorescence microscope and FV10-ASW software.
Quantitative Real-Time PCR Assays
Total RNA (2 μg) was extracted from liver tissues using TRIzol reagent, with the resulting RNA reverse transcribed to cDNA using PrimeScriptTM RT Master Mix Kit. Specific primers were synthesized by Tsingke Biotechnology (Beijing, China) (Table 1). RT-qPCR was performed in 20 μL reaction volumes including cDNA, ChamQ SYBR qPCR Master Mix and specific primers by the StepOne Plus Real-Time System. The expression of each gene was quantified based on its amplification cycle threshold (Ct) and normalized to that of β-actin mRNA using the formula 2−(ΔΔCt).
 |
Table 1 Specific Primer Sequences for Quantitate Levels of Gene Expression |
Western Blotting Assays
Liver tissues (20 mg) were homogenized on ice in RIPA lysis buffer containing protease and phosphatase inhibitors. Total proteins were extracted by centrifugation and quantified with a BCA kit (Thermo, USA, 23227). Protein samples were denatured for 5 min by boiling in loading buffer. Equal amounts of protein samples were separated by 10% SDS-PAGE (Beyotime, China, P0012A) and transferred to nitrocellulose membranes. The membranes were incubated for 1 h at room temperature in 5% non-fat dry milk and bovine serum albumin (BSA) dissolved in 1 × TBST at room temperature for 1 h to block non-specific binding. The membranes were subsequently incubated overnight at 4°C with primary antibodies against PDK1 (1:1000), p-PDK1 (1:1000), AKT (1:1000), p-AKT (1:1000), HIF-1α (1:500), GLUT1 (1:1000), LDLR (1:200) and β-actin (1:2000). The membranes were washed three times for 10 min each in 1 × TBST and incubated for 1 h at room temperature with HRP-labeled secondary antibody diluted in 5% non-fat dry milk at room temperature. Protein bands were visualized using an ECL kit and a chemiluminescence capture system and quantified using Image J software.
Statistical Analysis
Data were expressed as mean ± standard deviation (SD) and analyzed by one-way ANOVA followed by the LSD t-test. All statistical analyses were performed using GraphPad Prism Software (version 7), with P values < 0.05 considered statistically significant.
Results
RSV Improved General Characteristics and Blood Glucose and Lipid Levels
To determine the contribution of RSV to the overall improvement in diabetes, BW, L/W, FBG, TC, TG, LDL-C and HDL-C were measured. Compared with NC rats, rats in the DM group showed a significant decrement in BW, especially within 2 weeks of STZ injection (Figure 2A, P < 0.05). Following STZ injection, rats had an FBG >16.7 mmol/L, with no statistically significant differences between the DM and DR groups (Figure 2B, P>0.05). After 12 weeks, animals in the DM group showed significant increments in L/W and concentrations of FBG, TC, TG, and LDL-C compared with the NC group (P < 0.01) and a significant reduction in BW (Figure 2A–H, P < 0.05 vs NC). RSV administration did not alter BW in diabetic rats (Figure 2A, P>0.05), but significantly reduced FBG and L/W (Figure 2C and D), as well as TC, TG and LDL-C concentrations (Figure 2E–G, P < 0.05 or P < 0.01 vs DM). However, in contrast to previous findings,16 HDL-C levels were significantly higher in the DM and DR groups than in the NC group (P < 0.01), with no significant differences between the DM and DR groups (Figure 2H, P>0.05). Additionally, CV%, change% and confidence intervals of general characteristics and blood glucose and lipid levels were shown in Supplementary Table 1. Taken together, these results suggested that RSV could ameliorate liver index and improve glycolipid metabolism disorders in diabetic rats.
RSV Alleviated Pathological Injury in Diabetic Liver
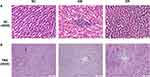
To assess the effect of RSV on pathological injury in diabetic liver, liver samples were subjected to HE and PAS staining assays. HE staining of liver tissues in DM rats showed vacuolar degeneration (indicated by blue arrows), inflammatory cell infiltration (indicated by green arrows) and lipid droplets (indicated by yellow arrows) in hepatocytes (Figure 3A). However, RSV treatment for 12 weeks decreased the numbers of fat vacuoles and lipid droplets and reduced inflammatory cell infiltration in diabetic rats.
PAS staining of the liver sections of rats in NC and DR groups showed glycogen accumulation in hepatocytes, as indicated by PAS-positive granules (indicated by black arrows). There were no PAS-positive granules in hepatocytes in DM rats, indicating the hepatic glycogen breakdown increased glucose output under diabetic conditions, a principal cause of hyperglycemia (Figure 3B). These results suggested that RSV could ameliorate hepatic steatosis and suppress hepatic glycogenolysis.
Impact of RSV on Levels of PDK1 and AKT in Diabetic Liver
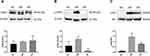
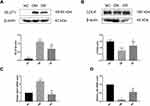
The ability of RSV to regulate the PDK1/AKT pathway in diabetes was assessed by measuring the levels of the proteins p-PDK1, p-AKT, total PDK1 and total AKT. Total PDK1 levels did not differ significantly in the three groups of rats (Figure 4A, P>0.05). Although the levels of p-PDK1 and p-AKT were significantly higher in DM than in NC rats (P < 0.05 or P < 0.01), these increments were reversed by RSV treatment (Figure 4B and C, P < 0.01).
Impact of RSV on HIF-1α mRNA and Protein Levels in Diabetic Liver
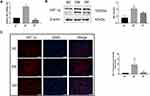
Because HIF-1α acts downstream of the AKT pathway and is implicated in the development of diabetes, its mRNA and protein levels in diabetic liver were determined by real-time PCR, immunofluorescence and Western blotting, respectively. The levels of HIF-1α mRNA and protein were markedly higher in DM rats than in NC rats (P < 0.01), suggesting that HIF-1α was activated under diabetes conditions (Figure 5A and B), with fluorescence intensity being significantly higher in the DM group than in the NC group (Figure 5C, P < 0.05). However, RSV treatment significantly reduced the HIF-1α mRNA and protein levels (P < 0.01 or P < 0.05). These findings indicated an association between diabetes and increased HIF-1α expression, and that RSV could inhibit the production of HIF-1α.
Impact of RSV on GLUT1 and LDLR mRNA and Protein in Diabetic Liver
To further clarify the mechanism of RSV in regulating glycolipid metabolism, the levels of expression of genes acting downstream of HIF-1α, including Glut1 and Ldlr, were measured. Compared with NC rats, GLUT1 protein expression was significantly higher and LDLR protein expression was significantly lower in DM rats (P < 0.01), findings consistent with their mRNA levels (Figure 6). However, RSV treatment significantly reduced GLUT1 level and significantly increased LDLR level (P < 0.05 or P < 0.01). These results suggested that RSV could regulate genes related to glucose and lipid metabolism, which might be closely related to HIF-1α.
Discussion
The present study showed that RSV can regulate glycolipid metabolism in diabetic rats. RSV was found to improve glycolipid metabolism disorders in diabetic rats by inhibiting PDK1/AKT phosphorylation and HIF-1α expression. These findings may provide new insight into treatment targets in diabetes therapy.
This study showed that diabetes can be successfully induced in rats by feeding an HFD and administering STZ (35 mg/kg). These treatments resulted in hyperglycemia, hyperlipidemia, hepatic inflammatory infiltration and hepatic glycogen breakdown, which indicated that glycolipid metabolism disorders occurred in DM rats. However, we found that HDL-C levels were significantly increased in DM rats. This was surprising because serum HDL-C levels are generally reduced under diabetic conditions, with higher HDL-C levels resulting in a lower cardiovascular risk.22 HDL-C levels were also found to be increased in mice with HFD-induced non-alcoholic fatty liver disease (NFALD)23 and in hepatic lipase and Ldlr knockout mice.24 Secondary non-genetic factors, such as drugs, alcohol intake, and liver diseases might also induce HDL-C increases.25 Thus, the increased HDL-C level observed in diabetic rats in the present study may be associated with downregulated LDLR levels and hepatic steatosis.
The ability of RSV to modulate glycolipid metabolism may involve the PDK1/AKT/HIF-1α pathway. HIF-1α was shown to be important in hypoxia, inflammation and autophagy influencing metabolic diseases. Under diabetic conditions, oxygen consumption is increased during glucose metabolism, resulting in increased oxygen demands by organs and cells. These increases can lead to hypoxia, which causes the stabilization and activation of HIF-1α.6 Although HIF-1α may be associated with diabetes development and progression,26 it is unclear whether HIF-1α activation is advantageous or detrimental in metabolic diseases. Disturbances of HIF-1α signaling have been reported to exert harmful effects, including inadequate insulin secretion by β-cells, insulin resistance, adipocyte dysfunction and inflammation.27 Paradoxically, hyperglycemia has been shown to inhibit HIF-1α in various tissues and cells, including in wounds, kidneys, the heart and pancreatic β-cells.11,28–30 HIF-1α activation was also found to be beneficial in diet-induced metabolic diseases.31,32 In contrast to these studies, the present study found that HIF-1α signals were activated in diabetic rats and that this effect was reversed by RSV. This may have resulted from the enhancement of mitochondrial metabolism as the process in adipose tissue and be regulated in a cell context-specific manner.9 In addition, downregulation of HIF-1α by RSV was also shown in endothelial injury of the thoracic aorta in diabetes, alcoholic fatty liver disease (AFLD) and adipose fibrosis.33–35 Furthermore, a recent study found that a HIF-1α inhibitor PX-478 was shown to act as an anti-diabetic therapeutic agent,36 further supporting our findings and suggesting that inhibition of HIF-1α expression might be beneficial for the treatment of diabetes. Findings showing that either Hif-1α deletion or overproduction in the liver was detrimental in diabetes13,37,38 suggest that optimal metabolic health depends on balanced HIF-1α activity.
Activation of the AKT pathway has been shown to increase the rate of HIF-1α protein production.39 AKT, as a key target in the HIF-1α pathway, exerts great effects on regulating glycolipid metabolism, apoptosis and angiogenesis.40 However, AKT activation, especially phosphorylation at Thr308, was catalyzed by PDK1. Moreover, phosphorylation of Ser473 is also necessary for AKT to be fully functionally activated.1 RSV treatment has been found to downregulate p-PDK1 and p-AKT (Ser473), which were similar to other previous findings.41,42 Taken together, these results suggested that RSV could reduce HIF-1α expression by inhibiting the overactivation of the PDK1/AKT pathway.
The present study also analyzed the effects of RSV on downstream target genes related to glycolipid metabolism. AKT has been reported to lead to HIF-1α-dependent Glut1 transcription and cap-dependent Glut1 mRNA translation.43 Thus, HIF-1α can regulate the gene encoding Glut1, a protein involved in glucose transport, thereby affecting the occurrence and progression of metabolic diseases.44 GLUT1 is also important in the cellular response to hypoxia by promoting anaerobic metabolism.45 RSV was found to show an anti-glycolytic effect associated with the downregulation of GLUT1 in H9c2 rat myoblast cells.46 The present study showed that RSV could reduce GLUT1 mRNA and protein levels, which was probably associated with the inhibition of HIF-1α expression. This finding suggested a method by which RSV could reduce FBG concentration. In contrast, LDLR, which regulates lipid metabolism, is an important receptor widely distributed in the liver that plays essential roles in the transport and clearance of cholesterol.47 RSV treatment was found to increase LDLR protein levels in FFA-induced steatotic L02 cells by controlling the sterol-regulatory element binding proteins (SREBPs) pathway.48 However, no direct evidence has reported a connection between HIF-1α and LDLR to date. HIF-1α has been reported to activate insulin-induced gene 2 (INSIG-2) transcription, preventing the excretion of SCAP-SREBP complexes from the endoplasmic reticulum.49 The reduction in active SREBP-2 results in a decrease in LDLR protein level, owing to the reduced binding of SREBP-2 to the cognate sterol regulatory element-1 (SRE-1) in the LDLR promoter.50 These findings suggested that LDLR might act downstream of the HIF-1α pathway. Furthermore, silencing of HIF-1α was found to increase LDLR levels in HepG2 cells stimulated with oleic acid and palmitic acid.51 Similarly, the present study first preliminarily demonstrated that HIF-1α overexpression could down-regulate LDLR expression in diabetic rats, with the latter probably being responsible for the elevation in serum LDL-C levels. These reactions could be reversed by RSV treatment. In summary, the present study found that RSV could regulate GLUT1 and LDLR levels and ameliorate glycolipid metabolism in diabetic rats, probably by inhibiting PDK1/AKT phosphorylation and HIF-1α expression.
However, the potential exact mechanism of the inhibition of the PDK1/AKT/HIF-1α pathway by RSV should require further research. Thereby further studies should research in vitro and in vivo genetic knock-out experiments, which would better elucidate the mechanisms of RSV for improving glycolipid metabolism in T2DM.
Conclusion
In conclusion, as indicated in Figure 7, the present study first preliminarily demonstrated that PDK1/AKT was phosphorylated and HIF-1α was activated in STZ-induced diabetic rats and that these reactions could be inhibited by RSV treatment with ameliorating anaerobic metabolism and cholesterol intake. This study clarified a certain relationship between HIF-1α and diabetes in the liver and proposed a possible new mechanism of RSV for treating or preventing diabetes in the future. Furthermore, RSV may have the potential to become a HIF-1α inhibitor for clinical therapy of metabolic diseases.
Ethics Statement
Animal experiments were approved by Southern Medical University Animal Ethics Committee (Guangzhou, China, No. L2020066) following the China Animal Welfare Legislation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [granted number 81603355] and the Hospital Pharmacy Foundation of Guangdong Province [granted number 2023A07].
Disclosure
The authors declare no conflict of interest.
References
1. James DE, Stockli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol. 2021;22(11):751–771. doi:10.1038/s41580-021-00390-6
2. Cheng Z, Qiao D, Zhao S, Zhang B, Lin Q, Xie F. Whole grain rice: updated understanding of starch digestibility and the regulation of glucose and lipid metabolism. Compr Rev Food Sci Food Saf. 2022;21(4):3244–3273. doi:10.1111/1541-4337.12985
3. Li KX, Ji MJ, Sun HJ. An updated pharmacological insight of resveratrol in the treatment of diabetic nephropathy. Gene. 2021;780:145532. doi:10.1016/j.gene.2021.145532
4. Miao R, Fang X, Wei J, et al. Target for Metabolic Syndrome. Front Physiol. 2022;13:822333. doi:10.3389/fphys.2022.822333
5. Di Blasio L, Gagliardi PA, Puliafito A, Primo L. Serine/Threonine Kinase 3-Phosphoinositide-Dependent Protein Kinase-1 (PDK1) as a key regulator of cell migration and cancer dissemination. Cancers. 2017;9(3):25. doi:10.3390/cancers9030025
6. Gonzalez FJ, Xie C, Jiang C. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol. 2018;15(1):21–32. doi:10.1038/s41574-018-0096-z
7. LaGory EL, Giaccia AJ. The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol. 2016;18(4):356–365. doi:10.1038/ncb3330
8. Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21(12):3995–4004. doi:10.1128/MCB.21.12.3995-4004.2001
9. Lee YS, Kim JW, Osborne O, et al. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell. 2014;157(6):1339–1352. doi:10.1016/j.cell.2014.05.012
10. Mesarwi OA, Moya EA, Zhen X, et al. Hepatocyte HIF-1 and intermittent hypoxia independently impact liver fibrosis in murine nonalcoholic fatty liver disease. Am J Respir Cell Mol Biol. 2021;65(4):390–402. doi:10.1165/rcmb.2020-0492OC
11. Dodd MS, Sousa Fialho MDL, Montes Aparicio CN, et al. Fatty acids prevent hypoxia-inducible factor-1alpha signaling through decreased succinate in diabetes. JACC Basic Transl Sci. 2018;3(4):485–498. doi:10.1016/j.jacbts.2018.04.005
12. Halberg N, Khan T, Trujillo ME, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29(16):4467–4483. doi:10.1128/MCB.00192-09
13. Ochiai D, Goda N, Hishiki T, et al. Disruption of HIF-1alpha in hepatocytes impairs glucose metabolism in diet-induced obesity mice. Biochem Biophys Res Commun. 2011;415(3):445–449. doi:10.1016/j.bbrc.2011.10.089
14. Jardim FR, de Rossi FT, Nascimento MX, et al. Resveratrol and brain mitochondria: a review. Mol Neurobiol. 2018;55(3):2085–2101. doi:10.1007/s12035-017-0448-z
15. Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32(7):537–541. doi:10.1016/j.nutres.2012.06.003
16. Ding S, Jiang J, Wang Z, et al. Resveratrol reduces the inflammatory response in adipose tissue and improves adipose insulin signaling in high-fat diet-fed mice. PeerJ. 2018;6:e5173. doi:10.7717/peerj.5173
17. Wang XL, Wu LY, Zhao L, et al. SIRT1 activator ameliorates the renal tubular injury induced by hyperglycemia in vivo and in vitro via inhibiting apoptosis. Bio Pharm. 2016;83:41–50. doi:10.1016/j.biopha.2016.06.009
18. Wang X, Meng L, Zhao L, et al. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res Clin Pract. 2017;126:172–181. doi:10.1016/j.diabres.2016.12.005
19. Gheibi S, Kashfi K, Ghasemi A. A practical guide for induction of type-2 diabetes in rat: incorporating a high-fat diet and streptozotocin. Bio Pharm. 2017;95:605–613. doi:10.1016/j.biopha.2017.08.098
20. Den Hartogh DJ, Tsiani E. Health benefits of resveratrol in kidney disease: evidence from in vitro and in vivo studies. Nutrients. 2019;11(7):1624. doi:10.3390/nu11071624
21. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi:10.4103/0976-0105.177703
22. Di Angelantonio E, Sarwar N, Perry PL, et al.; Emerging Risk Factors C. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi:10.1001/jama.2009.1619
23. Zhao L, Chen Y, Tang R, et al. Inflammatory stress exacerbates hepatic cholesterol accumulation via increasing cholesterol uptake and de novo synthesis. J Gastroenterol Hepatol. 2011;26(5):875–883. doi:10.1111/j.1440-1746.2010.06560.x
24. Barcat D, Amadio A, Palos-Pinto A, et al. Combined hyperlipidemia/hyperalphalipoproteinemia associated with premature spontaneous atherosclerosis in mice lacking hepatic lipase and low density lipoprotein receptor. Atherosclerosis. 2006;188(2):347–355. doi:10.1016/j.atherosclerosis.2005.11.022
25. Giammanco A, Noto D, Barbagallo CM, et al. Hyperalphalipoproteinemia and Beyond: the role of HDL in cardiovascular diseases. Life. 2021;11(6):581. doi:10.3390/life11060581
26. Catrina SB, Zheng X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia. 2021;64(4):709–716. doi:10.1007/s00125-021-05380-z
27. Girgis CM, Cheng K, Scott CH, Gunton JE. Novel links between HIFs, type 2 diabetes, and metabolic syndrome. Trends Endocrinol Metab. 2012;23(8):372–380. doi:10.1016/j.tem.2012.05.003
28. Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A. 2008;105(49):19426–19431. doi:10.1073/pnas.0805230105
29. Cheng K, Ho K, Stokes R, et al. Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets. J Clin Invest. 2010;120(6):2171–2183. doi:10.1172/JCI35846
30. Gu HF, Zheng X, Abu Seman N, et al. Impact of the hypoxia-inducible factor-1 alpha (HIF1A) Pro582Ser polymorphism on diabetes nephropathy. Diabetes Care. 2013;36(2):415–421. doi:10.2337/dc12-1125
31. Michailidou Z, Morton NM, Moreno Navarrete JM, et al. Adipocyte pseudohypoxia suppresses lipolysis and facilitates benign adipose tissue expansion. Diabetes. 2015;64(3):733–745. doi:10.2337/db14-0233
32. Rahtu-Korpela L, Karsikas S, Horkko S, et al. HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes. 2014;63(10):3324–3333. doi:10.2337/db14-0472
33. Li X, Li J, Wang L, et al. The role of metformin and resveratrol in the prevention of hypoxia-inducible factor 1alpha accumulation and fibrosis in hypoxic adipose tissue. Br J Pharmacol. 2016;173(12):2001–2015. doi:10.1111/bph.13493
34. Ma Z, Zhang Y, Li Q, Xu M, Bai J, Wu S. Resveratrol improves alcoholic fatty liver disease by downregulating HIF-1alpha expression and mitochondrial ROS production. PLoS One. 2017;12(8):e0183426. doi:10.1371/journal.pone.0183426
35. Sha W, Liu M, Sun D, et al. Resveratrol ameliorated endothelial injury of thoracic aorta in diabetic mice and Gly-LDL-induced HUVECs through inhibiting TLR4/HIF-1alpha. J Cell Mol Med. 2021;25(13):6258–6270. doi:10.1111/jcmm.16584
36. Lebeau PF, Byun JH, Platko K, et al. Caffeine blocks SREBP2-induced hepatic PCSK9 expression to enhance LDLR-mediated cholesterol clearance. Nat Commun. 2022;13(1):770. doi:10.1038/s41467-022-28240-9
37. Kucejova B, Sunny NE, Nguyen AD, et al. Uncoupling hypoxia signaling from oxygen sensing in the liver results in hypoketotic hypoglycemic death. Oncogene. 2011;30(18):2147–2160. doi:10.1038/onc.2010.587
38. Wang XL, Suzuki R, Lee K, et al. Ablation of ARNT/HIF1beta in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab. 2009;9(5):428–439. doi:10.1016/j.cmet.2009.04.001
39. Pez F, Dayan F, Durivault J, et al. The HIF-1-inducible lysyl oxidase activates HIF-1 via the Akt pathway in a positive regulation loop and synergizes with HIF-1 in promoting tumor cell growth. Cancer Res. 2011;71(5):1647–1657. doi:10.1158/0008-5472.CAN-10-1516
40. Vasudevan KM, Garraway LA. AKT signaling in physiology and disease. Curr Top Microbiol Immunol. 2010;347:105–133. doi:10.1007/82_2010_66
41. Brito PM, Devillard R, Negre-Salvayre A, et al. Resveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cells. Atherosclerosis. 2009;205(1):126–134. doi:10.1016/j.atherosclerosis.2008.11.011
42. Xu F, Wang Y, Cui W, et al. Resveratrol prevention of diabetic nephropathy is associated with the suppression of renal inflammation and mesangial cell proliferation: possible roles of Akt/NF-kappaB pathway. Int J Endocrinol. 2014;2014:289327. doi:10.1155/2014/289327
43. Taha C, Liu Z, Jin J, Al-Hasani H, Sonenberg N, Klip A. Opposite translational control of GLUT1 and GLUT4 glucose transporter mRNAs in response to insulin. Role of mammalian target of rapamycin, protein kinase b, and phosphatidylinositol 3-kinase in GLUT1 mRNA translation. J Biol Chem. 1999;274(46):33085–33091. doi:10.1074/jbc.274.46.33085
44. Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J. 1998;17(17):5085–5094. doi:10.1093/emboj/17.17.5085
45. Li RL, He LY, Zhang Q, et al. HIF-1alpha is a potential molecular target for herbal medicine to treat diseases. Drug Des Devel Ther. 2020;14:4915–4949. doi:10.2147/DDDT.S274980
46. Naito K, Kanki K. Glycolytic inhibition by resveratrol prevents myoblast cell death caused by glucose deprivation and hypoxia; a possible application to the three-dimensional tissue construction. J Biosci Bioeng. 2021;131(1):90–97. doi:10.1016/j.jbiosc.2020.08.010
47. Chandra NC. A comprehensive account of insulin and LDL receptor activity over the years: a highlight on their signaling and functional role. J Biochem Mol Toxicol. 2021;35(9):e22840. doi:10.1002/jbt.22840
48. Jing Y, Hu T, Lin C, et al. Resveratrol downregulates PCSK9 expression and attenuates steatosis through estrogen receptor alpha-mediated pathway in L02cells. Eur J Pharmacol. 2019;855:216–226. doi:10.1016/j.ejphar.2019.05.019
49. Hwang S, Nguyen AD, Jo Y, Engelking LJ, Brugarolas J, DeBose-Boyd RA. Hypoxia-inducible factor 1alpha activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J Biol Chem. 2017;292(22):9382–9393. doi:10.1074/jbc.M117.788562
50. Bjune K, Wierod L, Naderi S. Inhibitors of AKT kinase increase LDL receptor mRNA expression by two different mechanisms. PLoS One. 2019;14(6):e0218537. doi:10.1371/journal.pone.0218537
51. He Y, Yang W, Gan L, et al. Silencing HIF-1alpha aggravates non-alcoholic fatty liver disease in vitro through inhibiting PPAR-alpha/ANGPTL4 singling pathway. Gastroenterol Hepatol. 2021;44(5):355–365. doi:10.1016/j.gastrohep.2020.09.014
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.