Back to Journals » Drug Design, Development and Therapy » Volume 14
Resveratrol Protects Osteoblasts Against Dexamethasone-Induced Cytotoxicity Through Activation of AMP-Activated Protein Kinase
Authors Wang L, Li Q, Yan H, Jiao G, Wang H, Chi H, Zhou H, Chen L, Shan Y, Chen Y
Received 7 June 2020
Accepted for publication 23 September 2020
Published 23 October 2020 Volume 2020:14 Pages 4451—4463
DOI https://doi.org/10.2147/DDDT.S266502
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Anastasios Lymperopoulos
Liang Wang,1– 3,* Qiushi Li,4,* Haibo Yan,3 Guangjun Jiao,1 Hongliang Wang,1 Hai Chi,5 Hongming Zhou,1,6 Lu Chen,1 Yu Shan,1 Yunzhen Chen1,2
1Department of Orthopedics, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China; 2Shandong University Spine and Spine Cord Disease Research Center, Shandong University, Jinan, Shandong, People’s Republic of China; 3Department of Internal Medicine, Shandong Medical College, Linyi, Shandong, People’s Republic of China; 4Department of Orthopedics, Linyi People’s Hospital, Linyi, Shandong, People’s Republic of China; 5Department of Traumatic Orthopedics, Shandong Provincial ENT Hospital (Affiliated to Shandong University), Jinan, Shandong, People’s Republic of China; 6Department of Emergency Trauma Surgery, Linyi Central Hospital, Linyi, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yunzhen Chen
Department of Orthopedics, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China
Tel/Fax +86-531-82166531
Email [email protected]
Purpose: Glucocorticoids are used for the treatment of inflammatory diseases, but glucocorticoid treatment is associated with bone damage. Resveratrol is a phytoalexin found in many plants, and we investigated its protective role on dexamethasone-induced dysfunction in MC3T3-E1 cells and primary osteoblasts.
Materials and Methods: MC3T3-E1 cells and primary osteoblasts were treated with dexamethasone in the presence/absence of different doses of resveratrol for 24 or 48 h. Then, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium (MTT) and lactate dehydrogenase (LDH) assays were used to evaluate cell viability. Apoptosis was analyzed by a flow cytometry. An alkaline phosphatase (ALP) activity assay and Alizarin Red S staining were used to study osteoblast differentiation. Expression of osteoblast-related genes was measured by real-time reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The AMP-activated protein kinase (AMPK) signaling pathway and mitochondrial expression of superoxide dismutase were evaluated by Western blotting. Intracellular reactive oxygen species (ROS), adenosine triphosphate (ATP) content, mitochondrial-complex activity, and mitochondrial DNA content were measured to evaluate mitochondrial function.
Results: Resveratrol induced the proliferation and inhibited apoptosis of osteoblasts in the presence of dexamethasone. Resveratrol increased the ALP activity and mineralization of osteoblasts. Resveratrol also attenuated dexamethasone-induced inhibition of mRNA expression of osteogenesis maker genes, including bone morphogenetic protein-2, osteoprotegerin, runt-related transcription factor-2, and bone Gla protein. Resveratrol alleviated dexamethasone-induced mitochondrial dysfunction. Resveratrol strongly stimulated expression of peroxisome proliferator–activated receptor-γ coactivator 1α and sirtuin-3 genes, as well as their downstream target gene superoxide dismutase-2. Resveratrol induced phosphorylation of AMPK and acetyl-CoA carboxylase (ACC). Blockade of AMPK signaling using compound C reversed the protective effects of resveratrol against dexamethasone.
Conclusion: Resveratrol showed protective effects against dexamethasone-induced dysfunction of osteoblasts by activating AMPK signaling.
Keywords: resveratrol, dexamethasone, osteoblasts, AMPK, mitochondrial function
Introduction
Bone is an important and dynamic tissue which has critical roles in maintaining structural integrity, shape, and preserving skeletal size as well as mineral homeostasis. Bone metabolism is controlled tightly, and is responsible for the growth and development of bone under physiologic conditions, or bone remodeling under pathologic conditions. Homeostasis of bone mass during bone metabolism is regulated mainly by bone formation and bone resorption.
Increasing evidence suggests that decreased bone formation and increased bone resorption results in systemic bone loss in different pathologic conditions, such as chronic inflammation, rheumatoid arthritis, osteonecrosis of the femoral head, aging-related bone loss, sex corticosteroid deficiency-associated bone loss, drug-induced bone loss, and after ovariectomy. In particular, glucocorticoid-induced disorders of bone metabolism have attracted much attention recently, such as dexamethasone-induced osteoporosis.
Glucocorticoids are used widely for treatment of inflammatory and autoimmune disorders, but long-term or high-doses administration of glucocorticoids can cause several severe adverse effects, including secondary osteoporosis and osteonecrosis.1,2 Glucocorticoids inhibit bone formation and promote bone resorption via different signaling pathways, subsequently resulting in a negative bone balance in osteoblasts, osteoclasts, and osteocytes. Among these signaling pathways, the AMP-activated protein kinase (AMPK) is one of the most important and frequently studied metabolic effectors in bone homeostasis.
AMPK has emerged over recent decades and has been shown to be an important energy sensor in the regulation of cellular energy homeostasis. As a highly conserved serine/threonine kinase, AMPK can sense the ATP/AMP ratio and switch-on and switch-off catabolic or anabolic pathways in cells. Recent studies have demonstrated that AMPK has critical roles in osteoblastic and adipocytic differentiation, as well as the convergence between bone metabolism and fat metabolism.
Osteoprotegerin (OPG) inhibits osteoclast differentiation and bone resorption by regulating the AMPK signaling pathway.3 C1q/tumor necrosis factor-related protein-3 acts as a negative regulator of osteoclastogenesis via AMPK/c-Fos signaling.4 Interestingly, it has been reported that AMPK promotes osteogenesis directly and inhibits adipogenesis through an AMPK/osteopontin axis.5 AMPK activity regulates chondrogenic differentiation.6 AMPK is involved in the hypoxia inducible factor 1-mediated post-implantation survival of bone cells.7 These data suggest that AMPK is very important in bone metabolism-related pathogenesis, and indicate that AMPK may be an important drug target for the treatment of bone-related disorders.
Resveratrol is a naturally occurring polyphenol found in red wine. Recent studies have demonstrated that resveratrol showed antioxidant, anti-inflammatory, and lifespan-extending properties through diverse mechanisms. Resveratrol can suppress alveolar bone loss by inhibiting osteoclast differentiation.8 Moreover, a double-blind, randomized-controlled trial in patients with type-2 diabetes mellitus indicated that supplementation with resveratrol (500 mg) prevented loss of bone density.9 Another recent study showed that resveratrol could ameliorate bone loss and promote osteogenesis in ovariectomized mice via activation of the sirtuin 1 (SIRT1) pathway.10 Resveratrol treatment protects osteoblasts in osteoporosis rats via SIRT1 and PI3K/AKT/mTOR signaling pathway.11 Resveratrol also promotes differentiation of MC3T3-E1 cells via potentiation of the calcineurin/NFATc1 signaling pathway.12 These results strongly suggest that resveratrol may have protective roles in glucocorticoid-induced bone loss.
In the present study, we investigated the protective effects of resveratrol on dexamethasone-induced osteoblast dysfunction and explored the potential mechanisms of action.
Materials and Methods
Chemicals, Reagents and Antibodies
Resveratrol (purity ≥99%; catalog number: R5010) and dexamethasone (≥98%; D1756) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Antibodies against p-AMPK (1:1000 dilution; 50081), AMPK (1:1000; 5832), p-ACC (1:1000; 11818), and ACC (1:1000; 3676) were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies against β-Actin (1:1000; sc-47778), SOD1 (1:1000; sc-271014), and SOD2 (1:1000; sc-133134) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against PGC-1ɑ (1:500; AB3242) were from Millipore (Burlington, MA, USA). SIRT3 (1:1000; WL03840), Runx2 (1:1000; WL03358) and receptor activator of nuclear factor-κB ligand (RANKL; 1:600; WL00285) were from Wanleibio (Shenyang, China). MTT (ST1537) and Alizarin Red S (ST1078) were purchased from Beyotime Institute of Biotechnology (Shanghai, China). Minimum essential medium-alpha modification (α-MEM; 32571101), Dulbecco’s modified Eagle’s medium (DMEM; 10566016) and fetal bovine serum (10270–106) were purchased from Gibco (Carlsbad, CA, USA). TRIzol® Reagent (15596026) was obtained from Thermo Fisher Scientific (Waltham, MA, USA).
Cell Culture
MC3T3-E1 cells were purchased from Procell Life Science & Technology (Wuhan, China) and maintained in α-MEM (Gibco) supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37°C in a humidified atmosphere of 5% CO2. For osteogenic differentiation, cells were maintained in osteogenic-inducing medium (MUXMT-90021; Cyagen Biosciences, Suzhou, China) containing dexamethasone (100 nM), β-glycerophosphate (10 mM), and ascorbic acid (50 mg/mL) for the indicated days. For real-time reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and mineralization assays, cells were treated with dexamethasone or dexamethasone with different concentrations of resveratrol in osteogenic-inducing medium for 48 h and 21 days, respectively. For protein-expression and alkaline-phosphatase assays, cells were treated with dexamethasone or dexamethasone with different concentrations of resveratrol in osteogenic-inducing medium for 7 days.
For isolation and culture of primary osteoblasts, the calvaria of C57BL/6 male mice was obtained 24 h after birth. Osteoblasts were isolated by sequential digestions for 30 min at 37°C in a mixture of collagenase I (0.1%) and trypsin (0.25%). The study protocol was approved by the Animal Experiments Committee of Shandong Medical College (sdmc2018006) in Shandong, China. Experiments were undertaken in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA). Cells from the third and subsequent digestions were collected and plated in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Cells were incubated at 37°C in an atmosphere of 5% CO2 and 95% humidity. Cells underwent subculture every 72 h and used from passages 3 to 5. The osteoblast phenotype was confirmed in cells by characterizing expression of Runx2 and alkaline phosphatase (ALP).
Cell-Viability Assay
MC3T3-E1 cells were seeded in 96-well plates. After 24 h, cells were treated with dexamethasone (200 μM) in the presence/absence of resveratrol (0, 5, 10 μM) for 48 h. After treatment, 20 μL of MTT (5 mg/mL) was added to each well and samples were incubated in the dark for an additional 4 h at 37°C. The medium was discarded and the precipitated formazan was dissolved in 150 μL of dimethyl sulfoxide. The absorbance of the solution was measured using a microplate reader (Envision 2105; PerkinElmer, Waltham, MA, USA) at 570 nm.
Assay to Measure Lactate Dehydrogenase (LDH)
MC3T3-E1 cells were treated with dexamethasone (200 μM) in the presence/absence of resveratrol for 48 h. Then, LDH in the medium was measured with an LDH assay kit (C0016; Beyotime Institute of Biotechnology, Shanghai, China) according to manufacturer instructions.
ALP Assay
MC3T3-E1 cells were seeded in 12-well plates in osteogenic-inducing medium and treated with dexamethasone (200 μM) in the presence/absence of resveratrol for 7 days. Then, cells were washed twice and lysed with assay lysis buffer (100 μL). The ALP activity was measured with an ALP reagent kit according to manufacturer instructions (P0321S; Beyotime Institute of Biotechnology).
Quantification of the Mineralization Assay
MC3T3-E1 cells were seeded in six-well plates. After 24 h, the medium was replaced with osteogenic-inducing medium and cells were treated with dexamethasone (200 μM) in the presence/absence of resveratrol for 21 days. Then, cells were rinsed twice with phosphate-buffered saline (PBS) followed by fixation with 4% paraformaldehyde for 30 min at room temperature. Next, cells were stained with 2% Alizarin Red S (pH 4.2) for 20 min at room temperature and rinsed extensively with distilled water. Finally, the bound stain was eluted with 10% cetylpyridinium chloride (H108696; Aladdin, Shanghai, China,) and the absorbance of supernatants was measured by a microplate reader (Envision 2105; PerkinElmer) at 570 nm.
Flow Cytometry Analysis of Osteoblast Apoptosis
MC3T3-E1 cells were cultured in six-well plates and treated with dexamethasone (200 μM) in the presence/absence of resveratrol for 48 h. Then, cells were harvested and resuspended in binding buffer (300 μL) containing annexin V-FITC (5 μL) and propidium iodide (5 μL), and then rinsed with PBS. After washing twice with PBS, cells were placed on ice for 30 min and analyzed using a flow cytometer (FACSCelesta; BD Biosciences, Franklin Lakes, NJ, USA).
Measurement of Intracellular Reactive Oxygen Species (ROS)
A stock solution (10 mM) of 2ʹ,7ʹ-dichlorodihydrofluorescein-diacetate (DCFH-DA; S0033S) was obtained from Beyotime Institute of Biotechnology. In brief, MC3T3-E1 cells were cultured and treated with dexamethasone (200 μM) in the presence/absence of resveratrol for 6 h. Then, cells were incubated with DCFH-DA (10 μM) for 30 min. The green fluorescence of DCF was recorded at an excitation wavelengths of 485 nm and emission wavelength of 535 nm using a microplate reader (Envision 2105; PerkinElmer).
Determination of ATP Content
MC3T3-E1 cells were cultured in six-well plates in osteogenic-inducing medium and treated with dexamethasone (200 μM) in the presence/absence of resveratrol for 48 h. ATP content in MC3T3-E1 cells was determined by an Enhanced ATP assay kit (S0026; Beyotime Institute of Biotechnology) according to manufacturer instructions.
Measurement of Mitochondrial DNA Content
MC3T3-E1 cells were cultured in six-well plates in osteogenic-inducing medium and treated with dexamethasone (200 μM) in the presence/absence of resveratrol for 7 days. Then, total DNA was isolated using a DNA extraction kit (51304; Qiagen, Hilden, Germany) according to manufacturer instructions. A serial-dilution standard curve was prepared from a pool of all samples and RT-qPCR was carried out. The number of mitochondrial DNA copies was calculated from the ratio of cyclo-oxygenase I (mitochondrial-encoded gene)/cyclophilin A (nuclear-encoded gene). Primer sequences are shown in Table 1.
 |
Table 1 Primer Sequences Used in RT-qPCR |
RT-qPCR
Total RNA was isolated using TRIzol® Reagent (15596026; Thermo Fisher Scientific) according to manufacturer instructions. Complementary DNA was generated from RNA (2 μg) with a cDNA synthesis kit (1708841; Bio-Rad Laboratories, Hercules, CA, USA).
RT-qPCR was carried out using SYBR Green (1725124; Bio-Rad Laboratories) on aCFX Connect PCR system (Bio-Rad Laboratories) with a time–temperature profile of: one cycle of initial activation of 2 min at 95°C, followed by 40 cycles of 3 s at 95°C, and 30 s at 60°C. The profile of the melting curve consisted of a 30-s hold on the first step and then 5-s hold for every half degree from 65°C to 95°C. The results were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or cyclophilin A using the 2−ΔΔCt method. Primer sequences are shown in Table 1.
Western Blotting
Cells were lysed in 100 μL of lysis buffer (50 mM Tris, pH 7.4, 1 mM EDTA, 150 mM NaCl, 0.25% sodium deoxycholate, 1% NP40) containing protease inhibitors and phosphatase inhibitors (cOmplete, 5892970001; PhosSTOP, 4906837001; Roche, Penzberg, Germany). Samples were incubated at 4°C for 1 h and then centrifuged at 12000 × g for 15 min, and the supernatant was collected for analyses. Lysates were resolved by SDS-PAGE and then transferred to polyvinylidene difluoride membranes. The latter were blocked with 3% bovine serum albumin (for p-AMPK and p-ACC), or 5% nonfat milk (for all other antibodies) prepared in 1× TBST (10 mM Tris-HCl, 100 mM NaCl, 0.1% Tween 20, pH 7.5) for 1 h at room temperature, and then incubated with specific antibodies against p-AMPK, AMPK, p-ACC, ACC, SOD1, SOD2, PGC-1α, SIRT3, Runx2, RANKL, or β-Actin. Peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin secondary antibody was used. Proteins were visualized by enhanced chemiluminescence.
Determination of Activity of Mitochondrial Complex
Mitochondria in cultured cells were isolated as described previously.8 Briefly, cells were collected and resuspended in 1.0 mL of hypotonic buffer (10 mM NaCl, 2.5 mM MgCl2, 10 mM Tris base, pH 7.5) and homogenized on ice with a glass homogenizer. Then, homogenates were centrifuged at 1300 × g for 5 min at 4°C. The supernatant was centrifuged at 17,000 × g for 15 min at 4°C, and the mitochondria pellet resuspended in 100 μL of isotonic buffer (210 mM mannitol, 70 mM sucrose, 5 mM Tris base, 1 mM EDTA, pH 7.5). Assays to measure the activity of reduced nicotinamide adenine dinucleotide-ubiquinone reductase (“complex I”), succinate-CoQ oxidoreductase (“complex II”), cytochrome c oxidase (“complex IV”), and Mg2+-ATPase (“complex V”) were undertaken according to methods described previously.13
Statistical Analyses
Statistical analyses were undertaken using SPSS 19.0 (IBM, Armonk, NY, USA). Data are the mean ± SD and were compared by ANOVA followed by the Student’s t-test. p < 0.05 was considered significant. All experiments were repeated independently at least thrice.
Results
Protective Effects of Resveratrol on the Viability of MC3T3-E1 Cells Exposed to Dexamethasone
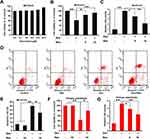
We wished to ascertain if resveratrol had protective effects upon MC3T3-E1 cells treated with dexamethasone. An MTT assay was employed and cellular viability was evaluated. An obvious cytotoxic effect was not observed in MC3T3-E1 cells treated with different concentrations of resveratrol, whereas treatment with high-dose (30 µM) resveratrol promoted significant proliferation of MC3T3-E1 cells (p < 0.05) (Figure 1A). Dexamethasone (200 µM) treatment decreased cell viability markedly (p < 0.05), whereas combined treatment with resveratrol attenuated this effect notably (p < 0.05 or 0.01) (Figure 1B). The LDH-release assay also suggested that resveratrol treatment attenuated dexamethasone-induced cytotoxicity in MC3T3-E1 cells significantly (p < 0.05 or 0.01) (Figure 1C). Next, we undertook a fluorescence-activated cell sorting (FACS) assay. Dexamethasone treatment caused a noticeable increase in apoptosis of MC3T3-E1 cells (p < 0.05) (Figure 1D and E), which was almost reversed by resveratrol. Similar results were obtained in the primary cultured of murine osteoblasts; resveratrol displayed protective effects against the dexamethasone-induced reduction in viability and increase in LDH release (Figure 1F and G). These data strongly suggested that resveratrol attenuated dexamethasone-induced osteoblast damage efficiently.
Protective Effects of Resveratrol on Osteogenic Differentiation in Dexamethasone-Treated MC3T3-E1 Cells
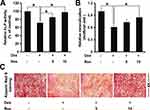
ALP is considered to be a marker of osteoblast differentiation. Next, we investigated if resveratrol could regulate ALP activity in dexamethasone-treated MC3T3-E1 cells. Dexamethasone exposure reduced ALP activity markedly (p < 0.05) (Figure 2A), a finding that is consistent with data from Wang and colleagues.14 However, resveratrol improved ALP activity against dexamethasone significantly, suggesting that resveratrol could reverse the inhibitory influence of dexamethasone on osteoblast differentiation. Formation of mineralized bone nodules is employed frequently to analyze osteoblastic maturation.We studied the effects of resveratrol on matrix mineralization in MC3T3-E1 cells exposed to dexamethasone. Staining using Alizarin Red S indicated that dexamethasone treatment caused a significant decrease in formation of calcified deposits from the mineralized extracellular matrix in MC3T3-E1 cells, whereas resveratrol strongly enhanced nodule formation against dexamethasone treatment (p < 0.05) (Figure 2B and C).
RT-qPCR was done to study the way resveratrol promotes osteoblast differentiation and nodule formation in the presence of dexamethasone. mRNA expression of the key osteogenic genes bone morphogenetic protein (BMP)-2 (p < 0.05), OPG, Runx2 (p < 0.05), and bone gla protein (p < 0.05 or 0.01) was decreased in MC3T3-E1 cells treated with dexamethasone (Figure 3A–D, respectively), whereas further incubation with resveratrol rescued the inhibitory effect of dexamethasone on expression of these osteogenic markers. mRNA expression of RANKL remained unchanged after dexamethasone treatment, but further treatment with resveratrol decreased its mRNA expression significantly (p < 0.05) (Figure 3E). Similar results were also observed regarding the protein expression of Runx2 and RANKL (Figure 3F).
Protective Effects of Resveratrol on Mitochondrial Function and Oxidative Stress in Dexamethasone-Treated MC3T3-E1 Cells
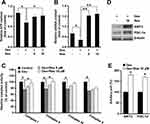
To determine whether resveratrol had a protective effect on dexamethasone-induced mitochondrial dysfunction, ATP production and mitochondrial biogenesis were measured. The ATP content in MC3T3-E1 cells decreased (p < 0.05) after exposure to dexamethasone, but incubation with resveratrol significantly prevented the reduction in intracellular ATP caused by dexamethasone (p < 0.05) (Figure 4A). Furthermore, dexamethasone exposure led to a decrease in mitochondrial DNA content (p < 0.05), whereas the intracellular mitochondrial DNA level was recovered completely (p < 0.01) (Figure 4B). This finding suggested that mitochondrial biogenesis might mediate the protective effect of resveratrol on dexamethasone-induced damage to MC3T3-E1 cells. Next, we measured the activity of mitochondrial complexes. The activity of complex I and II was decreased (p < 0.05) after treatment with dexamethasone, which was attenuated upon further treatment with resveratrol (p < 0.05) (Figure 4C). Resveratrol showed no effect on the activity of complex IV or V, although their activities were inhibited significantly (p < 0.05) by dexamethasone treatment (Figure 4C). We also found that resveratrol treatment induced protein expression of peroxisome proliferator-activated receptor γ coactivator-1ɑ (PGC-1ɑ) and SIRT3, two critical regulators for mitochondrial biogenesis and ROS production, in MC3T3-E1 cells (p < 0.05) (Figure 4D and E).
Recent studies have demonstrated that ROS production and oxidative stress contribute to dexamethasone-induced cell death.15 We revealed that resveratrol treatment decreased dexamethasone-induced ROS production significantly (p < 0.05) (Figure 5A). Interestingly, this effect of resveratrol could be largely blocked by AMPK inhibition (data not shown). The nuclear factor erythroid 2-related factor 2/heme oxygenase-1 (NRF2/HO1) signaling pathway has critical roles in regulating oxidative stress and maintaining normal mitochondrial functions. Therefore, we evaluated the role of resveratrol in modulating expression of NRF2 and HO1 in the presence of dexamethasone in MC3T3-E1 cells. Resveratrol administration to MC3T3-E1 cells showed no effect on mRNA expression of NRF2, but increased mRNA expression of HO1 significantly (p < 0.05) after treatment with dexamethasone, which was consistent with the result of the ROS assay (Figure 5B and C). Although mRNA expression of SOD1 remained unchanged, mRNA expression of SOD2 was increased markedly (p < 0.05) after treatment with resveratrol in the presence of dexamethasone (Figure 5D and E). Consistently, protein expression of SOD1 was increased modestly (p = 0.06), whereas protein expression of SOD2 was increased significantly (p < 0.05) (Figure 5F and G).
AMPK Mediates the Protective Effects of Resveratrol Against the Dexamethasone-Induced Cytotoxicity of Osteoblasts
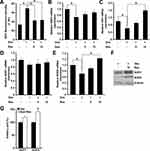
Recent studies have suggested that AMPK activation regulates bone formation and bone mass.16 Next, we examined if resveratrol could stimulate phosphorylation of AMPK. Western blotting indicated that resveratrol could promote activation of AMPK and its downstream target ACC in a dose-dependent manner (Figure 6A) in MC3T3-E1 cells. Similar results were observed in primary osteoblasts (Figure 6B), which suggested that the AMPK/ACC axis was involved in the protective effects of resveratrol on dexamethasone-induced osteoblast damage. To further confirm that AMPK activation is required for the resveratrol-mediated protective effects against dexamethasone, we used compound C (which has been demonstrated to inhibit phosphorylation of AMPK specifically). Resveratrol-induced AMPK activation in primary osteoblasts was prevented significantly by using compound C (Figure 6C).
Finally, administration of compound C markedly inhibited the resveratrol-mediated protective effects against dexamethasone-induced decrease in cell viability (p < 0.01) (Figure 7A) and mineralization (p < 0.05) (Figure 7B), as well as antioxidant genes and osteogenesis marker genes (p < 0.05 or 0.01) (Figure 7C–G). These data suggested that AMPK signaling might have critical roles in resveratrol-induced protection in MC3T3-E1 cells in the presence of dexamethasone.
Discussion
As a glucocorticoid, dexamethasone has been used widely to regulate the inflammatory response,17,18 and has shown strong immunosuppressive effects.19,20 Recently, it was reported that early treatment with dexamethasone improves the outcome in adults with acute bacterial meningitis.21 Dexamethasone combined with carfilzomib treatment has resulted in marked improvement in progression-free survival in patients with relapsed multiple myeloma.22 However, extensive clinical use of dexamethasone can cause severe side effects. High-dose dexamethasone treatment results in a high prevalence of severe side-effects in patients with spinal-cord compression from epidural metastases.23 Bordag and colleagues showed that dexamethasone use could induce metabolome changes in healthy males.24 Early research on dexamethasone demonstrated that it could rapidly induce osteoblast differentiation of human bone marrow osteogenic stromal cells and rat bone marrow stromal cells.25,26 However, a high dose of dexamethasone suppresses the proliferation of human osteoblast precursors.27 Numerous recent studies have suggested that dexamethasone can induce osteoblast cell death, bone loss, and osteoporosis.28,29 Some compounds have been shown preventive effects against dexamethasone-induced bone damage.30–32
Murgia and colleagues reported that resveratrol may be a promoter of osteoblast proliferation and antagonist of osteoclast differentiation.33 Moreover, it has been shown that resveratrol supplementation protects bone in different animal models.34,35 However, whether resveratrol has positive effects on dexamethasone-induced osteoblasts is not known.
First, we investigated if resveratrol could reverse the dexamethasone-induced death of MC3T3-E1 cells. Resveratrol treatment ameliorated dexamethasone-induced cytotoxicity significantly, which suggests that resveratrol may have multiple protective effects on dexamethasone-treated MC3T3-E1 cells. Matsuda and colleagues reported that resveratrol can prevent inflammation and mechanical stress-induced alveolar bone resorption and bone loss, but they did not investigate the protective effects of resveratrol on osteoblasts.8 One clinical trial strongly suggested that supplementation with resveratrol (500 mg) elicited beneficial effects on bone density in patients with type-2 diabetes mellitus, data that are consistent with our results.9 Jiang and coworkers demonstrated that resveratrol ameliorated bone loss and promoted osteogenesis by decreasing oxidative stress through SIRT1 in ovariectomized mice,10 but whether SIRT3/SOD2 was also involved in this process was not revealed.
Osteoblasts and osteoclasts have high energy demands, and abundant mitochondria have been found during osteoblast differentiation.36 The biogenesis and respiration of mitochondria have been shown to be associated with energy production and osteoblast function.36 In addition, mitochondrial dysfunction induces macrophage activation and systemic inflammation, subsequently leading to impaired function of osteoblasts and osteoclasts and bone resorption.37 A high dose of dexamethasone induces osteoblast dysfunction by interfering with mitochondrial function.38 We found that resveratrol treatment reversed the dexamethasone-induced decrease in mitochondrial biogenesis and ATP production markedly. These results strongly suggest that mitochondria mediated dexamethasone-induced osteoblast injury, and resveratrol mitigated dexamethasone-induced mitochondrial dysfunction in MC3T3-E1 cells.
Studies have shown that increased levels of ROS and oxidative stress in osteoblasts have critical roles in the pathogenesis of bone-metabolism disorders,39,40 whereas mitochondria are the major source of ROS and ATP production. We found that resveratrol protected against dexamethasone-induced ROS production in MC3T3-E1 cells. Cao and colleagues demonstrated that Mn(III)tetrakis (4-ben-zoic acid) porphyrin decreased bone loss in ovariectomized rats by reducing mitochondrial oxidative stress in osteoblasts, data that are in accordance with our results.41 Numerous investigations have suggested that NRF2 is an important transcription factor mediating protection against oxidants by stimulating transcription of antioxidant genes.42 Mitochondrial ROS production can be regulated through the NRF2 signaling pathway.43 As its downstream target gene, HO-1 has been proposed to mediate the effects of NRF2 on the oxidative stress response stimulated by exogenous substances, including dexamethasone.44 Our results revealed that the NRF2/HO-1 axis in MC3T3-E1 cells was impaired by dexamethasone treatment and that resveratrol administration significantly induced activation of this pathway. Hence, the NRF2/HO-1 pathway contributed to the protective effects of resveratrol on osteoblasts.
PGC-1ɑ has been shown to induce expression of most mitochondrial proteins and to stimulate mitochondrial biogenesis.45 However, PGC-1ɑ can also induce expression of genes that decrease ROS formation, such as GPx1 and SOD2.45 Kong and collaborators demonstrated that PGC-1ɑ was a suppressor of ROS production through induction of SIRT3 expression.46 Our data suggest that resveratrol stimulated expression of PGC-1ɑ and its downstream target genes, including SIRT3 and SOD2.
BMP and Runx2 signaling pathways have been shown to regulate the proliferation and differentiation of osteoblasts, and to have critical roles in regulating bone formation.47,48 Osteoblasts also modulate bone resorption through regulating OPG expression to maintain bone homeostasis.49 Resveratrol administration attenuated the dexamethasone-induced decrease in expression of BMP-2, Runx2, and OPG, indicating that resveratrol attenuated alteration of biomarkers specific for the formation and resorption of bone. Although we revealed that AMPK was involved in the protective effects of resveratrol on dexamethasone-induced osteoblasts, we cannot exclude the possibility that other signaling pathways upstream of BMP-2 and Runx2 may also have had important roles in this process.
Conclusions
In summary, our data using MC3T3-E1 and primary osteoblasts suggest that resveratrol has positive effects against dexamethasone-induced inhibition of differentiation and damage of osteoblasts. Our findings also suggest that treatment with resveratrol protects osteoblasts by activating AMPK, stimulating SIRT3-PGC-1α-SOD2 axis, and subsequently improving oxidative stress and mitochondrial dysfunction. Further study is needed to determine if resveratrol has protective role on differentiation and function of osteoclasts. Resveratrol may be a potential strategy for treating dexamethasone-induced osteoblast injury.
Abbreviations
LDH, lactate dehydrogenase; ALP, alkaline phosphatase; AMPK, AMP-activated protein kinase; ROS, reactive oxygen species; BMP-2, bone morphogenetic protein 2; OPG, osteoprotegerin; Runx2, runt related transcription factor 2; BGP, bone Gla protein; PGC-1ɑ, peroxisome proliferator–activated receptor γ coactivator 1α; SIRT3, sirtuin 3; SOD2, superoxide dismutase 2; SOD1, superoxide dismutase 1; ACC, acetyl-CoA carboxylase; SIRT1, sirtuin 1; PBS, phosphate buffered saline; COX I, cytochrome c oxidase subunit I; RANKL, receptor activator of nuclear factor-κB ligand; NRF2, nuclear factor erythroid 2-related factor 2; HO1, heme oxygenase-1.
Acknowledgments
This work was supported by the Jinan Science and Technology Project (grant 201805042) and the Program of Linyi Science and Technology Innovation Development (grant 201919058).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457–465. doi:10.1517/14740338.2016.1140743
2. Zhang S, Liu Y, Liang Q. Low-dose dexamethasone affects osteoblast viability by inducing autophagy via intracellular ROS. Mol Med Rep. 2018;17:4307–4316.
3. Tong X, Gu J, Song R, et al. Osteoprotegerin inhibit osteoclast differentiation and bone resorption by enhancing autophagy via AMPK/mTOR/p70S6K signaling pathway in vitro. J Cell Biochem. 2019;120:1630–1642.
4. Kim JY, Min JY, Baek JM, et al. CTRP3 acts as a negative regulator of osteoclastogenesis through AMPK-c-Fos-NFATc1 signaling in vitro and RANKL-induced calvarial bone destruction in vivo. Bone. 2015;79:242–251.
5. Wang YG, Qu XH, Yang Y, et al. AMPK promotes osteogenesis and inhibits adipogenesis through AMPK-Gfi1-OPN axis. Cell Signal. 2016;28:1270–1282.
6. Bandow K, Kusuyama J, Kakimoto K, Ohnishi T, Matsuguchi T. AMP-activated protein kinase (AMPK) activity negatively regulates chondrogenic differentiation. Bone. 2015;74:125–133.
7. Stegen S, van Gastel N, Eelen G, et al. HIF-1α promotes glutamine-mediated redox homeostasis and glycogen-dependent bioenergetics to support postimplantation bone cell survival. Cell Metab. 2016;23:265–279.
8. Matsuda Y, Minagawa T, Okui T, Yamazaki K. Resveratrol suppresses the alveolar bone resorption induced by artificial trauma from occlusion in mice. Oral Dis. 2018;24(3):412–421.
9. Bo S, Gambino R, Ponzo V, et al. Effects of resveratrol on bone health in type 2 diabetic patients. A double-blind randomized-controlled trial. Nutr Diabetes. 2018;8(1):51.
10. Jiang Y, Luo W, Wang B, Wang X, Gong P, Xiong Y. Resveratrol promotes osteogenesis via activating SIRT1/FoxO1 pathway in osteoporosis mice. Life Sci. 2020;246:117422. doi:10.1016/j.lfs.2020.117422
11. Yang X, Jiang T, Wang Y, Guo L. The Role and Mechanism of SIRT1 in Resveratrol-regulated Osteoblast Autophagy in Osteoporosis Rats. Sci Rep. 2019;9(1):18424. doi:10.1038/s41598-019-44766-3
12. Huang Y, Huo J, Liu FQ, et al. Resveratrol Promotes in vitro Differentiation of Osteoblastic MC3T3-E1 Cells via Potentiation of the Calcineurin/NFATc1 Signaling Pathway. Biochemistry (Mosc). 2019;84(6):686–692. doi:10.1134/S0006297919060117
13. Gao J, Feng Z, Wang X, et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018;25(2):229–240. doi:10.1038/cdd.2017.144
14. Wang N, Wang F, Gao Y, et al. Curcumin protects human adipose-derived mesenchymal stem cells against oxidative stress-induced inhibition of osteogenesis. J Pharmacol Sci. 2016;132(3):192–200. doi:10.1016/j.jphs.2016.10.005
15. Yang M, Huang Y, Chen J, Chen Y-L, Ma -J-J, Shi P-H. Activation of AMPK participates hydrogen sulfide-induced cyto-protective effect against dexamethasone in osteoblastic MC3T3-E1 cells. Biochemical and Biophysical Research Communications. 2014;454(1):42–47. doi:10.1016/j.bbrc.2014.10.033
16. Shah M, Kola B, Bataveljic A, et al. AMP-activated protein kinase (AMPK) activation regulates in vitro bone formation and bone mass. Bone. 2010;47(2):309–319. doi:10.1016/j.bone.2010.04.596
17. Tsurufuji S, Sugio K, Takemasa F. The role of glucocorticoid receptor and gene expression in the anti-inflammatory action of dexamethasone. Nature. 1979;280(5721):408–410. doi:10.1038/280408a0
18. Liu C, Yang N, Chen X, et al. The Flavonoid 7, 4′‐Dihydroxyflavone Prevents Dexamethasone Paradoxical Adverse Effect on Eotaxin Production by Human Fibroblasts. Phytother Res. 2017;31:449–458.
19. Giles AJ, Hutchinson M-KND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. doi:10.1186/s40425-018-0371-5
20. Kunicka JE, Talle MA, Denhardt GH, Brown M, Prince LA, Goldstein G. Immunosuppression by glucocorticoids: inhibition of production of multiple lymphokines by in vivo administration of dexamethasone. Cellular Immunology. 1993;149(1):39–49. doi:10.1006/cimm.1993.1134
21. De Gans J, van de Beek D. Van de Beek D. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347(20):1549–1556. doi:10.1056/NEJMoa021334
22. Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. doi:10.1056/NEJMoa1411321
23. Heimdal K, Hirschberg H, Slettebø H, Watne K, Nome O. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141–144. doi:10.1007/BF00172664
24. Bordag N, Klie S, Jürchott K, et al. Glucocorticoid (dexamethasone)-induced metabolome changes in healthy males suggest prediction of response and side effects. Sci Rep. 2015;5(1):15954. doi:10.1038/srep15954
25. Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone.. Endocrinology. 1994;134(1):277–286. doi:10.1210/endo.134.1.8275945
26. Bhattarai G, Poudel SB, Kook S-H, Lee J-C. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016;29:398–408. doi:10.1016/j.actbio.2015.10.031
27. Walsh S, Jordan GR, Jefferiss C, Stewart K, Beresford JN. High concentrations of dexamethasone suppress the proliferation but not the differentiation or further maturation of human osteoblast precursors in vitro: relevance to glucocorticoid‐induced osteoporosis. Rheumatology. 2001;40:74–83.
28. Inkielewicz-Stepniak I, Radomski MW, Wozniak M. Fisetin prevents fluoride-and dexamethasone-induced oxidative damage in osteoblast and hippocampal cells. Food Chem Toxicol. 2012;50:583–589.
29. Wang L, Heckmann BL, Yang X, Long H. Osteoblast autophagy in glucocorticoid‐induced osteoporosis. J Cell Physiol. 2019;234:3207–3215.
30. Zhao Y, Wang H, Li T, Yang F, Tzeng C. Baicalin Ameliorates Dexamethasone-Induced Osteoporosis by Regulation of the RANK/RANKL/OPG Signaling Pathway. Drug Des Devel Ther. 2020;14:195–206.
31. Xu YY, Chen FL, Ji F, Fei HD, Xie Y, Wang SG. Activation of AMP-activated protein kinase by compound 991 protects osteoblasts from dexamethasone. Biochem Biophys Res Commun. 2018;495:1014–1021.
32. Liu S, Fang T, Yang L, Chen Z, Mu S, Fu Q. Gastrodin protects MC3T3-E1 osteoblasts from dexamethasone-induced cellular dysfunction and promotes bone formation via induction of the NRF2 signaling pathway. Int J Mol Med. 2018;41:2059–2069.
33. Murgia D, Mauceri R, Campisi G, De Caro V. Advance on Resveratrol Application in Bone Regeneration: progress and Perspectives for Use in Oral and Maxillofacial Surgery. Biomolecules. 2019;9:94.
34. Tou JC. Resveratrol supplementation affects bone acquisition and osteoporosis: pre-clinical evidence toward translational diet therapy. Biochim Biophys Acta. 2015;1852:1186–1194.
35. Qin N, Wei L, Li W, et al. Local intra-articular injection of resveratrol delays cartilage degeneration in C57BL/6 mice by inducing autophagy via AMPK/mTOR pathway. J Pharmacol Sci. 2017;134:166–174.
36. Lee WC, Guntur AR, Long F, Rosen CJ. Energy Metabolism of the Osteoblast: implications for Osteoporosis. Endocr Rev. 2017;38(3):255–266.
37. Jin Z, Wei W, Yang M, Du Y, Wan Y. Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 2014;20:483–498.
38. Ma J, Fu Q, Wang Z, et al. Sodium hydrosulfide mitigates dexamethasone‐induced osteoblast dysfunction by interfering with mitochondrial function. Biotechnol Appl Biochem. 2019;66:690–697.
39. Ishii KA, Fumoto T, Iwai K, et al. Coordination of PGC-1β and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med. 2009;15:259–266.
40. Liang J, Zhang XY, Zhen YF, et al. PGK1 depletion activates Nrf2 signaling to protect human osteoblasts from dexamethasone. Cell Death Dis. 2019;10:888.
41. Cao X, Luo D, Li T, et al. MnTBAP inhibits bone loss in ovariectomized rats by reducing mitochondrial oxidative stress in osteoblasts. J Bone Miner Metab. 2020;38:27–37.
42. Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221–3247.
43. Kovac S, Angelova PR, Holmström KM, Zhang Y, Dinkova-Kostova AT, Abramov AY. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta. 2015;1850:794–801.
44. Han D, Gu X, Gao J, et al. Chlorogenic acid promotes the Nrf2/HO-1 anti-oxidative pathway by activating p21Waf1/Cip1 to resist dexamethasone-induced apoptosis in osteoblastic cells. Free Radic Biol Med. 2019;137:1–12.
45. St-Pierre J, Lin J, Krauss S, et al. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem. 2003;278(29):26597–26603.
46. Kong X, Wang R, Xue Y, et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5(7):e11707.
47. Wang EA, Rosen V, D’Alessandro JS, et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Nati Acad Sci. 1990;87:2220–2224.
48. Komori T. Regulation of bone development and maintenance by Runx2. Front Biosci. 2008;13:898–903.
49. Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.