Back to Journals » OncoTargets and Therapy » Volume 12
Resveratrol Inhibits Proliferation in HBL-52 Meningioma Cells
Authors Hu S, Wei W, Yuan J, Cheng J
Received 24 August 2019
Accepted for publication 7 November 2019
Published 30 December 2019 Volume 2019:12 Pages 11579—11586
DOI https://doi.org/10.2147/OTT.S228513
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Shun-an Hu,1 Wei Wei,2 Jia Yuan,2 Jin Cheng3
1Department of Neurosurgery, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei 441021, People’s Republic of China; 2Department of Oncology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei 441021, People’s Republic of China; 3Department of Pharmacy, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei 441021, People’s Republic of China
Correspondence: Jin Cheng
Department of Pharmacy, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, 136 Jingzhou Road, Xiangyang, People’s Republic of China
Tel +8618327506946
Email [email protected]
Jia Yuan
Department of Oncology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei 441021, People’s Republic of China
Email [email protected]
Objective: To investigate the effects of resveratrol on apoptosis and proliferation in meningioma cells and characterize the underlying molecular mechanism.
Methods: HBL-52 meningioma cells were treated with resveratrol at doses of 10, 50, 100, 200, and 400 μM for 24, 36, and 48 hrs. Inhibition of proliferation was measured by CCK8 assay, and apoptosis was determined by annexin V staining and flow cytometry. Expression of apoptosis-associated proteins (cleaved-caspase-3, pro-caspase-3) and Bcl-2 were measured by Western blot. Levels of miR-34a-3p and Bcl-2 mRNA were analyzed by reverse transcriptase PCR. A dual luciferase assay was used to determine whether miR-34a-3p binds to the 3ʹUTR of Bcl-2.
Results: Resveratrol reduces proliferation and increases apoptosis in HBL-52 cells. These effects increase with increasing resveratrol concentration and exposure time. Resveratrol increases levels of cleaved-caspase 3 protein as well as decreases levels of pro-caspase 3 protein and Bcl-2 mRNA. The 3ʹUTR of Bcl-2 contains putative binding sites for miR-34a-3p, and these binding sites can regulate the expression of a luciferase reporter. Overexpression of miR-34a-3p reduces Bcl-2 protein levels in HBL-52 cells.
Conclusion: Resveratrol suppresses proliferation and induces apoptosis in meningioma cells by upregulating miR-34a-3p, which in turn downregulates Bcl-2. Resveratrol may be a useful drug for treating meningiomas.
Keywords: resveratrol, meningioma, miR-34a-3p, apoptosis
Introduction
Meningioma is a common benign tumour with high morbidity and mortality in over the world. The 5-year relative survival rate of patients with meningeal tumor is only 19.5% for males and females.1 The common method of treating meningioma is surgery, when accompanied by adjuvant chemotherapy and radiotherapy, the prognosis of patients with meningeal tumor may be significantly improved.2 Chemotherapy is an effective mean to reducing pain and prolonging the life expectancy of subjects with meningeal cancer. Nevertheless, the clinical efficacy of chemotherapeutic drugs was significantly decreased due to the reverse action and multiple drug resistance.3,4 Previous studies have demonstrated that plant-derived drugs have significant antitumor activities, less side effects and wide range of targets, so they have been extensively observed.5–7
Resveratrol is a natural phytoalexin product, and is widely distributed in various plants, such as grapes and berries.8 As an important component of red wine, resveratrol has always been thought to exert a role in anti-cardiovascular diseases, and also show phytoestrogenic and antioxidant properties.9,10 Previous researches have also found that resveratrol could prolong lifespan and reduce the risk of tumors.11,12 Moreover, resveratrol has been reported to decrease the proliferation and induce the apoptosis of breast cancer stem cell-like cell via inhibiting the Wnt/β-actin cascade.12 Although a large number of researches showed the antitumor effect of resveratrol, the detailed underlying mechanisms need to be further observed.
MicroRNAs are about 19–25 nucleotide long, small non-coding RNAs with an important role in the post-transcriptional regulation of gene expression. MicroRNAs bind to specific sequences in the 3ʼ untranslated region (3ʼ UTR) of mRNAs resulting either to translation inhibition of the target gene.13 Previous findings have displayed that miRNAs play an important role in various tumors associated with cellular cascades involving in regulating the proliferation, invasion, migration, apoptosis and epithelial-mesenchymal transition (EMT).14 Furthermore, the down-regulation of miR-34a-3p in meningioma cells has been found, and its up-regulation could inhibit the expression level of B-cell CLL/lymphoma 2 (BCL2), which is an important regulator of apoptosis.15
In the current research, meningioma HBL-52 cell line was used to observe the effect and acting mechanism of resveratrol on cell proliferation and apoptosis. Our findings may provide a key understanding of the effect of resveratrol in the treatment of meningioma.
Materials and Methods
Drugs and Reagents
Resveratrol was purchased from Merck KGaA (Darmstadt, Germany), and it was dissolved in dimethyl sulfoxide (DMSO) to form a 10 mM stock solution. CCK8 kit and Annexin V-FITC/PI kit were obtained from Sigma-Aldrich. Dulbecco’s Modified Eagle Medium was purchased from HyClone and fetal bovine serum (FBS) was purchased from Gibco. Primary antibodies against cleaved-caspase-3 (dilution 1:1000), pro-caspase-3 (dilution 1:1000), Bcl-2 (dilution 1:1000), and β-actin (dilution 1:1000) were purchased from Abcam. PolyFect transfection reagent was obtained from Hilden (Qiagen, Germany). Dual-Luciferase® Reporter Assay System was purchased from Mannheim (Promega, Germany).
Cells Culture
HBL-52 cell line was obtained from the Bafeier Biological Co. Ltd (Wuhan, China) and cultured in DMEM medium supplemented with 10% FBS and antibiotics (100 U/mL streptomycin and 100 U/mL penicillin) in 25-cm2 culture flasks. Cultivation conditions set to 37°C in a humidified atmosphere containing 5% CO2. HBL-52 cells were subcultured when they reached exponential growth phase.
CCK8 Assay
The viability of HBL-52 cells was observed by CCK8 kit to assess the effects of resveratrol on the proliferation activity of cells. HBL-52 cells were grown in 96-well culture plates with DMEM medium at a density of 1×104 cells/mL. After incubation for 12 hrs at 37°C in a humidified atmosphere containing 5% CO2, HBL-52 cells were intervened with varying doses of resveratrol (0, 10, 50, 100, 200, and 400 μM) for 24, 36 and 48 hrs. Then, 10 μL of CCK8 reagent was separately added into each well, and cells were incubated for another 1.5 hrs. Finally, the absorbance of cells was measured at 490 nm using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The inhibition rate of resveratrol against the HBL-52 cells was calculated using the equation: inhibition rate (%)=(ODcontrol─ODdrug)/ODcontrol ×100%.
Apoptosis Assay
The effect of resveratrol on the apoptosis of HBL-52 cells was assessed by flow cytometry. HBL-52 cells were grown in 6-well culture plates with DMEM medium at a density of 2×105 cells/mL. After incubation for 24 hrs at 37°C in a humidified atmosphere containing 5% CO2, HBL-52 cells were intervened with varying doses of resveratrol (0, 50, 100, and 200 μM) for 36 hrs; then, cells were recovered and prepared as cell suspension. Next, HBL-52 cells (1×106 cells/mL) were resuspended in 200 μL staining buffer. 2 μL of Annexin V was added into the suspension; then, the mixture was slightly mixed and incubated in the dark at 2~8°C for 15 mins. 4 μL of PI solution was then added into the suspension, the mixture was slightly mixed and incubated in the dark at 2~8°C for 5 mins. The apoptotic rate of HBL-52 cells were determined by a flow cytometer (BD Accuri C6; BD Biosciences, Franklin Lakes, NJ, USA).
RNA Extraction and Real-Time - PCR Assay
Total RNA from HBL-52 cells was extracted by TRIZOL (Invitrogen, Carlsbad, CA) WITH RNase-free DNase. Reverse transcription was carried out according to the manufacturerʼs proposal. Real-time PCR was carried out to measure the expression levels of miR-34a-3p and Bcl-2 mRNA. The forward primers and reverse primers of each gene were designed as follows: miR-34a-3p (F: 5ʹ-CGGGATCCGCAGCCTCTCCATCTTC-3ʹ; R: 5ʹ-GGAATTCGGCTAGGAGGATCAACACAC-3ʼ), Bcl-2 (F: 5ʹ-TTCTTTGAGTTCGGTGGGGTC-3ʹ, R: 5ʹ-TGCATATTTGTTTGGGGCAGG-3ʹ) and β-actin (F: CTCCATCCTGGCCTCGCTGT-3ʹ, R: 5ʹ-GCTGTCACCTTCACCGTTCC-3ʹ). Measurement of relative expression of miR-34a-3p was carried out by a High-Specificity miR-34a-3p qRT-PCR Detection Kit (Stratagene Corp. La Jolla, CA, USA) in conjunction with an ABI 7500 thermal cycler (Applied Bio- systems, USA). All protocols were carried out based on the manufacturer’s instructions. We used U6 small nuclear RNA (U6 snRNA) for normalization. To measure the relative expression of Bcl-2 mRNA, the qRT-PCR tests were carried out by ABI TaqMan PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). β-actin was used as an internal control. The corresponding CT values were recorded with ABI 7500 software, and then the relative expression levels were calculated according to the formula 2−ΔΔCt. All tests were carried out in triplicate.
Western Blot Assay
Western blot was used to detect the expression levels of Cleaved caspase-3, pro-caspase-3 and Bcl-2. Cells were harvested after intervention and cell pellets were lyzed with 2×sample buffer (130 mM Tris/HCl, 6% SDS, 10% 3-mercapto-1,2-propanediol, 10% glycerol). Proteins (40 μg) were subjected to 12% of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After separation, the target proteins were transferred to a nitrocellulose membrane. After blocking, the membranes were incubated overnight at 4°C with diluted antibodies against Cleaved caspase-3 (dilution 1:1000), pro-caspase-3 (dilution 1:1000) and Bcl-2 (dilution 1:1000). Blots were washed and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:3000). Proteins were visualized using a chemiluminescence detection system (Thermo Scientific, USA).
Plasmids
The miR-34a-3p plasmid was generated using de novo synthesis of the sequence for its precursor (nucleotides 9151617–9151816 on chromosome 1 (hg19)), and this fragment was then ligated into pSG5 (Agilent Technologies, USA) by EcoRI and BglII restriction sites using Eurofins Genomics (Ebersberg, Germany). A fragment of Bcl-2 3ʼUTR (NM_000633.2, nucleotides 1796–2511) was de novo generated and ligated into pEX-A2 plasmid using Eurofins Genomics (Ebersberg, Germany). The potential binding sites for miR-34a-3p in the BCL2 3ʹUTR were mutated in an overlap extension PCR.
Transfection and Dual Luciferase Assays
HEK293T cells (4×104) were plated in 24-well plates, and transfected with 0.2 μg reporter gene and 0.8 μg miR-34a-3p precursor plasmid per well by PolyFect transfection reagent (Qiagen, Hilden, Germany) after one day. Dual luciferase assay was carried 48 hrs after transfection by the Dual-Luciferase® Reporter Assay System (Promega, Mannheim, Germany) based on manufacturer’s proposals.
Statistics
Each test was performed in triplicate. Data were displayed as the mean ± standard deviation. The differences between our data were assessed by one-way analysis of variance followed by a least significant difference post hoc test using SPSS 19.0. P<0.05 was considered as a statistical difference.
Results
Resveratrol Suppressed the Proliferative Activity of HBL-52 Cells
HBL-52 cells were intervened by varying concentrations of resveratrol for 24, 36, and 48 hrs, respectively. Proliferation inhibitory rates of HBL-52 cells were measured using CCK8 assay. As displayed in Figure 1, the inhibition of cell proliferation was greatly increased in HBL-52 cell line in response to resveratrol with a concentration- and time-dependent manner when compared to the control group (0 μM of resveratrol).
Resveratrol Induced the Apoptosis of HBL-52 Cells
HBL-52 cells were treated with different concentrations of resveratrol for 36 hrs, labeled using Annexin V-FITC/PI and determined using a flow cytometer. The staining of early-stage apoptotic cells was marked using Annexin V staining, and the staining of late-stage apoptotic cells was labeled by Annexin V and PI staining. As shown in Figure 2, in the control group, no obvious apoptotic changes were found using flow cytometric analyses. Nevertheless, the number of apoptotic cells was greatly increased following resveratrol treatment with a concentration-dependent manner.
Effect of Resveratrol on the Expression of Cleaved Caspase-3 and Pro-Caspase-3
The effects of resveratrol on the expression levels of apoptosis-associated proteins were assessed by Western blot. As shown in Figure 3, resveratrol treatment increased the level of cleaved-caspase-3 and decreased the expression of pro-caspase-3. Furthermore, the impacts of different concentrations of resveratrol on the expression levels of cleaved-caspase-3 and pro-caspase-3 protein were significant with a concentration-dependent manner. Our results indicated that resveratrol-induced HBL-52 cells death is associated with the expression levels of apoptosis-related proteins.
Impact of Resveratrol on miR-34a-3p/Bcl-2 Pathway
The effects of resveratrol on the expression levels of miR-34a-3p and Bcl-2 mRNA were determined using real-time PCR. As shown in Figure 4, our findings suggested that 50, 100, and 200 μM of resveratrol significantly induced the upregulation of miR-34a-5p expression (P<0.05). Additionally, the anti-apoptotic protein expression of Bcl-2 was greatly decreased in HBL-52 cells treated with different concentrations of resveratrol compared with control group (P<0.05). In addition, the effects of different concentrations of resveratrol on the expression levels of miR-34a-3p and Bcl-2 mRNA were significant with a concentration-dependent manner.
Bcl-2 Is the Direct Target of miR-34a-3p
miR-34a-3p was displayed to have conserved binding sites for the 3ʼ UTR of Bcl-2 (Figure 5A). To experimentally conform Bcl-2 as a target of miR-34a-3p, we constructed the miR-34a-3p binding sites in the 3ʼUTR of Bcl-2 genes into the luciferase-expressing reporter plasmid to observe the impacts of miR-34a-3p on reporter activity in HEK293T cells. As displayed in Figure 5B, co-transfection of miR-34a-3p with the plasmid resulted in the less luciferase activity than transfection of the plasmid alone. However, the mutation of binding sites in the 3ʼUTR of Bcl-2 genes eliminated the influence of miR-34a-3p. These data indicate that miR-34a-3p could directly interact with the binding sites in the 3ʼUTR of Bcl-2 gene.
Over-Expression of miR-34a-3p Down-Regulated Bcl-2 Expression
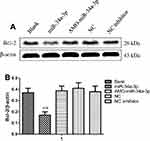
Previous study has supported the ideal that the expression level of miR-34a-3p in meningioma cells is downregulated.16 In order to assess the impacts of miR-34a-3p on the expression of Bcl-2, we determined the protein expression of Bcl-2 in HBL-52 cells. Bcl-2 expression was evaluated using Western blot 48 hrs after transfection with miR-34a-3p or anti-miR-34a-3p (inhibitor). Representative Western blot for Bcl-2 was shown in Figure 6A. After overexpression of miR-34a-3p, the protein level of Bcl-2 was significantly decreased compared with the control group (P<0.01, Figure 6B), indicating that upregulation of miR-34a-3p could inhibit the expression level of Bcl-2.
Discussion
These data of the current research suggested that resveratrol was able to greatly suppress the proliferation and increase the apoptosis of HBL-52 cells in a concentration- and time-dependent manner within a certain range. Our results are consistent with the findings of previous studies on other cancers.17,18
By comparing the results of the CCK8 test, it has shown that when the dose of resveratrol was more than 10 μM (except 100 μM), the inhibitory rate of cell proliferation at 48 hrs was not significantly different from those at 24 hrs or 36 hrs at the same concentration. In addition, when the dose of resveratrol was more than 50 μM (except 200 μM), the inhibitory rate of cell proliferation at 36 hrs was significantly different from those at 24 hrs at the same concentration. Therefore, we make a hypothesis that once the dose had increased to a certain point, prolonging the duration of agents therapy did not make a difference to the suppression of cell proliferation following resveratrol treatment.
Reduced cell viability is a comprehensive response, which represent functional states in proliferation, induction of apoptosis or autophagy and cytotoxic necrosis. An earlier study suggested that resveratrol reduces cell viability via inducing the apoptosis.19 Nevertheless, some researchers demonstrated that resveratrol leads to the death of ovarian cancer A2780 cells through a mechanism distinct from apoptosis.20 Both apoptotic cascades correlated with Bcl-2 and activation of caspase-3 were suggested to be required for the resveratrol-induced apoptosis in SGC-7901 gastric cancer cells.17 The initiation and execution of endogenous and exogenous apoptosis are mediated by Bcl-2 and caspase family proteins.21,22 Abnormal caspase level and activation have participated in a variety of tumors.22
In the current research, Western blot assay suggested that the expression of cleaved caspase-3 protein in HBL-52 cells greatly increased following resveratrol intervention. In addition, the level of pro-caspase-3 was significantly reduced after treatment with resveratrol. Moreover, a higher ratio of cleaved caspase-3/pro-caspase-3 and increased caspase-3 activation induced by resveratrol may lead to the apoptosis of HBL-52 cells.
Previous research has found the downregulation of miR-34a-3p in higher-grade meningiomas,15 and we identified that Bcl-2 is a predicted target of miR-34a-3p. Our study put forward a view that downregulation of miR-34a-3p in HBL-52 cells may contribute to increased cell proliferation and decreased apoptosis of tumor cells via the high expression of Bcl-2. The overexpression of miR-34a-3p in meningioma also inhibited the cell proliferation and induced the apoptosis.
Our results indicated that resveratrol could induce the expression level of miR-34a-3p mRNA and further suppress the expression of Bcl-2 mRNA with a dose-dependent manner. In some tumor cells, the inactivation of Bcl-2 is triggered by chemotherapeutic drugs that accompany the apoptosis of cancer cell lines.23 Some scholars thought that resveratrol treatment was able to arrest cells at G1- and S-phases, and led to the decreased expression of anti-apoptotic protein Bcl-2.24 In meningioma cells, caspase-3 activation was investigated to be associated with resveratrol-induced apoptosis.25 In the current research, resveratrol was able to downregulate expression and activation of Bcl-2 in HBL-52 cells via upregulating miR-34a-3p expression.
Conclusions
In conclusion, these findings of the current study suggested that resveratrol was able to inhibit the viability and induce the apoptosis of HBL-52 cells by inducing miR-34a-3p expression. Therefore, a potential application of resveratrol may be as an anticancer drug for the treatment of meningioma.
Acknowledgments
The authors would like to thank the Department of Pharmacy, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science. Shun-an Hu and Wei Wei are co-first authors. Jia Yuan and Jin Cheng are co-corresponding authors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Taylor AJ, Frobisher C, Ellison DW, et al. Survival after second primary neoplasms of the brain or spinal cord in survivors of childhood cancer: results from the British Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(34):5781–5787. doi:10.1200/JCO.2009.22.4386
2. Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncol. 2018;14(21):2161–2177. doi:10.2217/fon-2018-0006
3. Jiang C, Song T, Li J, et al. Promotes proliferation and resistances to apoptosis in meningioma. Mol Neurobiol. 2017;54(1):779–787. doi:10.1007/s12035-016-9763-z
4. Moazzam AA, Wagle N, Zada G. Recent developments in chemotherapy for meningiomas: a review. Neurosurg Focus. 2013;35(6):E18. doi:10.3171/2013.10.FOCUS13341
5. James MI, Iwuji C, Irving G, et al. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 2015;364:135–141. doi:10.1016/j.canlet.2015.05.005
6. He B, Wei W, Liu J, Xu Y, Zhao G. Synergistic anticancer effect of curcumin and chemotherapy regimen FP in human gastric cancer MGC-803 cells. Oncol Lett. 2017;14:3387–3394. doi:10.3892/ol.2017.6627
7. Tran KQ, Tin AS, Firestone GL. Artemisinin triggers a G1 cell cycle arrest of human Ishikawa endometrial cancer cells and inhibits cyclin dependent Kinase-4 promoter activity and expression by disrupting NF-kB transcriptional signaling. Anticancer Drugs. 2014;25:270–281. doi:10.1097/CAD.0000000000000054
8. Rauf A, Imran M, Suleria HAR, Ahmad B, Peters DG, Mubarak MS. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017;8(12):4284–4305. doi:10.1039/C7FO01300K
9. Thaung Zaw JJ, Howe PRC, Wong RHX. Does phytoestrogen supplementation improve cognition in humans? A systematic review. Ann N Y Acad Sci. 2017;1403(1):150–163. doi:10.1111/nyas.13459
10. Xia N, Daiber A, Förstermann U, Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br J Pharmacol. 2017;174(12):1633–1646. doi:10.1111/bph.v174.12
11. Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;296–300. doi:10.1016/j.cub.2005.12.038
12. Fu Y, Chang H, Peng X, et al. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/β-catenin signaling pathway. PLoS One. 2014;9:e102535. doi:10.1371/journal.pone.0102535
13. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi:10.1016/S0092-8674(04)00045-5
14. Cheng Q, Yi B, Wang A, Jiang X. Exploring and exploiting the fundamental role of microRNAs in tumor pathogenesis. Onco Targets Ther. 2013;6:1675–1684. doi:10.2147/OTT.S52730
15. Kouhsar M, Azimzadeh Jamalkandi S, Moeini A, Masoudi-Nejad A. Detection of novel biomarkers for early detection of non-muscle-invasive bladder cancer using competing endogenous RNA network analysis. Sci Rep. 2019;9(1):209–311. doi:10.1038/s41598-019-44944-3
16. Werner TV, Hart M, Nickels R, et al. MiR-34a-3p alters proliferation and apoptosis of meningioma cells in vitro and is directly targeting SMAD4, FRAT1 and BCL2. Aging (Albany NY). 2018;9(3):932–954. doi:10.18632/aging.101201
17. Wu X, Xu Y, Zhu B, Liu Q, Yao Q, Zhao G. Resveratrol induces apoptosis in SGC-7901 gastric cancer cells. Oncol Lett. 2018;16(3):2949–2956. doi:10.3892/ol.2018.9045
18. Ding XZ, Adrian TE. Resveratrol inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Pancreas. 2002;25:e71–e76. doi:10.1097/00006676-200211000-00024
19. Yu XD, Yang JL, Zhang WL, Liu DX. Resveratrol inhibits oral squamous cell carcinoma through induction of apoptosis and G2/M phase cell cycle arrest. Tumour Biol. 2016;37:2871–2877. doi:10.1007/s13277-015-3793-4
20. Opipari AW
21. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi:10.1016/S0092-8674(04)00046-7
22. Galluzzi L, Vitale I, Abrams JM, et al. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19:107–120. doi:10.1038/cdd.2011.96
23. Geng M, Wang L, Li P. Correlation between chemosensitivity to anticancer drugs and Bcl-2 expression in gastric cancer. Int J Clin Exp Pathol. 2013;6(11):2554–2559.
24. Sun C, Hu Y, Liu X, et al. Resveratrol downregulates the constitutional activation of nuclear factor-kappaB in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet Cytogenet. 2006;165:9–19. doi:10.1016/j.cancergencyto.2005.06.016
25. Shimizu T, Nakazato T, Xian MJ, Sagawa M, Ikeda Y, Kizaki M. Resveratrol induces apoptosis of human malignant B cells by activation of caspase-3 and p38 MAP kinase pathways. Biochem Pharmacol. 2006;71:742–750. doi:10.1016/j.bcp.2005.12.018
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.