Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
Resting-state functional connectivity changes within the default mode network and the salience network after antipsychotic treatment in early-phase schizophrenia
Authors Wang Y , Tang W, Fan X, Zhang J, Geng D, Jiang K, Zhu D, Song Z, Xiao Z, Liu D
Received 30 September 2016
Accepted for publication 4 January 2017
Published 7 February 2017 Volume 2017:13 Pages 397—406
DOI https://doi.org/10.2147/NDT.S123598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Wai Kwong Tang
Yingchan Wang,1 Weijun Tang,2 Xiaoduo Fan,3 Jianye Zhang,1 Daoying Geng,2 Kaida Jiang,1 Dianming Zhu,1 Zhenhua Song,1 Zeping Xiao,1 Dengtang Liu1
1First-Episode Schizophrenia and Early Psychosis Program, Division of Psychotic Disorders, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, 2Department of Radiology, Huashan Hospital, Fu Dan University, Shanghai, People’s Republic of China; 3Psychotic Disorders Program, UMass Memorial Medical Center, UMass Medical School, Worcester, MA, USA
Objective: Abnormal resting-state functional connectivity (FC), particularly in the default mode network (DMN) and the salience network (SN), has been reported in schizophrenia, but little is known about the effects of antipsychotics on these networks. The purpose of this study was to examine the effects of atypical antipsychotics on DMN and SN and the relationship between these effects and symptom improvement in patients with schizophrenia.
Methods: This was a prospective study of 33 patients diagnosed with schizophrenia and treated with antipsychotics at Shanghai Mental Health Center. Thirty-three healthy controls matched for age and gender were recruited. All subjects underwent functional magnetic resonance imaging (fMRI). Healthy controls were scanned only once; patients were scanned before and after 6–8 weeks of treatment.
Results: In the DMN, the patients exhibited increased FC after treatment in the right superior temporal gyrus, right medial frontal gyrus, and left superior frontal gyrus and decreased FC in the right posterior cingulate/precuneus (P<0.005). In the SN, the patients exhibited decreased FC in the right cerebellum anterior lobe and left insula (P<0.005). The FC in the right posterior cingulate/precuneus in the DMN negatively correlated with the difference between the Clinical Global Impression (CGI) score pre/post-treatment (r=–0.564, P=0.023) and negative trends with the difference in the Positive and Negative Syndrome Scale (PANSS) total score pre/post-treatment (r=–0.475, P=0.063) and the difference in PANSS-positive symptom scores (r=–0.481, P=0.060).
Conclusion: These findings suggest that atypical antipsychotics could regulate the FC of certain key brain regions within the DMN in early-phase schizophrenia, which might be related to symptom improvement. However, the effects of atypical antipsychotics on SN are less clear.
Keywords: schizophrenia, fMRI, default network, salience network, antipsychotics
Introduction
Functional magnetic resonance imaging (fMRI) allows the in vivo study of the relationship between altered cerebral activation patterns and psychopathological and cognitive features of psychiatric disorders.1 Different brain networks have been identified using resting-state fMRI, including the default mode network (DMN), the central executive network (CEN), and the salience network (SN).2–6 In particular, the DMN and SN are two important networks identified to be involved in schizophrenia.6–9
The DMN was first proposed by Raichle et al2 in 2001, who noted that the DMN is “active” in the resting state and “deactive” during tasks. The DMN primarily includes the posterior medial cortex, posterior lateral cortex, ventral medial prefrontal cortex (VMPFC), and frontal–dorsal medial prefrontal cortex.10 Although the exact functions of the DMN are unclear, it may be involved in monitoring the external environment, maintaining self-awareness,11 generating spontaneous thoughts,12 mind-wandering, and episodic memory.13 Some studies have reported DMN abnormalities in schizophrenia,14–20 and others have suggested that the abnormal functional activity within certain DMN brain regions is associated with the symptoms of schizophrenia.14,19–21 Abnormal connectivity in the prefrontal cortex may be associated with the inability to allocate resources properly between internal thoughts and external stimuli,19 and thought disturbances such as delusions, which are closely related to spontaneous thoughts, may be the result of abnormal DMN function in schizophrenia.12 The SN has been proposed to function in the identification of internal and external stimuli (salience) and the shifting of brain function from DMN to CEN activities.7,9 Hallucinations, delusions, disorganization, and psychomotor poverty in schizophrenia may be related to SN deficits.9,22–24
Palaniyappan et al25 reported reduced surface area across all three intrinsic networks (DMN, SN, and CEN) in schizophrenia; furthermore, the residual symptom burden was more pronounced in patients with lower DMN and SN surface areas. Orliac et al26 observed reduced functional connectivity (FC) in both the DMN and SN in patients with schizophrenia and that decreased connectivity in paracingulate cortex correlated with difficulty in abstract thinking, whereas decreased connectivity in the left striatum correlated with delusion and depression. Snitz et al27 reported that patients with schizophrenia exhibited hypoactivation in the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) and that 4 weeks of antipsychotic treatment significantly improved ACC function. Lui et al observed that the amplitude of low-frequency fluctuations (ALFF) in the DMN of patients with first-episode schizophrenia was increased significantly, particularly in the bilateral prefrontal and parietal cortex, left superior temporal cortex, and right caudate nucleus, after 6 weeks of atypical antipsychotic treatment. This increased regional ALFF was associated with symptom improvement.28
We hypothesized that 1) there exists abnormal FC within the DMN and SN in patients with early-phase schizophrenia, which might be closely related to positive symptoms such as delusions and hallucinations, and 2) antipsychotics such as atypical agents can regulate the abnormal FC of certain brain regions within the DMN or SN in schizophrenia patients and accordingly contribute to symptom improvement after treatment with medications.
Materials and methods
Study design
This was a prospective study of patients diagnosed with schizophrenia or schizophreniform disorder and treated with second-generation antipsychotics (SGAs). The patients were recruited between April 2013 and January 2014 at the Shanghai Mental Health Center. The study was approved by the Institutional Review Board of Shanghai Mental Health Center. Written informed consent was obtained from each participant.
Participants
To be included, patients had to have been diagnosed with schizophrenia or schizophreniform disorder according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR) criteria. The diagnosis was confirmed by a research psychiatrist (YCW) using MINI plus v 5.0.29 Patients diagnosed with schizophreniform disorder at study enrollment were subsequently diagnosed with schizophrenia after 6 months of illness duration. All patients were first-episode, drug-naïve psychosis or schizophrenia patients who relapsed following drug withdrawal >6 months. No subject was treated with long-acting antipsychotic medications, and all subjects were antipsychotic-free for at least 12 weeks at study enrollment. All patients were aged 18–40 years and in the early stage of illness, with a total duration no more than 5 years.30,31
The exclusion criteria were 1) inability to provide informed consent; 2) psychotic patients in unstable clinical conditions (eg, aggressive and uncooperative); 3) current substance abuse; 4) any other psychiatric diagnosis; 5) significant medical conditions, including neurological, severe cardiovascular, hepatic, or renal diseases; 6) pregnancy or breastfeeding; 7) electroconvulsive therapy within 6 months; or 8) patients with MRI contraindications.
Thirty-three healthy controls matched for age and gender were recruited from the local community through advertisement and were screened by the same research psychiatrist (YW) using MINI plus v 5.0. Individuals with any psychiatric disease, neurological disease, or a positive family history of psychiatric disease were excluded. The T1- and T2-weighted MRI results were reviewed by an experienced neuroradiologist, and no gross abnormalities were observed for all subjects.
Antipsychotic treatment
All patients were treated with SGAs (risperidone, paliperidone, olanzapine, quetiapine, aripiprazole, and ziprasidone). The choice of drug and dose was based on the treating psychiatrist’s clinical judgment. Usually, the dosage increased during the first 2 weeks of treatment and then remained constant for 4–6 weeks until the follow-up scan. Clinical symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS),32 and the severity of illness was also assessed by the Clinical Global Impressions (CGI) – severity scale.33,34
MRI assessment
Healthy controls were scanned only once to define the range of normal function. All patients were scanned and evaluated twice (pre- and post-treatment). The post-treatment assessment was performed after 6–8 weeks of treatment.
Imaging data acquisition
All subjects were scanned using a Siemens Verio 3.0 T MRI scanner. Foam pads and ear plugs were used to limit head motion and reduce machine noise. Before scanning, the participants were instructed to keep his/her eyes closed, to relax, to refrain from moving, to stay awake, and to let his/her thoughts come and go.26
Structure images were acquired with a fast spin echo (SE) sequence using the following parameters: repetition time (TR) =2,300 ms, echo time (TE) =2.98 ms, matrix 240×256, flip angle 9°, field of view =256 mm, voxel size =1×1×1 mm3, slice thickness =1 mm, gap =0 mm, and 196 slices. The blood oxygenation level dependent (BOLD) fMRI images were obtained using a gradient-echo (GRE) echo-planar imaging (EPI) sequence with the following parameters: TR =2,000 ms, TE =35 ms, matrix 64×64, flip angle 90°, field of view =256 mm, voxel size =1×1×1 mm3, slice thickness =4 mm, gap =0 mm, and 33 slices.
Preprocessing
The fMRI data were preprocessed using the Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm/), including slice timing, realignment, and normalization. The first 10 time points from each functional image were discarded to ensure the stability of the magnetic field. The participants included in the analysis exhibited <2 mm of maximum displacement in the x, y, or z direction and <2 rad of angular rotation about each axis. After slice timing and realignment, the data were normalized to standard Montreal Neurological Institute space, resampled to 3×3×3 mm3, and spatially smoothed with a 4×4×4 mm3 full width at half maximum Gaussian kernel. Finally, the smoothed images were band-pass filtered (0.01–0.08 Hz) using the REST software (http://restfmri.net/) to remove interference signals caused by machine signal drift and physiological noises such as those caused by breathing and heartbeats.
Independent component analysis (ICA)
The Group ICA fMRI Toolbox software (GIFT) (http://icatb.sourceforge.net) was used to analyze the fMRI data with spatial ICA. A minimum description length (MDL)35 algorithm was used to determine the optimal number of spatially independent components using the fMRI data of all participants. The mean dimension estimation was 22.45 (standard deviation [SD] =4.32); consequently, 22 components were decomposed from the images by GIFT.
The reference map for the DMN was provided by the GIFT software template (rDMN_ICA_REST 3×3×3.nii), which was obtained from 42 healthy participants by ICA using the posterior cingulate gyrus (BA 23/31), posterior parietal cortex (BA 7/39/40), superior frontal gyrus (BA 8/9/10), and ACC (BA 11/32) brain areas as a DMN mask.36 The reference template for the SN was constructed by selecting the bilateral insula and anterior cingulate areas7–9,22–24 on the IBASM-71 standard brain atlas in the WFU_PICKATLAS software (http://www.fmri.wfubmc.edu/cms/software). The components with high correlation to the maps were considered as the DMN and the SN of the participants. Accordingly, component 3 (r=0.40) and component 6 (r=0.38) were identified as the DMN component and the SN component.
Statistical analyses
Demographic characteristics were compared between patients with schizophrenia and healthy controls using t-test for continuous variables and chi-square test for categorical variables. Because the education levels of the patients and healthy controls differed (P=0.015), education level was controlled as a covariate in subsequent analyses. Individual DMN and SN component GIFT maps were entered into SPM8 for group analyses. Comparisons of schizophrenia patients and healthy controls were performed using two-sample t-tests, and patients were also compared before and after treatment using paired t-tests. Clusters with 20 voxels or greater surviving a false discovery rate (FDR) with an uncorrected threshold of P<0.005 were considered significant. Pearson correlations were used to examine the relationships: 1) at baseline, the mean Z values in the region clusters that significantly differed between schizophrenia patients and healthy controls correlated with psychopathology ratings, and 2) within schizophrenia patients, the mean Z values in the region clusters that were significantly altered after antipsychotic treatment correlated with the changes in psychopathology.
Results
Demographic and clinical characteristics
Thirty-six patients and 33 healthy controls were enrolled in this study. Three patients were excluded due to excessive head motion or technical failure. After 6–8 weeks of treatment, 26 patients fulfilled the follow-up visits and performed another MRI scan.
Table 1 shows the demographic and clinical characteristics of the two groups. No significant difference was observed between the schizophrenia patients and healthy controls in terms of age and gender; however, the difference in education between groups was significant (P=0.015) and education was controlled for in the subsequent analyses. Since SN deficits have been suggested to lead to psychosis symptoms, particularly reality distortion, delusions (item P1 of the positive symptoms subscale in PANSS) and hallucinations (item P3 of the positive symptoms subscale in PANSS) were specifically evaluated.7–9,22–24
Baseline ICA analysis (patients vs controls)
Abnormal resting-state FC in patients at baseline
Compared with healthy controls, the DMN of patients with schizophrenia exhibited increased FC in the right cerebellum posterior lobe, right inferior parietal lobule, and right posterior cingulate/precuneus and decreased FC in the left medial frontal gyrus, left precuneus, and left superior frontal gyrus (P<0.005, uncorrected, cluster ≥20) (Figure 1).
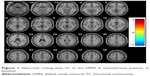  | Figure 1 Abnormal resting-state FC in the DMN in schizophrenia patients at baseline. |
Most of the brain areas in the SN exhibited decreased FC in patients with schizophrenia, including the left lentiform nucleus, left anterior cingulate gyrus, right middle frontal gyrus, and left middle frontal gyrus; only one brain area (left superior temporal gyrus) exhibited increased FC (P<0.005, uncorrected, cluster ≥20) (Figure 2).
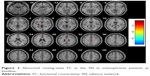  | Figure 2 Abnormal resting-state FC in the SN in schizophrenia patients at baseline. |
Correlation analysis: correlation with clinical characteristics
Correlation analyses were performed to investigate the correlation of FC with psychopathology in patients with schizophrenia. In the DMN, the FC of the left precuneus significantly and inversely correlated with PANSS total score (r=−0.632, P=0.009) and negative symptoms score (r=−0.610, P=0.012). The FC of the right posterior cingulate/precuneus significantly positively correlated with delusions (P1) (r=0.573, P=0.020), hallucinations (P3) (r=0.549, P=0.028), and reality distortion (P1+P3) (r=0.678, P=0.004). The FC of the left superior frontal gyrus negatively correlated with negative symptoms (r=−0.542, P=0.030), and the FC of the left medial frontal gyrus negatively correlated with general psychopathology (r=−0.496, P=0.051).
In the SN, the FC of the left lentiform nucleus significantly negatively correlated with positive symptoms (r=−0.440, P=0.010), and the FC of the left superior temporal gyrus exhibited a marginal significant correlation with PANSS total score (r=0.335, P=0.056) and with CGI-SI score (r=0.334, P=0.057).
ICA analysis (interpatient, pre- vs post-treatment)
FC changes within DMN and SN pre- and post-treatment
Twenty-six patients fulfilled the follow-up visits and performed another MRI scan. Table 2 shows the clinical changes of the patients with schizophrenia pre- and post-treatment. All patients received atypical antipsychotics, 18 patients received monotherapy: paliperidone (n=5), risperidone (n=4), olanzapine (n=4), ziprasidone (n=2), quetiapine (n=2), and aripiprazole (n=1), eight patients received combined therapy, and the average dose in chlorpromazine equivalence (CPZ eq) was 541±146 mg/day.37 During the treatment, seven patients received benzhexol hydrochloride for the side effects of the extra-pyramidal symptoms, and the serum prolactin level was significantly increased in four patients who received paliperidone or risperidone treatment.
After 6–8 weeks of treatment, in the DMN, the patients exhibited increased FC in the right superior temporal gyrus, right medial frontal gyrus, and left superior frontal gyrus and decreased FC in the right posterior cingulate/precuneus (P<0.005, uncorrected, cluster =20) (Figure 3). In the SN, the patients exhibited decreased FC in the right cerebellum anterior lobe and left insula (P<0.005, uncorrected, cluster =20) (Figure 4).
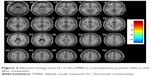  | Figure 3 Altered resting-state FC in the DMN in schizophrenia patients before and after treatment. |
Treatment effects: altered FC with symptom improvement
The altered mean Z in the right posterior cingulate/precuneus within DMN significantly negatively correlated with CGI score change (pre- and post-treatment) (r=−0.564, P=0.023) and marginally significantly correlated with PANSS total score change (r=−0.475, P=0.063) and positive symptom change (r=−0.481, P=0.060).
Discussion
The aim of this study was to examine the effects of atypical antipsychotics on DMN and SN and the relationship between these effects and symptom improvement in patients with schizophrenia. We observed both increased and reduced FC within the DMN and SN in unmedicated schizophrenia patients. The patients exhibited FC changes within the two networks after 6–8 weeks of atypical antipsychotic treatment.
In this study, the patients exhibited decreased FC in the prefrontal cortex (left and right medial frontal gyrus and left superior frontal gyrus) before treatment. The FC in the left superior frontal gyrus negatively correlated with negative symptoms in schizophrenia, and the FC in the prefrontal brain regions generally increased after antipsychotic treatment. This result is consistent with most previous DMN studies.20,38,39 Tang et al39 observed that patients with early-onset schizophrenia exhibited increased FC between the medial frontal gyrus and other areas of the DMN, and partial correlation analyses indicated that the FC of the medial frontal gyrus significantly correlated with PANSS-positive symptoms. The authors suggested that these abnormalities were a source of abnormal introspectively oriented mental activities. The prefrontal cortex is the key brain region associated with episodic memory, working memory, self-inhibition capability, and other functions. If the prefrontal function is impaired or if linked brain areas are damaged, personality changes such as emotional blunting, initiative, and sluggish behavior (termed frontal lobe syndrome) may arise. A quantitative meta-analysis of resting-state brain activity in schizophrenia revealed hypoactivation in the ventromedial prefrontal cortex (vmPFC) and noted that the vmPFC had previously been identified as a crucial area for self-referential processing and may represent a target for increasing the diagnostic validity of resting-state activity for disorders with dysfunctions of the self.40 The cingulate cortex and the precuneus were identified as important in this study, similar to observations reported by Mingoia et al.20 Lower connectivity of the ACC within the anterior DMN component and precuneus within the posterior DMN component has been reported for patients with poor insight compared with those with good insight; connectivity between the anterior and posterior parts of the DMN was lower in patients than in controls and qualitatively different between the good and poor insight patient groups.41 The precuneus and posterior cingulate are considered the most critical brain regions of the DMN. The precuneus has been suggested to be closely related to the functions of self-awareness, self-centered mental imagery, and extraction of episodic memory, and it is the earliest brain region to recover consciousness in patients in a vegetative state.10 The cingulate gyrus is the center node of the whole-brain cortical structure.20 A positron emission tomography (PET) study revealed that the metabolic activity in the resting state is higher in the precuneus and posterior cingulate cortex than in other areas of the brain.10 Complex network analyses of resting-state brain function have indicated that the precuneus and posterior cingulate are the key brain regions or node contacts with other brain areas within the DMN and between the DMN and other networks.10,42
The effect of antipsychotic drugs, particularly atypical antipsychotic drugs, on the resting-state DMN remains unclear. A multisite fMRI study suggested that antipsychotics diminish neural activation in the motor (cortical and subcortical) and DMN in patients with schizophrenia and that antipsychotics may be a potential confounding limitation to the interpretation of fMRI studies on the disease process in medicated patients with schizophrenia.43 In this study, we observed FC abnormalities in the DMN of patients with schizophrenia. The FC of the anterior–posterior cingulate and precuneus was altered after 6–8 weeks of atypical antipsychotic treatment, and the FC in these two brain regions exhibited a trend of correlation with changes in psychiatric symptoms. Based on these results, FC in the medial frontal cortex, posterior cingulate/precuneus, and other important areas of the brain can be considered the key nodes in DMN function that lead to psychotic symptoms in schizophrenia, and atypical antipsychotics may modulate the FC of these DMN key nodes such as the prefrontal cortex and right posterior cingulate/precuneus, to alter the level of function of the DMN and eventually improve mental symptoms.
This study found that the SN mainly includes the bilateral insula, anterior cingulate gyrus, temporal lobe (superior temporal gyrus, middle temporal gyrus), frontal gyrus (middle frontal gyrus, inferior frontal gyrus), and part of the limbic system parts. Like other resting-state brain networks, the specific brain regions related to the SN have not yet been confirmed, and most scholars consider the bilateral anterior insula and anterior cingulate the key brain regions of the SN.7–9,23–26 Additional brain areas have also been proposed to participate in the SN. Based on ICA analysis, White et al22 concluded that the SN includes the bilateral insula, anterior cingulate gyrus, superior temporal gyrus, precentral gyrus, left superior frontal gyrus, and right medial frontal gyrus. However, Garcia-Garcia et al44 reported that the SN also includes the inferior frontal gyrus, middle temporal gyrus, superior parietal lobule, angular gyrus, putamen, globus pallidus, and tonsils as well as the insula and anterior cingulate. Palaniyappan and Liddle9 noted that the right anterior insula and dorsal anterior cingulate gyrus serve as key areas of the SN, including the limbic and subcortical and other major areas of the brain network system.
This study proposes that the limbic system and the left superior temporal gyrus regions are important brain regions in the SN. Schizophrenia patients exhibited decreased FC in the left lentiform nucleus, and the FC significantly negatively correlated with PANSS-positive symptoms. The lenticular nucleus is part of the striatum, one of the important brain areas of the limbic system. The resting fMRI study of 26 schizophrenics and 26 healthy controls revealed reduced FC in the bilateral striatum (mainly in the putamen and globus pallidus). The FC in the paracingulate cortex has been correlated with difficulties in abstract thinking, and FC in the left striatum has been correlated with delusion and depression scores.26 Another study determined that patients with schizophrenia performing a reward signal task exhibited activation of key brain areas of the SN such as the insular and anterior cingulate but no activation of other brain regions related to the reward system such as the striatum, amygdala, hippocampus, and midbrain. In response to rewards, the control subjects activated task-related regions such as the striatum, amygdala/hippocampus, and midbrain.45
The role of limbic system brain areas such as the striatum in the SN is not clear, but these areas are involved in many physiological functions such as the regulation of visceral activities, regulation of sensory information in the central nervous system, generation or alteration of emotions, and participation in learning and memory. Thus, we can conclude that limbic system brain regions may play a crucial role in the recognition and performance of salience stimulation to affect the ability of the SN to recognize internal and external stimuli, thus weakening the ability to “switch” between the DMN and the CEN, ultimately resulting in psychotic symptoms.
In this study, the left superior temporal gyrus also exhibited abnormal connectivity in the SN of patients, and the FC of this brain region exhibited a correlation trend with the PANSS total score. A review of region of interest volumetric studies of schizophrenia patients revealed that 35 (total 46) reported reduced gray matter volume in the superior temporal gyrus or its substructures, and the change in the left side volume particularly correlated with positive symptoms such as hallucinations and thought disorder.46 A multimodal morphometry and fMRI study in schizophrenia identified areas with coexistence gray matter reductions and emotional activation in the bilateral middle temporal and superior temporal gyri and identified the left superior and middle temporal gyri as relevant areas for the understanding of auditory hallucinations in schizophrenia.47 Straube et al48 observed that in schizophrenia, the left superior temporal sulcus is misconnected to the inferior frontal gyrus, particularly during the processing of metaphoric gestures, and thus, dysfunctional integration of gestures in an abstract sentence context may underlie certain interpersonal communication problems in patients with schizophrenia. The left superior temporal gyrus may be a key brain region in the process of schizophrenia pathology, but the relationship between this brain area and the SN during the pathological course of the disease requires further study and validation in a larger sample.
In this study, after 6–8 weeks of treatment with atypical antipsychotics, schizophrenia patients exhibited decreased FC in the right cerebellum anterior lobe and left insula. A disturbed prefronto–thalamo–cerebellar circuit has been proposed to play a role in the pathophysiology of schizophrenia,49 but the role of the cerebellum in the SN remains unknown. A study of resting-state FC of the vermal and hemispheric subregions of the cerebellum in 228 healthy adults indicated that the FC of different parts of the cerebellum is related to the visual and auditory networks and the DMN and SN. These results confirm the existence of both functional integration and segregation among cerebellar subregions and greatly improve our understanding of the functional organization of the human cerebellum.50 The insula is the key brain area in the SN.7–9,22–26 This study revealed decreased FC in the insula of schizophrenia patients after treatment. Nevertheless, the FC in the two brain areas was not associated with the relief of psychiatric symptoms.
Studies of the resting-state SN in schizophrenia have yielded inconsistent findings. Woodward et al51 observed that functional resting-state networks were differentially affected in schizophrenia, with no differences in the SN. However, some studies have suggested that abnormal FC in the SN primarily occurs in the insular areas, particularly in the right insular in schizophrenia patients.52,53 No conclusion on the impact of antipsychotics on the SN has been drawn. This study reveals that patients with schizophrenia exhibit abnormal connectivity in the striatum (lenticular nucleus) within the SN. Therefore, we propose that the midbrain limbic system brain areas involved in the schizophrenia dopamine hypothesis may be closely related to the SN. However, the FC in the brains of schizophrenia patients was not altered after 6–8 weeks of treatment with antipsychotic drugs. This lack of change may be attributable to a variety of factors, including the sample choice, the uncertainty of the resting state, and the short duration of drug treatment.
It is important to note several limitations in this study. First, we used a relatively small sample size, and the reference map for the SN was not provided by GIFT but rather was constructed using the IBASM-71 standard brain atlas of WFU_PICKATLAS software. The use of different network templates could have impacted the results. Second, the healthy controls participating in this study were expected to be matched with the patients, but the level of education differed significantly between the groups and was controlled for in the analyses. Third, to avoid interference with treatment, the patients were treated with different medications. The various types of antipsychotics may have different effects on resting-state brain networks.
Conclusion
This study provides evidence for resting-state functional abnormalities of the DMN and SN in untreated schizophrenia patients. The patients exhibited FC changes within the two networks after 6–8 weeks of atypical antipsychotic treatment. The prefrontal cortex (superior frontal gyrus and medial frontal gyrus) and the right posterior cingulate/precuneus may be the key brain regions of the DMN. The limbic system (left lentiform nucleus) and the left superior temporal gyrus may be important brain areas of the SN in addition to the insula and anterior cingulate. Future research should further explore whether specific abnormalities in resting-state FC in the DMN and SN can predict treatment responses in schizophrenia.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81371479), Shanghai Science and Technology Committee Foundations (15411964400, 16ZR1430500), Shanghai Municipal Commission of Health and Family Planning Foundations (20144Y0054, 2014ZYJB0002), and Shanghai Municipal Hospital Appropriate Technology Programme (SHDC12014214). It was also supported by Shanghai Key Laboratory of Psychotic Disorders (14K03), Shanghai Clinical Center for Mental Disorders (2014), and Early Psychosis Program of Shanghai Mental Health Center (2013-YJTSZK-05). These funding agents had no role in the study design, collection, analysis, and interpretation of the data, writing of the manuscript, or decision to submit the paper for publication.
Author contributions
Yingchan Wang conceived of the study, participated in the clinical treatment, and helped to draft the manuscript. Weijun Tang and Daoying Geng carried out the MRI assessment and imaging data acquisition. Xiaoduo Fan participated in the data analysis and manuscript editing. Jianye Zhang, Kaida Jiang, Dianming Zhu, and Zhenhua Song carried out the clinical treatment and data acquisition. Zeping Xiao and Dengtang Liu are the guarantors of integrity of the entire study and helped to revise the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Meisenzahl EM, Schlosser R. Functional magnetic resonance imaging research in psychiatry. Neuroimaging Clin N Am. 2001;11(2):365–374. | ||
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. | ||
Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. | ||
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. | ||
Cabral J, Kringelbach ML, Deco G. Exploring the network dynamics underlying brain activity during rest. Prog Neurobiol. 2014;114:102–131. | ||
Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14(6):277–290. | ||
Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. | ||
Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. | ||
Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37(1):17–27. | ||
Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. | ||
Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. | ||
Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. | ||
Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16(2):317–330. | ||
Zhou Y, Liang M, Tian L, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97(1–3):194–205. | ||
Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. | ||
Mannell MV, Franco AR, Calhoun VD, Canive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: a methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp. 2010;31(3):424–437. | ||
Bluhm RL, Miller J, Lanius RA, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33(4):1004–1012. | ||
Camchong J, MacDonald AW 3rd, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37(3):640–650. | ||
Jang JH, Jung WH, Choi JS, et al. Reduced prefrontal functional connectivity in the default mode network is related to greater psychopathology in subjects with high genetic loading for schizophrenia. Schizophr Res. 2011;127(1–3):58–65. | ||
Mingoia G, Wagner G, Langbein K, et al. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr Res. 2012;138(2–3):143–149. | ||
Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164(3):450–457. | ||
White TP, Joseph V, Francis ST, Liddle PF. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123(2–3):105–115. | ||
Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Reality distortion is related to the structure of the salience network in schizophrenia. Psychol Med. 2011;41(8):1701–1708. | ||
Pu W, Li L, Zhang H, et al. Morphological and functional abnormalities of salience network in the early-stage of paranoid schizophrenia. Schizophr Res. 2012;141(1):15–21. | ||
Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Regional contraction of brain surface area involves three large-scale networks in schizophrenia. Schizophr Res. 2011;129(2–3):163–168. | ||
Orliac F, Naveau M, Joliot M, et al. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res. 2013;148(1–3):74–80. | ||
Snitz BE, MacDonald A, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162(12):2322–2329. | ||
Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67(8):783–792. | ||
Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. quiz 34–57. | ||
Pinkham AE, Penn DL, Perkins DO, Graham KA, Siegel M. Emotion perception and social skill over the course of psychosis: a comparison of individuals “at-risk” for psychosis and individuals with early and chronic schizophrenia spectrum illness. Cogn Neuropsychiatry. 2007;12(3):198–212. | ||
Stefanovic MP, Petronijevic N, Dunjic-Kostic B, et al. Role of sICAM-1 and sVCAM-1 as biomarkers in early and late stages of schizophrenia. J Psychiatr Res. 2016;73:45–52. | ||
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. | ||
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28–37. | ||
Haro JM, Kamath SA, Ochoa S, et al. The Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand Suppl. 2003;416:16–23. | ||
Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14(3):140–151. | ||
Franco AR, Pritchard A, Calhoun VD, Mayer AR. Interrater and intermethod reliability of default mode network selection. Hum Brain Mapp. 2009;30(7):2293–2303. | ||
Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014;40(2):314–326. | ||
Mazza M, Catalucci A, Pino MC, et al. Dysfunctional neural networks associated with impaired social interactions in early psychosis: an ICA analysis. Brain Imaging Behav. 2013;7(3):248–259. | ||
Tang J, Liao Y, Song M, et al. Aberrant default mode functional connectivity in early onset schizophrenia. PLoS One. 2013;8(7):e71061. | ||
Kuhn S, Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull. 2013;39(2):358–365. | ||
Liemburg EJ, van der Meer L, Swart M, et al. Reduced connectivity in the self-processing network of schizophrenia patients with poor insight. PLoS One. 2012;7(8):e42707. | ||
Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. | ||
Abbott C, Juarez M, White T, et al. Antipsychotic dose and diminished neural modulation: a multi-site fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):473–482. | ||
Garcia-Garcia I, Jurado MA, Garolera M, et al. Alterations of the salience network in obesity: a resting-state fMRI study. Hum Brain Mapp. 2013;34(11):2786–2797. | ||
Gradin VB, Waiter G, O’Connor A, et al. Salience network-midbrain dysconnectivity and blunted reward signals in schizophrenia. Psychiatry Res. 2013;211(2):104–111. | ||
Sun J, Maller JJ, Guo L, Fitzgerald PB. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Res Rev. 2009;61(1):14–32. | ||
Garcia-Marti G, Aguilar EJ, Marti-Bonmati L, Escarti MJ, Sanjuan J. Multimodal morphometry and functional magnetic resonance imaging in schizophrenia and auditory hallucinations. World J Radiol. 2012;4(4):159–166. | ||
Straube B, Green A, Sass K, Kircher T. Superior temporal sulcus disconnectivity during processing of metaphoric gestures in schizophrenia. Schizophr Bull. 2014;40(4):936–944. | ||
Yeganeh-Doost P, Gruber O, Falkai P, Schmitt A. The role of the cerebellum in schizophrenia: from cognition to molecular pathways. Clinics (Sao Paulo). 2011;66(suppl 1):71–77. | ||
Sang L, Qin W, Liu Y, et al. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage. 2012;61(4):1213–1225. | ||
Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130(1–3):86–93. | ||
Moran LV, Tagamets MA, Sampath H, et al. Disruption of anterior insula modulation of large-scale brain networks in schizophrenia. Biol Psychiatry. 2013;74(6):467–474. | ||
Manoliu A, Riedl V, Zherdin A, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 2014;40(2):428–437. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.