Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Responsiveness of the Mini-Balance Evaluation System Test in Type 2 Diabetic Patients with Peripheral Neuropathy
Authors Phyu SN , Wanpen S, Chatchawan U
Received 6 October 2022
Accepted for publication 16 December 2022
Published 29 December 2022 Volume 2022:15 Pages 3015—3028
DOI https://doi.org/10.2147/JMDH.S392058
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Sitt Nyein Phyu,1,2 Sawitri Wanpen,3 Uraiwan Chatchawan2,3
1Human Movement Sciences, School of Physical Therapy, Faculty of Associated Medical Sciences, Khon Kaen University, Khon Kaen, Thailand; 2Research Center in Back, Neck, Other Joint Pain and Human Performance (BNOJPH), Faculty of Associated Medicine Sciences, Khon Kaen University, Khon Kaen, Thailand; 3School of Physical Therapy, Faculty of Associated Medical Sciences, Khon Kaen University, Khon Kaen, Thailand
Correspondence: Uraiwan Chatchawan, School of Physical Therapy, Faculty of Associated Medical Sciences, Khon Kaen University, Muang, Khon Kaen, Thailand, Tel/Fax +6643202085, Email [email protected]
Background: Mini-BESTest is an instrument for assessing the balance impairment; however, the use of the Mini-BESTest in type 2 diabetic patients with peripheral neuropathy is not well documented in the literature. The aim of this study was to examine the responsiveness and the minimal important change (MIC) of the Mini-BESTest after four weeks of the balance exercises.
Methods: A prospective single group pretest-posttest design was applied, and forty-eight type 2 diabetic patients with peripheral neuropathy were participated (mean age of 59.04 ± 7.533 years; 3 males and 45 females). All participants were given an intervention program including foot care and balance exercises (50-minute sessions, three times a week for four weeks). The responsiveness of the Mini-BESTest was determined using two approaches: 1) the distribution-based method evaluating the change scores (pre- and post-intervention), the effect size (ES), the standard response mean (SRM), the standard error of measurement (SEM) and the minimum detectable change (MDC95) and 2) the anchor-based method evaluating the MIC using the Global Rating of Change scale (GRC) as an external criterion.
Results: After the balance exercises treatment, the Mini-BESTest scores significantly improved (p < 0.001) with an ES of 3.9 and SRM of 4.32. SEM was 0.73 and MDC95 was 2.03 points. The area under the receiver operating characteristic (ROC) curve corresponded to 81%. The cutoff point of the Mini-BESTest was ≥ 5 points corresponding to the GRC ≤ 3 versus > 3 for the discrimination of the Mini-BESTest between improvement and no improvement after exercises.
Conclusion: The Mini-BESTest can be demonstrated as high responsiveness according to the determination of the distribution-based and the anchor-based methods. The MIC of the Mini-BESTest was taken as ≥ 5 points and could be used as an outcome measure for the discriminated evaluation of type 2 diabetic patients with peripheral neuropathy.
Keywords: balance, diabetic peripheral neuropathy, Mini-BESTest
Graphical Abstract:

Introduction
Diabetic peripheral neuropathy (DPN) is a chronic dysfunction or damage of the nerves caused by diabetes mellitus (DM),1 with higher occurrence in type 2 DM, which negatively affects patients’ quality of life.2,3 As the prevalence of diabetes rises around the world, so does the prevalence of DPN.4,5 The incidence of DPN in type 2 DM varies from 21.3% to 34.5%6–8 and risk factors such as age and DM duration contribute to developing DPN.9 The development of diabetic foot problems and traumatic falls is likely influenced by the biomechanical abnormalities brought on by DPN.10–12 According to a systematic review, diabetic neuropathy negatively affects gait kinematics and postural balance, which increases the risk of falling.13 Static, dynamic, and functional activity are affected by DPN due to impaired sensory function, proprioception, movement strategy and cognitive performance or the presence of disorientation and biomechanical structural disorders.14,15
In this context, the multidimensional balance tool, the Balance Evaluation Systems Test (BESTest) was performed.16 However, the evaluation is lengthy. Therefore, a short form of the tool, namely the Mini-Balance Evaluation System Test (Mini-BESTest) was produced.17 The Mini-BESTest examines almost all balance components such as static and dynamic stability, transfers, gait, variation of support surfaces, visual ability, obstacle negotiation, external forces and dual tasking, that reveals a broad range of activities corresponding to the entire spectrum of task and environment complexity.17–19 The Mini-BESTest demonstrated its excellent reliability, validity and responsiveness with the minimal important change (MIC) in a variety of brain diseases such as stroke, Parkinson’s disease, and mixed neurological diseases with different origins.18,20–24 Nevertheless, the study of the Mini-BESTest was very limited for use in peripheral nerve disorders such as the DPN in type 2 DM patients; however, it has been proven as a standardized balance measure with the excellent reliability and validity in type 2 DM patients with DPN.25 Its responsiveness and the MIC are still needed to study in DPN population which is a representation of the peripheral nerve disorder. Therefore, the main hypothesis of our study is that the balance test of the Mini-BESTest may respond more rapidly to peripheral nerve problems than to central nerve disorders when compared with the previous studies.
Responsiveness is the ability to detect true change over time to assess the effectiveness of interventions. In other words, the patient’s score changes according to his or her status and remains stable when the status is unchanged. This change should be large enough to be statistically significant for research purposes and precise enough to reflect meaningful change according to an external criterion for clinical application.26
To assess responsiveness, effect size (ES) and other similar measures are often used, which may take into account the overall ability of a given outcome measure to map the clinical course of a condition.27 Distribution-based approaches have the advantage of ease of use. Furthermore, however, methods based on anchors, which imply the use of an external criterion, are recommended.27 Responsiveness to meaningful change is necessary but not sufficient for estimating the smallest score changes regarded as important. When coupled with clinical anchors, this change in the score is also referred to as the MIC and occasionally the minimal clinically important difference.27 Both distribution-based and anchor-based methods have been used here and the MIC was estimated by comparing the distribution-based and anchor-based methods.
Balance exercises: practicing standing on one leg, exercises using balance equipment, lower-body and core resistance exercises, yoga, and tai chi improve balance with no set of the intensity. The frequency of balance training can be ≥2–3 times per week and progress as tolerated.28 A systematic review of Streckmann et al29 revealed that exercise is essential for promoting balance control leading to a decreased likelihood of falls in DPN patients. The objective of exercise training including functional strengthening exercises, walking training, balance retraining or Tai Chi, can promote the balance, gait, and daily living activity in diabetic neuropathy population.29 In addition, the systematic review of Gu and Dennis30 suggested that a multicomponent training programme promotes postural control and gait performance in DPN patients without serious risks. In this study, the intervention program builds on the program used in the previous study by Allet et al,31 the highest quality study from a systematic review of fall prevention programs in type 2 DM patients with DPN, assessed using the Physiotherapy Evidence Database (PEDro).30
Mini-BESTest is a simple, non-invasive validated tool that can assess the balance control system without the need for long sessions and although it is a useful clinical test that was verified by its excellent reliability and validity in type 2 DM patients with DPN,25 the responsiveness and the MIC of the Mini-BESTest are prerequisite to study in type 2 DM patients with DPN. Hence, this study aimed to investigate the responsiveness of the Mini-BESTest and its MIC in type 2 DM patients with DPN after four weeks of balance exercises intervention using two different approaches (the distribution-based and the anchor-based methods).
Materials and Methods
Study Design
A prospective single group pretest-posttest design (9/2021) was applied. Type 2 DM patients with DPN were recruited from the outpatient diabetic clinic at North Okkalapa General Hospital, (Yangon, Myanmar). All participants were requested to sign in a consent form before the screening and the data collection.
Participants
The participants were aged 40–70 and were diagnosed as type 2 DM with DPN by using the physical examination (cut-off point as ≥2.5) of the Michigan Neuropathy Screening Instrument (MNSI).32 The inclusion criteria were the following: 1) 5–15 years of DM, 2) controlled blood glucose levels based on three follow-up medical assessments from the medical records, 3) Mini-BESTest scores <20.533 and 4) being able to understand the instructions of the mini-mental state examination.15 The exclusion criteria were the following: 1) foot ulcers, 2) fracture of lower limbs within six months before the study, 3) peripheral venous insufficiency, 4) cardiac, renal or hepatic insufficiency, 5) uncontrolled hypertension or myopathy, 6) cerebrovascular insufficiency such as carotid artery insufficiency who suffers from vertigo and balance problems 7) central nervous system dysfunctions such as stroke, Parkinson’s disease, and cerebral ataxia, 8) partial or complete blindness and 8) severe auditory problems.33,34 All exclusion criteria were indicated by the medical records and/ or the physical examination by the medical doctor.
Procedure
A total of 55 participants were recruited; a part of the sample (n=14) had participated previously in the reliability and validity study,25 the rest were new recruitments. After signing the informed consent form, DPN was identified using MNSI, and the eligibility was determined by using the inclusion criteria. Forty-eight participants met the inclusion criteria and baseline assessments were conducted including 1) the collection of demographic data, 2) documentation of blood glucose levels and HbA1C using the last medical record, and 3) evaluation of balance with the Mini-BESTest. Then, the training programme for the foot care and balance exercises were given about 3 days by an expert physical therapist having 25-year experience of applying this programme at the hospital. After that, a 4-weeks intervention was performed, including an exercise programme that was conducted at the hospital (2 weeks) and home (2 weeks). After the 4-weeks of intervention, the Mini-BESTest and the Global Rating of Change (GRC) assessments were performed. All outcome measurements were assessed by the principal investigator (S.N.P). In addition, all participants were requested to document “the exercises they completed” in an exercise diary. There were no dropouts, and all participants underwent post-evaluation. A flow diagram of the study procedure can be seen in Figure 1.
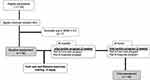 |
Figure 1 Study procedure. |
Sample Size
The estimation of the sample size was based on a previous study20 investigating the responsiveness of the Mini-BESTest in patients with balance disorders. The sample size was calculated with a formula for a single group of pretest-posttest design in the distribution-based method (major result), using the standard deviation of the Mini-BESTest after treatment (6.9 points), the difference between the mean values of pre-test and post-test (post-pre = 3.1 points), a power of 80%, a significance level of 5% and a drop-out rate of 10%. Therefore, 48 participants were required.
Assessment Tools
Mini-Balance Evaluation System Test (Mini-BESTest)
It consists of 14 items and lasts approximately 15 minutes. It focuses on four functions of the balance system: 1) anticipatory postural adjustments, 2) postural response to external perturbations, 3) sensory orientation and 4) stability in gait.17 Two items of the Mini-BESTest were assessed bilaterally, but only the lower score was added to the total score. The total score is 28 points; each item is assessed on a 3-point ordinal scale, ranging from 0 (“severe balance impairment”) to 2 (“no balance impairment”).35 A previous study found an excellent inter-rater (Intraclass correlation coefficient [ICC] = 0.95) and intra-rater reliability (ICC = 0.93) and a valid clinical application in type 2 DM patients with DPN.25
Global Rating of Change (GRC) Scale
For the anchor-based method, the GRC scale was used as an external criterion. This scale measures the important differences in the evaluated parameters using a 15-point scale ranging from −7 (extremely worse than baseline) to +7 (extremely better than baseline), with 0 representing “no change”, based on both the patients’ and their therapists’ ratings. Scales generally range from “a great deal worse” to “a great deal better”, with zero indicating no change.36 The GRC values >3 were considered a meaningful change or considerable improvement, while the GRC values ≤3 denoted a detrimental condition.20,26
Intervention Program
The intervention sessions lasted 50 minutes in total and included a 40-minutes balance exercises programme and a 10-minutes foot care programme. After screening, participants received 3 days of training on the foot care37,38 and the balance exercises programme30,31 by the expert physical therapist who has 25-year experience in Physical Therapy.
The intervention programme was performed 3 times per week for 4 consecutive weeks; the first half of the intervention was conducted under supervision at the hospital, while the second half was conducted without supervision at home. The hospital sessions were provided to groups of 5–6 participants under the supervision of the expert physical therapist.
All participants were instructed to perform the exercise programme at home following the instructions of the expert physical therapist and doing it near the stable handgrips or high stable seats or near the wall to restore balance to avoid falling when doing so, be sure to wear light and comfortable clothing and sports shoes. Additionally, exercise books and logbooks including records of medical conditions such as blood pressure, fainting, pain, etc., and emergency phone numbers (ie, emergency phone number and principal investigator’s phone number) were distributed to all participants.
The foot care programme consisted of a daily 9 steps foot examination, which also included recommendations and educational information (Box 1).37,38 The balance exercises programme was based on the intervention used in the previous studies.30,31 It included 8 steps of balance and walking exercises (Box 2).30,31
 |
Box 1 The Foot Care Programme |
 |
Box 2 The Balance Exercises Programme |
Data Analysis
Descriptive statistics were used for the demographic data analysis. Continuous data was presented as mean ± standard deviation (SD) while the categorical data was presented as a percentage (%).
For distribution-based responsiveness, the paired t-test was used to compare the difference between pre- and post-intervention (change score). The effect size (ES) and the standard response mean (SRM) were used to indicate the magnitude of responsiveness. ES was calculated by dividing the observed mean change score by the standard deviation of the baseline score for the same participants, while SRM was calculated by dividing the observed mean change score by the standard deviation of the change score for the same participants. Both ES and SRM were interpreted as indicating a large change when >0.8, a moderate change when 0.5–0.8, a small change when 0.2–0.5 and a trivial change when <0.2.39 The standard error of measurement (SEM) and the minimum detectable change (MDC95) were calculated based on ICC (3,1) and SD of 14 subjects (female-to-male ratio: 13:1) recruited from a previous study,25 after ensuring that they met the inclusion criteria for the current study. MDC95 was established as the result of the following multiplication: SEM × z-value × √2.
As for anchor-based responsiveness, the MIC was determined. The Mini-BESTest change score was compared to the GRC assessment as an external criterion. The Pearson correlation (r) was applied for the GRC scale ratings of the patients and their expert physical therapist, and the Mini-BESTest change scores and the mean GRC change scores. For all these correlations, r ≥ 0.30 was as acceptable values for the association between measures.27
The receiver operating characteristic (ROC) curve was used as a splitter to divide the participants into two groups: participants who presented changes and participants who did not present changes in the Mini-BESTest scores based on the average of the GRC (patients and their therapist) which classified to be two groups of GRC > 3 or GRC ≤ 3. Sensitivity referred to the number of participants correctly identified as improved based on the cut-off score divided by the number of participants identified as having undergone a meaningful change (GRC > 3). Specificity was calculated with the number of participants who were correctly identified as not improved based on the cut-off value divided by the number of participants who did not undergo a meaningful change (GRC ≤ 3). The optimal cut-off value was chosen as the point that jointly maximised sensitivity and specificity (associated with the lowest level of misclassification).20 The area under the ROC curve (AUC) represented the ability of the Mini-BESTest to discriminate the balance impairment; these referred to “change” and “no change” according to the score of the external criterion. Larger AUC areas denote a greater ability to distinguish patients who improved from those who did not improve. The AUC value ranges from 0 to 1, where a value >0.9 is considered outstanding, 0.8–0.9 is determined as excellent discrimination, 0.7–0.8 is acceptable, 0.5–0.6 is poor and <0.5 indicates no discrimination ability.40 In addition, the floor or ceiling effects were considered present if >15% of the participants achieved the lowest or highest possible score, respectively, both at baseline and post-intervention.41 All statistical analyzes were performed using Stata/SE software package version 14.0 (StataCorp LP, College Station, TX 77845, USA).
Results
All 55 participants were recruited, only of 48 participants (3 males and 45 females) with DPN and balance impairment (age: 59.04 ± 7.53) were met the inclusion criterion and completed with no drop-out until the end in the study (Figure 1). The demographic data and the medical history of all participants are presented in Table 1.
 |
Table 1 Baseline Characteristics of the Sample |
Responsiveness According to the Distribution-Based Method
Tables 2 and 3 present the numerical results of the Mini-BESTest and the GRC scale. The mean Mini-BESTest score was 17.02 ± 1.64 at baseline and 23.42 ± 2.34 post-intervention with the difference of 6.40 ± 1.48 (95% CI: [5.96–6.83] and p-value of <0.001; Figure 2). This improvement was accompanied by a large ES (3.9; 95% CI: [3.01–4.77]) and SRM (4.32). The reliability, SEM and MDC95 measurements for the Mini-BESTest using the data from the sub-cohort of 14 subjects recruited from a previous study are also shown in Table 3.
 |
Table 2 Results of the Mini-BESTest and the Global Rating of Change (GRC) Scale at Baseline and Post-Intervention |
 |
Table 3 Reliability and Responsiveness Indexes for Mini-BESTest |
 |
Figure 2 Comparison of mean Mini-BESTest scores between at baseline (white box) and after intervention (gray box). |
Responsiveness According to the Anchor-Based Method
The change score of the Mini-BESTest was ≥5 for 41 participants (85%) and <5 for 7 participants (15%). No participant was worsened by evaluation using the Mini-BESTest. The mean GRC was ≤3 in 7 participants (27%) and the mean GRC was >3 in 35 participants (73%). No participant presented worsened balance function as evaluated with either Mini-BESTest or GRC. Moreover, the lowest and the highest scores both at baseline and post-intervention corresponded to <15% of all participants (Table 2).
Table 3 shows the results corresponding to responsiveness according to the anchor-based method. The correlation between the participants’ and the therapist’s GRC ratings was moderate (r = 0.52; 95% CI: [0.28–0.70], p-value < 0.001). Moreover, the correlation between the mean GRC scores and the Mini-BESTest change scores was also moderate (r = 0.63; 95% CI: [0.42–0.77], p < 0.001).
Figure 3, when using the Mini-BESTest cut-off score (≥5) (in the blue circle) to distinguish the patients who improved (GRC > 3) from those who did not improve (GRC ≤ 3), the AUC was 0.81 (95% CI: [0.65–0.98]), representing the excellent discrimination ability with a sensitivity of 97.14% and a specificity of 46.15% (Table 4). In addition, for the participants with GRC > 3 and GRC ≤ 3, the Mini-BESTest change scores were 6.86 ± 1.21 and 5.15 ± 1.46, respectively, with a difference of 1.70 (95% CI: [0.86 to 2.54], p-value < 0.002).
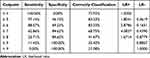 |
Table 4 Cut-off Value of the Mini-BESTest Change Scores According to the GRC Scores ≤3 versus >3 |
Discussion
The present study showed that the Mini-BESTest has high-quality outcome measurement and valid psychometric properties for the evaluation of balance in type 2 DM patients with DPN. This study confirmed that our hypothesis was in consistent with our results. The Mini-BESTest found the high responsiveness with high MIC which was confirmed by the distribution-based and the anchor-based approaches after 12 sessions of balance exercises in a 4-weeks intervention.
Responsiveness of the Mini-BESTest
The distribution-based approach was detected by the significant change scores showing that it was highly sensitive to change and the ES and SRM values also presented large changes in the current study. These results were larger than the previous studies on balance disorders,20 subacute cerebral infarction,24 Parkinson’s disease23 and subacute stroke.21,22 In addition, the current study also reported smaller SEM (0.73) and MDC95 (2.03) values than the previous studies where SEM was 1.26 and MDC95 in the range of 3.0–3.5.20,42,43 These smaller changes reflect true change rather than a measurement error. Moreover, the change score (6.40) was higher than MDC95, meaning that it was reliable and not due to an error. However, both SEM and MDC95 were estimated from a subgroup (n=14) which may imply a lower estimation than when including all participants.
As for the anchor-based approach, the Mini-BESTest showed the largest improvement in our study. Similar to the previous studies,20,23 the mean GRC scores was used as an external criterion. The mean GRC scores and the Mini-BESTest change scores significantly correlated (r = 0.63). This moderate correlation was similar but slightly smaller than that of a previous study (r = 0.72) in patients with balance disorders.20 These may be due to participants’ memory bias or understanding of enhancement. This was supported by the finding that there was a lower correlation between the GRC ratings given by the patients and the therapist in our study (r = 0.52) than in the most recent study (r = 0.61).20 However, the acceptable correlation r ≥ 0.30 between the mean GRC and the Mini-BESTest change scores in our study confirmed using the GRC as an anchor to assess the balance rehabilitation outcome.
MIC of the Mini-BESTest
Using the ROC, the relative discrimination between the Mini-BESTest and the GRC scores was excellent with an AUC of 0.81% at a cut-off Mini-BESTest ≥5. Sensitivity was 97.15% and specificity was 46.14%. It seemed that the Mini-BESTest may be good for screening rather than the confirmation of the impairment of the balance. However, 34 participants presented balance improvement in both GRC and Mini-BESTest measurements post-intervention. These findings were similar to the results of a recent study that found an AUC of 0.82% at a cut-off score of 5.5 (sensitivity: 73%; specificity: 93%) determined by the ambulatory independence with or without an assisting device (external criterion); but the average duration between two assessments was longer as 78 days (more than 2 months).24 The change score in the present study was higher than in previous studies that used the GRC as an external criterion. Godi et al20 found the Mini-BESTest change score of 4 points and an AUC of >80% in mixed neurological diseases, while the study of Winairuk et al24 found the Mini-BESTest change score of 3.5 with an AUC of >60% in cerebral infarction. Moreover, using patient-rated and therapist-rated GRC scores were used as external criterion, the change score of the Mini-BESTest was ranged from 3.4 to 4 points and an AUC from 75% to 82% in Parkinson’s diseases.23
Mini-BESTest showed the high responsiveness and was consistent in term of the MIC in both; as the mean change score of 6.40 ± 1.48 (95% CI: 5.96–6.83) for the distribution-based approach and at a cut-off ≥5 for the anchor-based approach. The current study showed the MIC (≥5 points) was higher than the value of MDC95 (2.03) according to the study of Turner et al.44 In term of the floor or ceiling effects, no such effect was found in our study, a finding that was consistent with previous research on patients with balance disorders in mixed neurological diseases.20 However, a significant floor effect of the Mini-BESTest was found in recent studies of subacute stroke patients.21,22 This may be because the Mini-BESTest contains items that might be difficult to perform in recent studies.21,22
Balance control includes continuous coordination of sensory, motor, and cognitive systems.45 Altered sensation processing from the senses and periphery in peripheral neuropathy in DM can influence motor planning, motor control, and motor responsiveness. Effective treatment of balance disorders depends on the cause of the impaired balance. The training regimen for balance exercises used in this study allows the central nervous system to process balance control through other systems (eg, sensory systems) that remain intact to maintain equilibrium.46,47
A high sensitivity to change was found in this study, probably due to the pathology of type 2 DM with DPN compared to brain disorders like stroke patients in previous studies.46,47 The recovery period for stroke patients may be longer than for individuals with peripheral nerve injury because balance impairment, in the first case, occurs due to a malfunction in the central nervous system that is unable to appropriately complete the sensory-motor integration and generate motor output. Consequently, the benefit of training to restore balance in stroke patients depends on the recovery of brain functioning through neuroplasticity, which relies on numerous factors, including the stroke severity and stage, as well as the presence of comorbidities.46,47
Strength
The balance exercises programme in the present study was a standardised intervention programme31 that covers all the dimensions of the balance system through balance and walking exercises, as well as functional strength and endurance exercises. These elements were consistent with the Mini-BESTest items. Therefore, the balance scores can be seen as a better improvement than that reported in previous studies that used conventional physical therapy rehabilitation (eg, passive stretching, active exercises such as bed mobility, transfer, balance, and functional training) for balance improvement. These programmes are not based on motor relearning, which may not be sufficient for balance improvement in stroke patients, a complicated condition that affects various brain areas, resulting in the disruption of brain networks and widespread dysfunctions.47 This is the strength of our study since it proves that the Mini-BESTest has high responsiveness specifically to peripheral nerve damage. Nonetheless, lower levels of improvement were found in another study of a population with brain disorders,20,23 implying that further research is needed to confirm its effects.
Limitation
As for the limitations of this study, the majority of the participants were females (93.8%) and the responsiveness of the Mini-BESTest should be compared with other standardised balance tests in type 2 DM with DPN population. Despite the fact that our study defined the specific duration of diabetes that was eligible, the wide age range of our participants may affect the balance test results. Nevertheless, the present results are in line with the recent studies, supporting that the Mini-BESTest is a valid outcome measurement for type 2 DM with DPN population. Furthermore, the high risk of foot ulcers and diabetic patients with peripheral artery disease/ lower limb ischaemia were excluded by using the subjective questionnaires in our study.
Conclusion
According to the findings of this study, the distribution-based and the anchor-based approaches indicated that the Mini-BESTest is highly responsive for the evaluation of balance change in type 2 DM patients with DPN after four weeks intervention, with an improvement of more than or equal to 5-points as an indicator of minimal importance in balance performance.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical Approval and Consent of Participants
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Human Ethics Committee of Khon Kaen University, Thailand (protocol number: HE632104) and the Institutional Review Board (IRB) of the University of Public health, Myanmar.
Informed consent was obtained from all subjects before participating in the study by signing the consent form before data collection.
Acknowledgments
The authors want to thank all the participants for their willingness to contribute to the study. We express our sincere gratitude to all physiotherapists who kindly accepted to administer the scales and the staff at North Okkalapa General Hospital (Yangon, Myanmar) for their help and cooperation.
Funding
This work was financially supported by the Research Centre in Back, Neck, Other Joint Pain and Human Performance (BNOJPH), Khon Kaen University (Khon Kaen, Thailand).
Disclosure
All authors declare that they have no conflicts of interest.
References
1. Belapurkar P, More S, Patil G, Mohan M. A review on diabetic neuropathy. Int J Adv Res Develop. 2018;3(2):202–212.
2. Papaspurou M, Laschou VC, Partsiopoulou P, et al. Fears and health needs of patients with diabetes: a qualitative research in rural population. Med Arch. 2015;69(3):190–195. doi:10.5455/medarh.2015
3. Jaiswal M, Divers J, Dabelea D, et al. Prevalence of and risk factors for diabetic peripheral neuropathy in youth with type 1 and type 2 diabetes: SEARCH for diabetes in youth study. Diabetes Care. 2017;40(9):1226–1232. doi:10.2337/dc17-0179
4. Yang H, Sloan G, Ye Y, et al. New perspective in diabetic neuropathy: from the periphery to the brain, a call for early detection, and precision medicine. Front Endocrinol. 2020:10. doi:10.3389/fendo.2019.00929
5. Ling E, Lepow B, Zhou H, Enriquez A, Mullen A, Najafi B. The impact of diabetic foot ulcers and unilateral offloading footwear on gait in people with diabetes. Clin Biomech. 2020;73:157–161. doi:10.1016/j.clinbiomech.2020.01.014
6. Kisozi T, Mutebi E, Kisekka M, et al. Prevalence, severity and factors associated with peripheral neuropathy among newly diagnosed diabetic patients attending Mulago hospital: a cross-sectional study. Afr Health Sci. 2017;17(2):463–473. doi:10.4314/ahs
7. wei PY, Lin CH, Lee IT, Chang MH. Prevalence and biochemical risk factors of diabetic peripheral neuropathy with or without neuropathic pain in Taiwanese adults with type 2 diabetes mellitus. Diabetes Metab Syndr Clin Res Rev. 2018;12(2):111–116. doi:10.1016/j.dsx.2017.09.013
8. Ponirakis G, Elhadd T, Chinnaiyan S, et al. Prevalence and risk factors for painful diabetic neuropathy in secondary healthcare in Qatar. J Diabetes Investig. 2019;10(6):1558–1564. doi:10.1111/jdi.13037
9. Win MMTM, Fukai K, Nyunt HH, Hyodo Y, Linn KZ. Prevalence of peripheral neuropathy and its impact on activities of daily living in people with type 2 diabetes mellitus. Nurs Health Sci. 2019;21(4):445–453. doi:10.1111/nhs.12618
10. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375. doi:10.1056/nejmra1615439
11. Du C, Wang H, Chen H, et al. The feasibility and effectiveness of wearable sensor technology in the management of elderly diabetics with foot ulcer remission: a proof-of-concept pilot study with six cases. Gerontology. 2021;67(4):493–502. doi:10.1159/000513729
12. Brown SJ, Handsaker JC, Bowling FL, Boulton AJM, Reeves ND. Diabetic peripheral neuropathy compromises balance during daily activities. Diabetes Care. 2015;38(6):1116. doi:10.2337/dc14-1982
13. Khan KS, Andersen H. The impact of diabetic neuropathy on activities of daily living, postural balance and risk of falls - a systematic review. J Diabetes Sci Technol. 2021;16:289–294. doi:10.1177/1932296821997921
14. Ahmad I, Hussain E, Singla D, Verma S, Ali K. Balance training in diabetic peripheral neuropathy: a narrative review. JSM Diabetol Manag. 2017;2(1):1002.
15. Roman de Mettelinge T, Cambier D, Calders P, Van Den Noortgate N, Delbaere K. Understanding the relationship between type 2 diabetes mellitus and falls in older adults: a prospective cohort study. PLoS One. 2013;8(6):e67055–e67055. doi:10.1371/journal.pone.0067055
16. Horak FB, Wrisley DM, Frank J. The balance evaluation systems test (BESTest) to differentiate balance deficits. Phys Ther. 2009;89(5):484–498. doi:10.2522/ptj.20080071
17. Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the balance evaluation systems test: the Mini-BESTest. J Rehabil Med. 2010;42(4):323–331. doi:10.2340/16501977-0537
18. Di Carlo S, Bravini E, Vercelli S, Massazza G, Ferriero G. The Mini-BESTest: a review of psychometric properties. Int J Rehabil Res. 2016;39(2):97–105. doi:10.1097/MRR.0000000000000153
19. Pardasaney PK, Slavin MD, Wagenaar RC, Latham NK, Ni P, Jette AM. Conceptual limitations of balance measures for community-dwelling older adults. Phys Ther. 2013;93(10):1351–1368. doi:10.2522/ptj.20130028
20. Godi M, Franchignoni F, Caligari M, Giordano A, Turcato AM, Nardone A. Comparison of reliability, validity, and responsiveness of the Mini-BESTest and berg balance scale in patients with balance disorders. Phys Ther. 2013;93(2):158–167. doi:10.2522/ptj.20120171
21. Chinsongkram B, Chaikeeree N, Saengsirisuwan V, Horak FB, Boonsinsukh R. Responsiveness of the balance evaluation systems test (BESTest) in people with subacute stroke. Phys Ther. 2016;96(10):1638–1647. doi:10.2522/ptj.20150621
22. Winairuk T, Pang MYC, Saengsirisuwan V, Horak FB, Boonsinsukh R. Comparison of measurement properties of three shortened versions of the balance evaluation system test (BESTest) in people with subacute stroke. J Rehabil Med. 2019;51(9):683–691. doi:10.2340/16501977-2589
23. Godi M, Arcolin I, Giardini M, Corna S, Schieppati M. Responsiveness and minimal clinically important difference of the Mini-BESTest in patients with Parkinson’s disease. Gait Posture. 2020;80:14–19. doi:10.1016/j.gaitpost.2020.05.004
24. Hasegawa S, Matsui T, Kishi M, et al. Sensitivity to change and responsiveness of the balance evaluation systems test (BESTest), Mini-BESTest, and Brief-BESTest in patients with subacute cerebral infarction. J Phys Ther Sci. 2021;33(1):69–74. doi:10.1589/jpts.33.69
25. Phyu SN, Peungsuwan P, Puntumetakul R, Chatchawan U. Reliability and validity of mini-balance evaluation system test in type 2 diabetic patients with peripheral neuropathy. Int J Environ Res Public Health. 2022;19(11):6944. doi:10.3390/ijerph19116944
26. Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. Vol. 892. Saddle River, NJ: Pearson/Prentice Hall Upper; 2009.
27. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–109. doi:10.1016/j.jclinepi.2007.03.012
28. Kanaley JA, Colberg SR, Corcoran MH, et al. Exercise/physical activity in individuals with type 2 diabetes: a consensus statement from the American College of Sports Medicine. Med Sci Sports Exerc. 2022;54(2):353–368. doi:10.1249/MSS.0000000000002800
29. Streckmann F, Zopf EM, Lehmann HC, et al. Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med. 2014;44(9):1289–1304. doi:10.1007/s40279-014-0207-5
30. Gu Y, Dennis SM. Are falls prevention programs effective at reducing the risk factors for falls in people with type-2 diabetes mellitus and peripheral neuropathy: a systematic review with narrative synthesis. J Diabetes Complications. 2017;31(2):504–516. doi:10.1016/j.jdiacomp.2016.10.004
31. Allet L, Armand S, de Bie RA, et al. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia. 2010;53(3):458–466. doi:10.1007/s00125-009-1592-4
32. Herman WH, Pop-Busui R, Braffett BH, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29(7):937–944. doi:10.1111/j.1464-5491.2012.03644.x
33. Marques A, Silva A, Oliveira A, Cruz J, Machado A, Jácome C. Validity and relative ability of 4 balance tests to identify fall status of older adults with type 2 diabetes. J Geriatr Phys Ther. 2017;40(4):227–232. doi:10.1519/JPT.0000000000000109
34. Chatchawan U, Eungpinichpong W, Plandee P, Yamauchi J. Effects of Thai foot massage on balance performance in diabetic patients with peripheral neuropathy: a randomized parallel-controlled trial. Med Sci Monit Basic Res. 2015;21:68–75. doi:10.12659/MSMBR.894163
35. King L, Horak F. On the mini-BESTest: scoring and the reporting of total scores. Phys Ther. 2013;93(4):571–575. doi:10.2522/ptj.2013.93.4.571
36. Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17(3):163–170. doi:10.1179/jmt.2009.17.3.163
37. Beuscher TL. Guidelines for diabetic foot care: a template for the care of all feet. J Wound Ostomy Cont Nurs. 2019;46(3):241–245. doi:10.1097/WON.0000000000000532
38. Howarth D. Preventing foot complications in people with diabetes mellitus. Nurs Standard. 2019;34(7):69–74. doi:10.7748/ns.2019.e11432
39. Cohen J. Statistical Power Analysis for the Behavioral Sciences New York. NY: Academic; 1988:54.
40. Jr DWH, Lemeshow S, Sturdivant RX. Applied Logistic Regression. John Wiley & Sons; 2013.
41. McHorney C, Tarlov A. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res. 1995;4(4):293–307. doi:10.1007/bf01593882
42. Tsang CSL, Liao LR, Chung RCK, Pang MYC. Psychometric properties of the mini-balance evaluation systems test (Mini-BESTest) in community-dwelling individuals with chronic stroke. Phys Ther. 2013;93(8):1102–1115. doi:10.2522/ptj.20120454
43. Löfgren N, Lenholm E, Conradsson D, Ståhle A, Franzén E. The Mini-BESTest - a clinically reproducible tool for balance evaluations in mild to moderate Parkinson’s disease? BMC Neurol. 2014;14(1):235. doi:10.1186/s12883-014-0235-7
44. Turner D, Schünemann HJ, Griffith LE, et al. Using the entire cohort in the receiver operating characteristic analysis maximizes precision of the minimal important difference. J Clin Epidemiol. 2009;62(4):374–379. doi:10.1016/j.jclinepi.2008.07.009
45. Shumway-Cook A, Woollacott MH. Motor Control: Translating Research into Clinical Practice. Lippincott Williams & Wilkins; 2007.
46. Alawieh A, Zhao J, Feng W. Factors affecting post-stroke motor recovery: implications on neurotherapy after brain injury. Behav Brain Res. 2018;340:94–101. doi:10.1016/j.bbr.2016.08.029
47. Su F, Xu W. Enhancing brain plasticity to promote stroke recovery. Front Neurol. 2020;11:554089. doi:10.3389/fneur.2020.554089
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

