Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Respiratory Oscillometry in Chronic Obstructive Pulmonary Disease: Association with Functional Capacity as Evaluated by Adl Glittre Test and Hand Grip Strength Test
Authors Ribeiro CO , Lopes AJ , Melo PL
Received 29 December 2021
Accepted for publication 3 April 2022
Published 4 May 2022 Volume 2022:17 Pages 1017—1030
DOI https://doi.org/10.2147/COPD.S353912
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Caroline Oliveira Ribeiro,1 Agnaldo José Lopes,2,3 Pedro Lopes de Melo1
1Biomedical Instrumentation Laboratory, Institute of Biology and Faculty of Engineering, State University of Rio de Janeiro, Rio de Janeiro, Brazil; 2Pulmonary Function Laboratory, State University of Rio de Janeiro, Rio de Janeiro, Brazil; 3Pulmonary Rehabilitation Laboratory, Augusto Motta University Center, Rio de Janeiro, Brazil
Correspondence: Pedro Lopes de Melo, Rua São Francisco Xavier 524, Pavilhão Haroldo Lisboa da Cunha, Sala 104, Maracanã, Rio de Janeiro, 20550-013, Brazil, Tel +55-21-2334-0705, Email [email protected]
Purpose: Respiratory oscillometry has emerged as a powerful method for detecting respiratory abnormalities in COPD. However, this method has not been widely introduced into clinical practice. This limitation arises, at least in part, because the clinical meaning of the oscillometric parameters is not clear. In this paper, we evaluated the association of oscillometry with functional capacity and its ability to predict abnormal functional capacity in COPD.
Patients and Methods: This cross-sectional study investigated a control group formed by 30 healthy subjects and 30 outpatients with COPD. The subjects were classified by the Glittre‑ADL test and handgrip strength according to the functional capacity.
Results: This study has shown initially that subjects with abnormal functional capacity had a higher value for resistance (p < 0.05), reactance area (Ax, p < 0.01), impedance modulus (Z4, p < 0.05), and reduced dynamic compliance (Cdyn, p < 0.05) when compared with subjects with normal functional capacity. This resulted in significant and consistent correlations among resistive oscillometric parameters (R=− 0.43), Cdyn (R=− 0.40), Ax (R = 0.42), and Z4 (R = 0.41) with exercise performance. Additionally, the effects of exercise limitation in COPD were adequately predicted, as evaluated by the area under the curve (AUC) obtained by receiver operating characteristic analysis. The best parameters for this task were R4-R20 (AUC = 0.779) and Ax (AUC = 0.752).
Conclusion: Respiratory oscillometry provides information related to functional capacity in COPD. This method is also able to predict low exercise tolerance in these patients. These findings elucidate the physiological and clinical meaning of the oscillometric parameters, improving the interpretation of these parameters in COPD patients.
Keywords: chronic obstructive pulmonary disease, COPD physiopathology, forced oscillation technique, respiratory impedance, Glittre-ADL test, handgrip strength, exercise limitation
Introduction
Chronic obstructive pulmonary disease (COPD) accounts for 6% of deaths worldwide, with more than 90% occurring in low- to middle-income countries.1 This disease is usually caused by exposure to noxious particles or gases, resulting in persistent respiratory symptoms and airflow limitation due to airway and alveolar abnormalities.2 At present, spirometry is considered the gold standard in assessing airflow limitation in COPD. However, spirometry requires high cooperation on the volunteers and great effort in executing the expiratory maneuver. This may result in variation in the quality of the test results since this procedure can temporarily cause several changes in the bronchomotor tone, leading to underdiagnosis.3–5 It was previously reported that only 30% of patients could perform maneuvers that meet all the quality standards defined by ERS/ATS, and the proportion is even lower in the elderly.6,7
The forced oscillation technique, also known as respiratory oscillometry, is a non-invasive approach to investigate mechanical properties of the respiratory system by assessing airway impedance (pressure/flow signal). The method is based on applying low amplitude oscillatory pressures into the airway entrance during spontaneous ventilation.8 Another significant advantage is that oscillometry can provide detailed information on the respiratory system’s mechanical characteristics that are complementary to that provided by spirometry. Due to these characteristics, oscillometry has emerged as a powerful method to extract clinically relevant information and provide insight into the mechanisms responsible for smoking-induced respiratory diseases. Since this method was introduced in the mid-1950s,9 it has been applied in COPD by many investigators.10 Areas of application included the diagnosis of precocious respiratory abnormalities in smokers and early-stage COPD,11–14 the categorization of airway obstruction level,15 and the evaluation of bronchodilator response.16 This method has been continuously improving in the last decades and has reached a high level of sophistication, currently representing the state-of-the-art in assessing lung function.17
Despite several attractive characteristics of oscillometry, this method has not been widely introduced into clinical practice.17 This limitation arises, at least in part, because the physiological or clinical meaning of the derived parameters is not clear. The functional abnormalities associated with COPD cause dyspnea on exertion, reduced exercise capacity, and poor quality of life.18
The ADL – Glittre test is an effective, easy-to-apply, valid, and reliable test for assessing functional capacity in COPD19 and interstitial lung disease.20 It can be considered more descriptive than the 6-minute walk test (6MWT) to assess functional capacity, as it involves, in addition to walking, activities such as sitting and getting up from a chair, going up and down steps, and arm movements with weight support, tasks considered difficult for these patients.21 This method is sensitive and specific to distinguish COPD patients with abnormal and normal functional capacity.22
The loss of muscle strength is directly linked to physical performance, mobility, and functionality in patients with COPD. The handgrip test proved to be valid in assessing the strength and is considered cheap, simple, easy and can be performed with a portable measurement tool.23–25 Therefore, the ADL – Glittre and the handgrip test have the potential to elucidate the physiological and clinical meaning of oscillometric parameters.
Some recent reports investigate the association of oscillometric indices with the change in 6MWT during and after pulmonary rehabilitation26 and the exercise tolerance in COPD.27,28 However, the relationships between oscillatory parameters and ADL – Glittre and the handgrip test were not evaluated, and the association of oscillometry and functional capacity is not fully understood.
Based on the abovementioned considerations, the objectives of the present study were (1) to investigate the association between oscillometric parameters and changes in ADL – Glittre and the handgrip test; (2) to evaluate the accuracy of oscillometry as a predictor of abnormal functional capacity in COPD.
Materials and Methods
Study Design
All analyses were conducted on the same examination day in this cross-sectional study. The subjects assessed symptoms, measurement of oscillometry, respiratory pressures, spirometry, handgrip test, and ADL–Glittre in that order. The research ethics board of the State University of Rio de Janeiro approved this research (protocol 2927/2011), and post-informed consent was obtained before inclusion in the study. The study was conducted following the principles of the Declaration of Helsinki.
Subjects
We studied subjects diagnosed according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria,2 and over 40 years old. They have no history of respiratory infections in the last thirty days at the time of exams and no history of cardiovascular or orthopedic diseases. Before the exams were performed, all patients took their usual medication, except for bronchodilators (BD), to avoid interference in the assessment, as established by the American Thoracic Society/European Respiratory Society (ATS/ERS).29
Healthy subjects were evaluated as a control group. They were clinically stable and with spirometric and oscillometric exams compatible with normality.30,31 They were also over 40 years old, non-smokers, with no history of respiratory infections in the last thirty days at the time of the exams, no cardiovascular, pulmonary, or orthopedic diseases.
The study involved a group of COPD patients with 30 subjects and a control group formed of 30 subjects. These numbers were based on similar previous works in the literature.26,32
Spirometry
Spirometry analysis was conducted following the standard protocols of the American Thoracic Society/European Respiratory Society.29,33 The analyzed parameters were the forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), the FEV1/FVC ratio, and the forced expiratory flow (FEF) between 25% and 75% of the FVC (FEF/FVC) ratio. These parameters were expressed as absolute values and as a percentage of the predicted values (% of predicted), and the reference values were obtained from the equations of Pereira et al.31 Forced expiratory maneuvers were repeated until three sequential measurements were obtained. The studied indexes were obtained using the best curve, which was selected based on the higher values of FEV1 plus FVC. Lung function data were collected at post-bronchodilator test.2
The software automatically detected non-acceptable maneuvers according to ATS criteria, providing quality control of the spirometric exams.
Oscillometry
These measurements were performed using a previously described instrument34 according to standard recommendations.8 Briefly, a pseudorandom sinusoidal signal with 2 cmH2O peak-to-peak of amplitude, containing all harmonic of 2 Hz between 4 and 32 Hz, was applied by a loudspeaker. The pressure input (P) was measured with a Honeywell 176 PC pressure transducer (Microswitch, Boston, MA, USA), and the airway flows (V’) with a screen pneumotachograph coupled to a similar transducer. The signals were digitized at a rate of 1024 Hz by a personal computer, and a fast Fourier transform (F) was computed using blocks of 4096 points with 50% overlap to evaluate respiratory impedance [Zrs=(F(P)/F(V’)]. Three acceptable tests of 16s were performed, and the result adopted was the mean score. To exclude outlying values, the coefficient of variation of respiratory resistance at the lowest oscillation frequency (4 Hz) for the three measurements was ≤10%. The test was considered acceptable if the volunteers presented stable tidal volumes and rates and were free of pauses. Common artifacts such as swallows, coughs, and leaks were identified by evaluating flow and pressure signals, and the acquisition was repeated until three stable and without artifacts were obtained. Only exams with coherence function ≥0.9 in the whole frequency range studied were accepted to reduce the influence of spontaneous breathing.
The interpretation of the oscillometric results was performed according to recent international standards.8 We evaluated the resistance at 4 and 20 Hz (R4 and R20, respectively), related to the airway and tissue Newtonian resistance and the delayed airway resistance resulting from the gas redistribution. The difference between R4 and R20 (R4-R20) was evaluated as an indicator of the frequency dependence of the respiratory resistance. This parameter is related to respiratory system non-homogeneities.
Reactive properties were analyzed using the respiratory system dynamic compliance (Cdyn), resonance frequency (fr), the area under the reactance curve (Ax), and the impedance module. Cdyn is related to the total compliance of the respiratory system. The reactance at 4 Hz was used to calculate Cdyn (Cdyn = 1/2πfX4). The frequency at which respiratory reactance becomes zero is known as the resonance frequency, which reflects the homogeneity of the respiratory system. Ax was analyzed using the area composed of the lowest frequency (4Hz), the associated reactance (X4), and the fr. The respiratory system’s total mechanical load was studied by analyzing the 4 Hz impedance module (Z4), which integrates resistive and elastic respiratory load.22
ADL - Glittre
The test started with the subjects seated on a chair.19 At a starting signal, they stood up and then walked 5 m, crossed over an interposed 2-step staircase, and walked another 5 m up to a 2-shelf fixture, which was adjusted individually to the shoulder and waist height of each subject. Three bags weighing 1 kg each positioned on the top shelf had to be moved one by one to the bottom shelf, down to the floor, back to the bottom shelf, and finally to the top shelf again. The subjects then walked back to the initial chair where they had started, crossed over the 2-step stairs, sat down, and immediately started the next lap by rising again. Each step of the stair was 17 cm high and 27 cm deep. The subjects were asked to complete five laps as quickly as possible, and the primary outcome of the test is time to perform its five laps. They were allowed to rest if necessary but were told to resume activity as soon as possible. The chronometer was not stopped, and the subjects carried a backpack containing 2.5 kg (women) or 5.0 kg (men). Results in COPD patients were compared with reference values.35
Handgrip Test
This analysis was performed using absolute and relative handgrip strength (HGS) measurements.36 The handgrip test was performed using a handheld hydraulic dynamometer (Saehan, SH 5001). Participants were instructed to sit with their elbows flexed at a 90° angle, using the dynamometer in their hands in a neutral position. They were asked to squeeze the dynamometer with the maximal force for up to 3 s. They were allowed at least 60s of rest between measurements. Three attempts were made with each hand, and the highest value was used for analysis. These measurements were compared with a reference population.37
Statistical Analysis
Data were initially tested for normality using the Shapiro–Wilk test (OriginLab Origin® 8.0, Microcal Software, Inc. Ostend, Belgium), and when the sample showed a normal (parametric) distribution behavior, the Two-Sample t-Test was used to analyze the groups. On the other hand, when the distribution presented a non-normal (non-parametric) characteristic, the Mann–Whitney test analyzed the groups.
Correlation analyses were performed using Pearson correlations for normally distributed data and Spearman correlations when the data were not normally distributed (GraphPad Prism 5.03, GraphPad Software, La Jolla, CA, USA). Those associations were classified as suggested by Dawson and Trapp.38 The p < 0.05 was used to consider the statistically significant differences.
The accuracy of oscillometry as a predictor of exercise tolerance in COPD was evaluated using receiver operator characteristic (ROC) analysis. These evaluations were performed for the two studied exercise tolerance outcomes. The cut-off value used for the Glittre‑ADL test was 210 s, which previous studies showed as sensitive and specific to distinguish COPD patients with an abnormal and normal functional capacity.22 In the handgrip analysis, we considered cut-off values of <30 kgf for men and <20 kgf for women (dominant hand) to identify dynapenia in COPD patients.39,40 We identified optimal prediction cut points as those yielding the best compromise between sensitivity and specificity. The area under the curve (AUC) was calculated to estimate the predictive capability of the poor functional performance and muscle function. In this analysis, AUCs >0.70 were considered adequate for predictive use,41 while values >0.90 were considered in the high predictive accuracy range.42 To complement ROC analysis, multivariate logistic regression (MLR) was used to determine the best oscillometric parameter to predict abnormal functional capacity in COPD.
Results
As shown in Table 1, we enrolled a group of 60 participants (30 controls and 30 patients). The airflow limitation severity was classified as mild in 4 patients, moderate in 16, severe in 7, and very severe in 3 patients. Spirometric parameters were significantly reduced in individuals with COPD. There was an increase in age and pack-years in the COPD group. Table 1 also shows biometric and spirometric characteristics in groups taking the Glittre-ADL test and Handgrip strength as reference. There was no change in height, body mass, and body mass index (BMI) in these groups.
Oscillometric Parameters Classified According to the Exercise Performance Tests
Taking the AVD-Glittre test as reference (Table 1), Figure 1 shows that the individuals with abnormal results presented significant changes (p < 0.01) in all parameters.
When the Handgrip test is used as a reference (Table 1), it observed increases in R4 and R4-R20 (Figure 2). Considering the reactive parameters, reduced Cdyn and increased values of fr, Ax, and Z4 were observed.
ADL – Glittre and Handgrip Strength Test
Table 2 depicts the results of the AVD-Glittre and Handgrip in the whole group of patients with COPD. There was a significant increase in performed time in the COPD group compared to predicted values (p < 0.0001). In similar comparisons, significant reductions were observed considering the handgrip strength (p < 0.01).
 |
Table 2 Predicted and Measured Values in Patients with COPD for Total Glittre-ADL Test Time and Handgrip Analysis |
Correlation Analysis
These evaluations were performed considering the group of COPD patients. As can be seen in Table 3, one resistive (R4) and three reactive (Cdyn, Ax, Z4) parameters presented significant (p < 0.05) direct associations with the ADL-Glittre test.
 |
Table 3 Correlation Analysis in Patients with COPD Describing the Association of Oscillometric Parameters with Total Glittre-ADL Test Time and Handgrip Analysis |
Significant direct correlations (p < 0.045) were also observed between resistive (R4) and reactive (fr and Z4) oscillometric parameters with the handgrip strength test in the dominant hand (Table 3). Considering the non-dominant hand, significant direct correlations (p < 0.045) were observed between R4, Cdyn, fr, Ax, and Z4.
Oscillometry as a Predictor of Exercise Tolerance in COPD
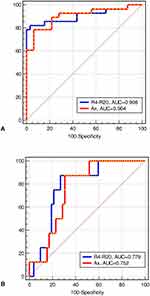
Of the 60 obtained values, 32 had a Glittre‑ADL test >210 s, in which thirty test times were estimated in the control group, and 2 were normal values measured in the COPD group. The 28 (46.7%) remaining values were obtained in COPD patients with a Glittre‑ADL test <210 s. Table 4 depicts the accuracy, sensitivity, specificity, and cut-off point for the oscillometric parameters in predicting abnormal functional capacity in COPD, as evaluated by the Glittre‑ADL test. Healthy subjects and COPD patients were used in this analysis. R4-R20, fr, and Ax achieved high prediction accuracy (AUC >0.90) among the studied parameters. Figure 3A shows the ROC curves of the two more accurate parameters obtained using the Glittre‑ADL test as a reference. In this case, MLR showed that R4-R20 was the best oscillometric parameter to predict abnormal function capacity (p = 0.0004).
 |
Table 4 Accuracy of Oscillometry in Predicting Abnormal Functional Capacity in COPD Based on Glittre-ADL Test |
Considering the Handgrip analysis as a reference for abnormal functional capacity, of the 60 obtained values, 52 were in the normal range. Thirty were estimated in the control group, and 22 were normal values measured in the COPD group. The 8 (13.3%) abnormal values were obtained in COPD patients. The accuracies and cut-off points for the studied parameters in predicting abnormal functional capacity in COPD, as evaluated by the handgrip analysis, are presented in Table 5. All studied parameters provided an adequate predictive accuracy (AUC >0.70), except for R20. R4-R20 achieved the highest accuracy (AUC = 0.779), as shown in Figure 3B, which presents the ROC curves of the two more accurate parameters obtained using the handgrip analysis as a reference. The MLR revealed that R4-R20 was the most predictive parameter in detecting abnormal function capacity (p = 0.0443).
 |
Table 5 Accuracy of Oscillometry in Predicting Abnormal Functional Capacity in COPD Based on Handgrip Evaluations |
Discussion
This study investigated the physiological and clinical meaning of the oscillometric parameters. It was evaluated whether oscillometry is correlated and would predict the poor exercise tolerance measured by the Glittre‑ADL test and handgrip strength in 30 patients with COPD and 30 controls. It was revealed a clear association between oscillometry and functional capacity. The ROC curve analysis demonstrated that R4-R20 and Ax accurately predicted poor Glittre‑ADL test and handgrip strength. These results provide clear evidence that oscillatory indices are related to physical performance and helpful in predicting poor exercise tolerance in COPD.
A functional limitation is a common and important finding in patients with COPD,2 which is directly related to increased mortality,43 a higher frequency of exacerbations and hospitalizations,44 and a reduction in quality of life.21 The decrease in exercise capacity in individuals with COPD (Table 2) may be explained by ventilatory limitation, abnormalities in gas exchanges, and cardiovascular dysfunction.
Figure 1 shows how the drop in physical performance detected by the Glittre-ADL test impacts the respiratory mechanics evaluated by oscillometry. It is generally agreed that abnormal changes affecting primarily the lungs will also have significant systemic effects. Figure 1 supports this hypothesis and adds new information, revealing increased resistance values (R4, R20) in patients with Glittre-ADL test >210 s. The observed increases in ventilation heterogeneity (R4-R20, fr), respiratory work (Z4), elastic properties (Ax), and reduced compliance (Cdyn) were also in line with the changes typically observed in patients with COPD and exercise limitations. These changes reflect the worsening of airway obstruction, increased secretion, and changes in the pulmonary parenchyma in these patients.2
Considering the Handgrip as a reference method for detecting functional abnormalities, Figure 2 shows that the changes observed in oscillometric parameters were similar to those observed using the Glittre-ADL test as a reference. Interestingly, changes observed in oscillometry using Glittre-ADL test and Handgrip analysis are in close agreement with that previously observed using the 6MWD as a reference.27
In agreement with the involved physiology,2 Table 3 shows reasonable and significant direct associations between Glittre-ADL test time, Handgrip analysis, and parameters related to airway obstruction (R4), elastic properties (Ax), and respiratory work (Z4). The inverse association observed with Cdyn and Glittre-ADL test time and the direct correlation observed with Handgrip analysis are also consistent with these principles. The systemic effects due to lung abnormalities may also explain these relationships. The results presented in Table 3 are in close agreement with that obtained recently by Yamamoto et al27 using the 6-minute walking distance (6MWD) as an index of exercise tolerance. Reasonable and significant associations were obtained among oscillometric parameters and 6MWD. The cited authors attributed the observed changes and associations to airflow limitation and dynamic hyperinflation as predominant factors defining exertional dyspnea and disease severity in COPD. These results also reflect Zeng et al,28 who recently found correlations between resistive and reactive parameters with 6MWD in COPD. They are also in line with that obtained by Yamamoto et al.45 The authors observed that oscillometric parameters correlated with exertional ventilatory parameters and reflected exercise tolerance in COPD subjects during cycle ergometer tests. Similar results were also observed in adults with sickle cell anemia, analyzing the correlations among 6MWD and oscillometric parameters.46 The new information in Table 3 shows that these correlations extend to the ADL – Glittre test and Handgrip analysis. The associations observed in Table 3 are also consistent with the weak to moderate correlations between respiratory mechanics evaluated by spirometric analysis and 6MWD47,48 and the ADL-Glittre test.19
Table 4 and Figure 3A show that R4-R20 achieved the highest accuracy among the oscillometric parameters in predicting exercise limitation, as evaluated by the ADL-Glittre test. MLR revealed that R4-R20 was the most predictive parameter in line with these findings. This high performance probably occurs because this parameter reflects ventilation heterogeneity, which results in abnormalities in gas exchanges. Other interesting findings were that fr and AX could also predict exercise limitation. It is also noteworthy that the three parameters mentioned achieved high accuracy in predicting exercise limitation (AUC >0.90).
As shown in Table 5 and Figure 3B, R4-R20 was the most accurate of the studied parameters in predicting abnormal functional capacity in COPD based on handgrip evaluations. MLR also described R4-R20 as the most predictive parameter. These findings are in close agreement with that obtained using ADL-Glittre test (Table 4), in which this parameter was also the most predictive. Another interesting finding was that Ax could also accurately predict using handgrip evaluations (Table 5) and the ADL-Glittre test (Table 4). A possible explanation for this might be that the changes in bronchial architecture resulting from COPD lead to an irregular ventilation distribution associated with imbalances in the pulmonary time constants.
There is currently a consensus in the literature on the need to develop new sensitive and non-invasive lung-function testing to allow early and accurate detection of pulmonary function decline.49–51 Respiratory oscillometry has been widely perceived as the state-of-the-art lung function analysis17 and one of the most promising emerging technologies in this area.52,53 However, although its advantages associated with a straightforward and detailed examination are particularly important, this method is not yet widely used. One of the main aspects limiting its wide routine application is that the obtained parameters are clinically challenging to interpret. In this practical context, the present study’s findings could help improve clinical practice showing that the ventilatory changes evaluated by oscillometry may accurately anticipate the limitations during exercise in COPD. This ability is related to the causal relationships between adequate ventilation, oxygen availability, and physical performance.
A critical analysis of the potential limitations of the present study is necessary. First, it was a single-center study; hence, the results may not represent the entire patient population. One could argue that we only recruited 60 subjects and that the exact prediction accuracy remains unknown. Future studies should include a larger number of subjects. However, this preliminary analysis significantly contributes to an important debate in the literature concerning the clinical and physiological meaning of the oscillometric parameters and provides support for using these indexes in predicting the exercise performance in COPD.
One could argue that the study and reference populations are not well matched for age. However, the analyzed groups can be considered homogeneous because height is the determinant parameter in oscillometry, and this parameter is homogeneous among the studied groups.
One could also argue that we used estimated values of the AVD-Glitre test and Handgrip analysis in the control group. Although in practice, some value outside the normal range may arise in actual measurements, this is a rare event, so the use of normal values should not significantly influence the results. It is essential to consider also that the present study used worldwide-recognized cut-off values to distinguish normal and abnormal functional capacity parameters, which enhanced its generalizability.
Comparing oscillometric and standard spirometric measures to predict poor functional capacity would be very useful. This is an important issue to be addressed in future research.
The present study was intended to explore a possible connection between oscillometric parameters and functional performance and the accuracy of oscillometry as a predictor of abnormal functional capacity in COPD. The study was not designed to test the obtained cut-offs. It is a natural continuation of the present study and will be investigated in the following stages of this research.
Lastly, this study focused on whole-breath impedance measurements. Within-breath analysis was not evaluated. The development of similar analysis for within-breath impedance parameters is a clear direction for future research.
Conclusion
In conclusion, this study evaluated the association of oscillometry and functional capacity in COPD. It has been shown that the oscillometric parameters adequately described the presence of exercise limitation. This resulted in significant and consistent correlations among these parameters and indexes of functional capacity. The ROC analysis demonstrated that oscillometric parameters achieved adequate accuracy and that R4-R20 and Ax achieved high accuracy in predicting poor Glittre‑ADL test and handgrip strength. These results support and add new information concerning Glittre-ADL and Handgrip analysis to previous findings using 6MWD. These results suggest that oscillometric parameters are associated with abnormal exercise performance in COPD, and may help predict poor functional performance in these patients.
Acknowledgments
This study was supported by the Brazilian Council for Scientific and Technological Development (CNPq), the Rio de Janeiro State Research Supporting Foundation (FAPERJ), and in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organisation. Health topics; 2021. Available from: https://www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_1.
2. Perez-Alba E, Nuzzolo-Shihadeh L, Espinosa-Mora JE, Camacho-Ortiz A. Use of self-administered surveys through QR code and same center telemedicine in a walk-in clinic in the era of COVID-19. JAMIA. 2020;27:985–986. doi:10.1093/jamia/ocaa054
3. Di Mango AM, Lopes AJ, Jansen JM, Melo PL. Changes in respiratory mechanics with increasing degrees of airway obstruction in COPD: detection by forced oscillation technique. Respir Med. 2006;100(3):399–410. doi:10.1016/j.rmed.2005.07.005
4. Karkhanis VS, Joshi JM. Spirometry in chronic obstructive lung disease (COPD). J Assoc Physicians India. 2012;60 Suppl:22–26.
5. Johannessen A, Lehmann S, Omenaas ER, Eide GE, Bakke PS, Gulsvik A. Post-bronchodilator spirometry reference values in adults and implications for disease management. Am J Respir Crit Care Med. 2006;173(12):1316–1325. doi:10.1164/rccm.200601-023OC
6. Tomalak W, Czajkowska-Malinowska M, Radlinski J. Application of impulse oscillometry in respiratory system evaluation in elderly patients. Pneumonol Alergol Pol. 2014;82(4):330–335. doi:10.5603/PiAP.2014.0041
7. Czajkowska-Malinowska M, Tomalak W, Radlinski J. Quality of spirometry in the elderly. Pneumonol Alergol Pol. 2013;81(6):511–517.
8. King GG, Bates J, Berger KI, et al. Technical standards for respiratory oscillometry. Eur Respir J. 2020;55(2):1900753. doi:10.1183/13993003.00753-2019
9. Dubois AB, Brody AW, Lewis DH, Burgess BF. Oscillation mechanics of lungs and chest in man. J Appl Physiol. 1956;8(6):587–594. doi:10.1152/jappl.1956.8.6.587
10. Faria ACD, Dames da Silva KK, Costa GM, Lopes AJ, Melo PL. Forced oscillation technique in the detection of smoking-induced respiratory changes. In: Hudak RP, Majernik M, editors. Biomedical Engineering - Technical Applications in Medicine. Vol. 1. Croatia: InTech; 2012.
11. Faria AC, Lopes AJ, Jansen JM, Melo PL. Evaluating the forced oscillation technique in the detection of early smoking-induced respiratory changes. Biomed Eng Online. 2009;8:22. doi:10.1186/1475-925X-8-22
12. Gao L, Wu Y, Wang H, et al. Forced oscillation technique for sensitive detection of early-stage chronic obstructive pulmonary disease. Eur Respir J. 2019;54(suppl 63):A2631.
13. Ribeiro CO, Faria AC, Lopes AJ, Melo PL. Forced oscillation technique for early detection of the effects of smoking and chronic obstructive pulmonary disease: contribution of fractional-order modeling. Int J COPD. 2018;13:3281–3295. doi:10.2147/COPD.S173686
14. Bhattarai P, Myers S, Chia C, et al. Clinical application of forced oscillation technique (FOT) in early detection of airway changes in smokers. J Clin Med. 2020;9(9):2778. doi:10.3390/jcm9092778
15. Amaral JL, Lopes AJ, Faria AC, Melo PL. Machine learning algorithms and forced oscillation measurements to categorise the airway obstruction severity in chronic obstructive pulmonary disease. Comput Methods Programs Biomed. 2015;118(2):186–197. doi:10.1016/j.cmpb.2014.11.002
16. da Costa GM, Faria AC, Di Mango AM, Lopes AJ, Lopes de Melo P. Respiratory impedance and response to salbutamol in healthy individuals and patients with COPD. Respir Int Rev Thoracic Dis. 2014;88(2):101–111. doi:10.1159/000362691
17. Bates JH, Irvin CG, Farre R, Hantos Z. Oscillation mechanics of the respiratory system. Compr Physiol. 2011;1(3):1233–1272.
18. Aarli BB, Calverley PM, Jensen RL, et al. The association of tidal EFL with exercise performance, exacerbations, and death in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2179–2188. doi:10.2147/COPD.S138720
19. Skumlien S, Hagelund T, Bjortuft O, Ryg MS. A field test of functional status as performance of activities of daily living in COPD patients. Respir Med. 2006;100(2):316–323.
20. Alexandre HF, Cani KC, Araujo J, Mayer AF. Reliability and validity of the Glittre-ADL test to assess the functional status of patients with interstitial lung disease. Chron Respir Dis. 2021;18:14799731211012962. doi:10.1177/14799731211012962
21. Dechman G, Scherer S. Outcome measures in cardiopulmonary physical therapy: focus on the Glittre ADL-test for people with chronic obstructive pulmonary disease. Cardiopulm Phys Ther J. 2008;19(4):115–118.
22. Gulart AA, Munari AB, Klein SR, Santos da Silveira L, Mayer AF. The Glittre-ADL test cut-off point to discriminate abnormal functional capacity in patients with COPD. COPD. 2018;15(1):73–78. doi:10.1080/15412555.2017.1369505
23. Strandkvist VJ, Backman H, Röding J, Stridsman C, Lindberg A. Hand grip strength is associated with forced expiratory volume in 1 second among subjects with COPD: report from a population-based cohort study. Int J Chron Obstruct Pulmon Dis. 2016;11:2527–2534. doi:10.2147/COPD.S114154
24. Jeong M, Kang HK, Song P, et al. Hand grip strength in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:2385–2390. doi:10.2147/COPD.S140915
25. Albarrati AM, Gale NS, Enright S, Munnery MM, Cockcroft JR, Shale DJ. A simple and rapid test of physical performance inchronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:1785–1791. doi:10.2147/COPD.S106151
26. Zimmermann SC, Thamrin C, Chan AS, Bertolin A, Chapman DG, King GG. Relationships between forced oscillatory impedance and 6-minute walk distance after pulmonary rehabilitation in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:157–166. doi:10.2147/COPD.S225543
27. Yamamoto A, Shirai T, Hirai K, et al. Oscillometry as a predictor of exercise tolerance in COPD. Copd. 2020;17(6):647–654. doi:10.1080/15412555.2020.1844176
28. Zeng G-S, Chen L-C, Fan H-Z, et al. The relationship between steps of 6MWT and COPD severity: a cross-sectional study. Int J Chronic Obstr. 2018;14:141–148.
29. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338.
30. Vetromille Ribeiro FC, Lopes AJ, de Melo PL. Reference values for respiratory impedance measured by the Forced Oscillation Technique in adult men and women. Clin Respir J. 2018;12:2126–2135. doi:10.1111/crj.12783
31. Pereira CACB, Simões SP, Pereira JG, Gerstler FWL, Nakatani JG. Reference values for spirometry in Brazilian adults. J Brasileiro de Pneumologia. 1992;18:10–22.
32. Karloh M, Araujo CL, Gulart AA, Reis CM, Steidle LJ, Mayer AF. The Glittre-ADL test reflects functional performance measured by physical activities of daily living in patients with chronic obstructive pulmonary disease. Braz J Phys Ther. 2016;20(3):223–230. doi:10.1590/bjpt-rbf.2014.0155
33. Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi:10.1164/rccm.201908-1590ST
34. de Melo PL, Werneck MM, Giannella-Neto A. New impedance spectrometer for scientific and clinical studies of the respiratory system. Rev Sci Instrum. 2000;71(7):2867–2872. doi:10.1063/1.1150705
35. Reis CMD, Karloh M, Fonseca FR, Biscaro RRM, Mazo GZ, Mayer AF. Functional capacity measurement: reference equations for the Glittre activities of daily living test. J brasileiro de pneumologia. 2018;44(5):370–377. doi:10.1590/s1806-37562017000000118
36. Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9(2):222–226. doi:10.1016/S0363-5023(84)80146-X
37. Novaes RD, Miranda AS, Silva JO, Tavares BVF, Dourado VZ. Reference equations for predicting of handgrip strength in Brazilian middle-aged and elderly subjects. Fisioterapia e Pesquisa. 2009;16(3):217–222.
38. Dawson B, Trapp RG. Basic & Clinical Biostatistics.
39. Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95(5):1851–1860. doi:10.1152/japplphysiol.00246.2003
40. Elbedewy RMS, El Said SMS, Taha RM. Indicators of abnormal hand grip strength among older Egyptian adults. J Multidiscip Healthc. 2020;13:387–392. doi:10.2147/JMDH.S240502
41. Goedhart DM, Zanen P, Kerstjens HA, Lammers JW. Discriminating asthma and COPD based on bronchodilator data: an improvement of the methods. Physiol Meas. 2005;26(6):1115–1123. doi:10.1088/0967-3334/26/6/020
42. Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285–1293. doi:10.1126/science.3287615
43. Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772. doi:10.1136/thx.2006.060145
44. Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129(3):536–544. doi:10.1378/chest.129.3.536
45. Yamamoto Y, Miki K, Matsuki T, et al. Evaluation of exertional ventilatory parameters using oscillometry in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:1697–1711. doi:10.2147/COPD.S260735
46. Marinho CL, Maioli MCP, Amaral J, Lopes AJ, Melo PL. Respiratory resistance and reactance in adults with sickle cell anemia: part 2-Fractional-order modeling and a clinical decision support system for the diagnosis of respiratory disorders. PLoS One. 2019;14(3):e0213257.
47. Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–1478. doi:10.1183/09031936.00150414
48. Rambod M, Porszasz J, Make BJ, Crapo JD, Casaburi R, Investigators CO. Six-minute walk distance predictors, including CT scan measures, in the COPDGene cohort. Chest. 2012;141(4):867–875. doi:10.1378/chest.11-0870
49. Ko FW, Hui DS, Lai CK. Worldwide burden of COPD in high- and low-income countries. Part III. Asia-Pacific studies. Int j Tuberc Lung Dis. 2008;12(7):713–717.
50. Fazleen A, Wilkinson T. Early COPD: current evidence for diagnosis and management. Ther Adv Respir Dis. 2020;14:1753466620942128. doi:10.1177/1753466620942128
51. Chukowry PS, Spittle DA, Turner AM. Small airways disease, biomarkers and COPD: where are we? Int J Chronic Obstr. 2021;16:351–365.
52. Brusasco V, Barisione G, Crimi E. Pulmonary physiology: future directions for lung function testing in COPD. Respirology. 2014;20:209–218.
53. MacIntyre NR. The future of pulmonary function testing. Respir Care. 2012;57(1):
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.