Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Respiratory Oscillometry and Functional Performance in Different COPD Phenotypes
Authors Teixeira EM, Ribeiro CO , Lopes AJ , de Melo PL
Received 7 November 2023
Accepted for publication 27 February 2024
Published 6 March 2024 Volume 2024:19 Pages 667—682
DOI https://doi.org/10.2147/COPD.S446085
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jill Ohar
Elayne Moura Teixeira,1 Caroline Oliveira Ribeiro,1 Agnaldo José Lopes,2,3 Pedro Lopes de Melo1,4
1Biomedical Instrumentation Laboratory, Institute of Biology and Faculty of Engineering, State University of Rio de Janeiro, Rio de Janeiro, Brazil; 2Pulmonary Function Laboratory, Pedro Ernesto University Hospital, Faculty of Medical Sciences, State University of Rio de Janeiro, Rio de Janeiro, Brazil; 3Pulmonary Rehabilitation Laboratory, Augusto Motta University Center, Rio de Janeiro, Brazil; 4Laboratory of Clinical and Experimental Research in Vascular Biology - Biomedical Center, State University of Rio de Janeiro, Rio de Janeiro, Brazil
Correspondence: Pedro Lopes de Melo, Rua São Francisco Xavier 524, Pavilhão Haroldo Lisboa da Cunha, Sala 104, Maracanã, Rio de Janeiro, RJ, 20550-013, Brazil, Tel +55-21-2334-0705, Email [email protected]
Purpose: Chronic obstructive pulmonary disease (COPD) phenotypes may introduce different characteristics that need to be known to improve treatment. Respiratory oscillometry provides a detailed analysis and may offer insight into the pathophysiology of COPD. In this paper, we used this method to evaluate the differences in respiratory mechanics of COPD phenotypes.
Patients and Methods: This study investigated a sample of 83 volunteers, being divided into control group (CG = 20), emphysema (n = 23), CB (n = 20) and asthma-COPD overlap syndrome (ACOS, n = 20). These analyses were performed before and after bronchodilator (BD) use. Functional capacity was evaluated using the Glittre‑ADL test, handgrip strength and respiratory pressures.
Results: Initially it was observed that oscillometry provided a detailed description of the COPD phenotypes, which was consistent with the involved pathophysiology. A correlation between oscillometry and functional capacity was observed (r=− 0.541; p = 0.0001), particularly in the emphysema phenotype (r = − 0.496, p = 0.031). BD response was different among the studied phenotypes. This resulted in an accurate discrimination of ACOS from CB [area under the receiver operating curve (AUC) = 0.84] and emphysema (AUC = 0.82).
Conclusion: These results offer evidence that oscillatory indices may enhance the comprehension and identification of COPD phenotypes, thereby potentially improving the support provided to these patients.
Keywords: asthma-COPD overlap, emphysema, chronic bronchitis, respiratory impedance, handgrip analysis, Glittre-ADL test, forced oscillation technique, bronchodilator response
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous lung condition.1 A phenotype is generally considered to be the physical appearance or biochemical characteristic resulting from an interaction between your genotype and the environment. In COPD, where the underlying genes are mostly unknown or poorly characterized, phenotype has become almost synonymous with clinical subgroup.2
Phenotyping allows selecting a uniform group of patients and evaluating the most important outcome measures in this group for therapeutic clinical trials.3 The Spanish guide to chronic obstructive pulmonary disease (GEsEPOC) recognizes 3 phenotypes: emphysema, chronic bronchitis and asthma associated with COPD.4 The differences between the three phenotypes are not precisely known.
The forced oscillation technique (FOT), also referred to as respiratory oscillometry, is a non-invasive method able to provide a detailed analysis of the respiratory system resistance and reactance.5 This method has high potential to increase our understanding of the differences between phenotypes, as well as in their differential diagnosis.
Individuals with COPD exhibit multiple systemic manifestations, including a direct association between the decline in respiratory and peripheral muscle strength and their physical performance and overall functionality. The measurement of the respiratory pressures represent an important procedure for the functional evaluation of the respiratory muscles.6 In addition, peripheral muscle strength may be evaluated by the handgrip test. It is recognized for its cost-effectiveness, simplicity, and a robust correlation with morbidity in chronic diseases.7–11
Exercise intolerance is a common feature in patients with COPD, contributing to reduce the ability to perform activities of daily living.12 These abnormalities may be evaluated by the ADL-Glittre test, which proved to be valid, reliable and capable of reflecting the perception of functional limitation.13
In this context, the current study has two main objectives (1) use respiratory oscillometry to investigate the differences among the COPD phenotypes, and (2) evaluate the association between these abnormalities and the decrease in the functional performance of these patients.
Materials and Methods
Study Design
The present work was developed at the Biomedical Instrumentation Laboratory of the State University of Rio de Janeiro. This research is a cross-sectional study that was approved by the Ethics Committee of the Pedro Ernesto University Hospital (protocol 456 - CEP/2018/HUPE). All individuals signed an informed consent form before performing the tests. The study was carried out in accordance with the Declaration of Helsinki and all measurements were performed on the same day. The subjects carried out respiratory oscillometry and spirometry measurements before and after using the BD. Manovacuometry test, palmar grip and ADL–Glittre, were also performed, in that order.
Subjects
The number of volunteers was calculated using the MedCalc version 12 using preliminary results.14 It were assumed type I and type II errors of 5%, which are usual values in the literature. For the control group, individuals with normal spirometry, non-smokers, without previous pulmonary diseases, and with BMI within the normal range were included. Our study involved individuals who were diagnosed in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD)1 criteria and were aged 40 years or older. All studied subjects had no recent history of respiratory infections within the preceding thirty days at the time of the examinations, and they also had no past history of cardiovascular, orthopedic diseases or COVID-19.
The emphysema phenotype,4,15–17 the chronic bronchitis phenotype18 and the ACOS phenotype19 were diagnosed according to previous studies. Before conducting the tests, all patients continued their regular medications, excluding bronchodilators, in order to prevent any interference in the evaluation, as recommended by the American Thoracic Society/European Respiratory Society (ATS/ERS).20
Spirometry
For spirometry, a computerized system (nSpire Health, Inc., 1830 Left hand Circle, Longmont, CO 80501) was used according to standard protocols.20,21 The parameters analyzed were forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and the ratio between forced expiratory flow (FEF) between 25% and 75% and FVC (FEF/FVC). These parameters were quantified in both absolute values and as a percentage of predicted values, with reference values derived from Pereira et al.22 Lung function data were acquired following post-bronchodilator testing.
Respiratory Oscillometry
The used instrument has been previously described23 and was employed in accordance with current recommendations.5 Pressure oscillations were applied in the frequency range of 4 to 32 Hz, with an amplitude of 2 cmH2O produced by a loudspeaker coupled to the respiratory system through a mouthpiece. The resulting flow and pressure signals were measured near the mouth by a pneumotachograph and a pressure transducer, respectively. During the exams, the volunteers remain seated, with their heads in a neutral position, use a nose clip, maintain spontaneous breathing through the mouthpiece and firmly supporting their cheeks and chin to minimize the shunt. A total of three acceptable tests, each comprising 16 seconds, were carried out, and the outcome considered was the average score. To eliminate any outlier values, only measurements with a coefficient of variation of respiratory resistance at the lowest frequency (4 Hz) equal to or less than 10% for all three tests were retained. Additionally, only examinations with a coherence function of 0.9 or greater across the entire frequency range were accepted, aiming to minimize the impact of spontaneous breathing.
The resistive properties were interpreted through the resistances at 4 Hz (R4), 12 Hz (R12), 20 Hz (R20) and the difference between R4 and R20 (R4-R20). The reactance results were interpreted using the mean (Xm), dynamic compliance (Cdyn), resonance frequency (fr) and area under the reactance curve (Ax). Cdyn is directly associated with the overall compliance of the respiratory system, and was computed using the reactance at 4 Hz (Cdyn=1/2πfX4). The resonance frequency, where respiratory reactance becomes zero, is an indicator of the homogeneity of the respiratory system. The parameter Ax was assessed by the area under the curve formed by the lowest frequency (4 Hz), the corresponding reactance (X4), and the resonance frequency (fr). To analyze the total mechanical load of the respiratory system, the impedance module at 4 Hz (Z4) was investigated, encompassing both the resistive and elastic components of the respiratory load.22
Manovacuometry
The maximum inspiratory pressure (MIP) and the maximum expiratory pressure (MEP) were measured. Measurements were performed five times, until three values were obtained with a variation of less than 5%, the highest value being considered for analysis. Predicted values were calculated using the formulas described in Black & Hyatt.6
Handgrip Test
The handgrip test was conducted using a handheld hydraulic dynamometer (Saehan, SH 5001). Participants were evaluated seated, with their elbows flexed at a 90° angle, holding the dynamometer in their hand in a neutral position. Three trials were performed with each hand, with a one-minute interval between measurements, and the highest value was used for analysis.24 Predicted values were derived from Novaes et al, 2009.25
ADL–Glittre
The ADL–Glittre test was performed as described in Skumlien et al 2006.26 Heart rate (HR), peripheral oxygen saturation (SpO2) and dyspnea index (Modified Borg Scale)27 were measured at the beginning, at each lap and at the end of the test. No verbal stimulus was offered throughout the test. The results obtained from patients with COPD were compared to reference values.28
Statistical Analysis
Data were initially tested for normality using the Shapiro–Wilk test (OriginLab® 8.0, Microcal Software, Inc. Ostend, Belgium), and when the sample showed a normal distribution behavior, the Two-Sample t-Test was used to analyze the groups. On the other hand, when the distribution presented a non-normal characteristic, the Mann–Whitney test was used to analyze the groups. The value of p < 0.05 was used to consider the statistically significant differences.
Correlation analyses were conducted using Pearson correlations for data that exhibited a normal distribution and Spearman correlations for data that did not adhere to a normal distribution. This analysis was carried out using Prism 5.03 (GraphPad Software, La Jolla CA, USA). The classification of these associations followed the guidelines proposed by Dawson and Trapp.29
The accuracy of oscillometry in distinguishing COPD phenotypes was assessed using receiver operating characteristic (ROC) analysis. Optimal prediction cut points were identified based on the optimal trade-off between specificity and sensitivity. The area under the curve (AUC) was computed to quantify the diagnostic accuracy, and AUC values greater than 0.80 were deemed suitable for diagnostic purposes.30 These results were presented as mean ± 95% of the confidence interval (CI). We evaluated oscillometry parameters pre and post bronchodilator, as well as the variations associated with the use of this drug (Δ=values post BD-values pre BD).
Results
The study included a cohort of 83 participants, comprising 20 control subjects and 63 patients with COPD (Table 1). Among these groups, no significant alterations were observed in terms of height, body mass, and body mass index (BMI). However, there was an increase in both age and pack-years within the COPD group.
 |
Table 1 Biometric and Spirometric Parameters of the Studied Groups |
Spirometric pre-bronchodilator (pre-BD) parameters exhibited significant reductions in individuals with COPD compared to the control group, as indicated in Table 1. Considering the BD effect, we observed a significantly higher change in ACOS in comparison with EMP and CB groups and that the EMP and CB groups presented similar modifications.
Oscillometric Parameters
Figure 1 depicts changes in resistive parameters. The values of R4 and R12 (Figures 1A and B, respectively) before BD use were significantly higher than those observed in the control group in all studied phenotypes. The use of the bronchodilator resulted in a significant reduction of R4 and R12 in patients with emphysema and ACOS, but not in patients with CB.
R4-R20 values (Figure 1C) before BD use were significantly higher than those observed in the control group in all studied phenotypes. Bronchodilator use resulted in a significant reduction of R4-R20 in patients with ACOS, but not in patients with emphysema or CB.
Considering the comparisons among the phenotypes, higher values of R4 before BD were observed in the ACOS group in comparison with the group with emphysema (Figure 1A). The ACOS group showed significantly higher values of R4-R20 before using BD than that observed in EMP and CB groups (Figure 1C).
Figure 2A–D, shows that fr, Cdyn, Ax, and Z4 (respectively) values were significantly different from that observed in the control group before BD use. These parameters showed no observable differences following bronchodilator administration in patients with CB. In contrast, patients with emphysema and ACOS exhibited significant changes following bronchodilator administration.
Xm values before BD use were more negative in all studied phenotypes than in the control group (Figure 2E). Bronchodilator administration resulted in significant increases in Xm in groups of patients with emphysema and ACOS. Patients with CB, however, do not present significant changes.
Functional Analysis Tests
Figure 3 describes predicted and measured values of handgrip test in each one of the studied subgroups of patients. Significant reductions were observed in all groups, both considering the dominant hand (Figure 3A) and the non-dominant hands (Figure 3B).
Similar comparisons considering the respiratory pressures are showed in Figure 4. Significant changes were observed in all groups, both in MIP (Figure 4A) and MEP values (Figure 4B). Considering the comparisons among the phenotypes, higher values of MIP after BD were observed in the CB group in comparison with the group with emphysema (Figure 4A).
Figure 5 depicts the results of the AVD-Glittre test. The performed time significantly increased in all studied subgroups of patients when compared to predicted values.
Correlation Analysis
Considering all COPD patients subgroups, almost all studied oscillometric parameters were associated with ADL-Glittre test time and handgrip analysis (Table 2). The exception was due to R4-R20. As can be seen in Table 2, no associations were observed among oscillometric parameters and respiratory pressures.
 |
Table 2 Correlation Analysis Between Total Glittre-ADL Test Time, Handgrip Analysis, Respiratory Pressures and Oscillometric Parameters in the Whole Group of Patients with COPD |
Considering only the emphysema phenotype (Table 3), Cdyn and Z4 showed significant inverse or direct associations, respectively (p < 0.05) with the ADL-Glittre test. There was no relationship between oscillometry and the palmar grip test in the dominant hand. With respect to the non-dominant hand, significant inverse correlations (p < 0.05) were observed between the resistive (R4) and reactive (fr) oscillometric parameters. There was no relationship between oscillometric parameters and manovacuometry.
 |
Table 3 Correlation Analysis Between Total Glittre-ADL Test Time, Handgrip Analysis, Respiratory Pressures and Oscillometric Parameters in the Emphysema Group |
When the correlation analysis included only CB patients, there was no relationship between the oscillometric parameters and the ADL-Glittre test (Table 4). No relationship was found with the palmar grip test in the dominant hand, and an inverse correlation was observed with R12 (p < 0.05). There was no relationship between oscillometric parameters and manovacuometry.
 |
Table 4 Correlation Analysis Between Total Glittre-ADL Test Time, Handgrip Analysis, Respiratory Pressures and Oscillometric Parameters in the Chronic Bronchitis Group |
Similar analysis considering only patients with the ACOS phenotype showed no relationship between the oscillometry and the ADL-Glittre test (Table 5). Concerning the palmar grip test, we do not observed associations with the dominant hand, while, significant inverse correlations (p < 0.05) were observed between R4 and R12 with the non-dominant hand. There was no relationship between oscillometric parameters and manovacuometry.
 |
Table 5 Correlation Analysis Between Total Glittre-ADL Test Time, Handgrip Analysis, Respiratory Pressures and Oscillometric Parameters in the ACOS Group |
Oscillometry Discriminating the Different Phenotypes
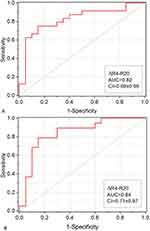
Oscillometric parameters pre and post bronchodilator do not present adequate values of AUC in discriminating the studied phenotypes (AUC < 0.80). The variations of R4-R20 due to the use of BD, on the other hand, provided an accurate discrimination of ACOS from emphysema (Figure 6A, AUC = 0.82, CI = 0.69±0.95) and chronic bronchitis (Figure 6B, AUC = 0.84, CI = 0.71±0.97).
Discussion
In this study, four major findings were obtained: 1) initially, that oscillometry provided a detailed and consistent description of the COPD phenotypes; 2) BD response was different among the studied phenotypes; 3) The study revealed an association between oscillometry and functional capacity, especially within the emphysema phenotype; and 4) ROC curve analysis showed that ΔR4-R20 effectively discriminated ACOS from chronic bronchitis and emphysema phenotypes.
Table 1 displays the biometric parameters of the groups under investigation. Although there was a significant difference in age and body mass between the control group and the ACOS group, the analysed groups can be considered homogeneous, since height is the most important parameter for defining impedance values, age and BMI do not significantly alter respiratory oscillometry parameters.31 This parameter did not exhibit statistically significant differences among the studied groups (Table 1).
In general, the observed increases in R4 and R12 described in Figure 1A and B may be associated with inflammation of the mucous glands due to high tobacco consumption, which results in airway obstruction.32,33 Considering the specific characteristics of the phenotypes, the increased values in emphysema in comparison with the control group may be explained by the destruction of the small airways and loss of the parenchymal tissue that keeps the airways open.33 The increase in resistance found in the CB group may be associated with the worsening of airflow obstruction, a result of the excess mucus caused by the increase in goblet cells in the small airways in these individuals.34 The similar increase observed in ACOS can be explained by the typical increased bronchial secretion and, consequently, greater narrowing of the airways.
The comparisons among phenotypes showed that ACOS presented higher R4 values before BD use than the group with emphysema predominance. This is in line with the results of Van Noord et al, which showed that airway resistance was significantly higher in asthma and chronic bronchitis than in emphysema.35 Further support to this finding is provided by 3D CT analyses in the third to sixth generation central bronchus, which showed that the ACOS had greater airway narrowing compared with COPD.36
R4-R20 is associated with the respiratory system homogeneity of ventilation.37 The results of the present study provide evidence of reduced ventilation homogeneity in all studied phenotypes (Figure 1C). In close agreement with these results, Su et al, showed associations between variations in resistance and the degree of morphological abnormalities of the small airways evaluated with endobronchial optical coherence tomography in COPD and heavy smokers.38
R4-R20 increased in ACOS in comparison with emphysema and CB (Figure 1C). This result agrees with that obtained using 3D computed tomography analyses of the airways in COPD and ACOS in the study of Karayama et al.36 This difference may be explained, at least in part, by the addition of the pathophysiological characteristics of asthma in these patients. In this disease, bronchial obstruction may result from bronchospasm, mucosal oedema and hypersecretion.39 Thus, the presence of these additional factors seems to introduce greater non-homogeneities in the ventilation of these patients than those caused in patients with predominance of emphysema or CB.
Some authors claim that bronchodilation in patients with COPD causes an increase in the diameter of the airways.40,41 In line with previous studies,41,42 we found a reduction in respiratory resistance (Figures 1A and B) in individuals classified as having emphysema and ACOS after using a bronchodilator. It was interesting to observe that similar alterations did not occur in the group with a preponderance of CB. This indicates that the smooth muscle relaxation introduced by BD use do not result in significant changes in this group. Since the main mechanism of respiratory obstruction in these patients refers to excessive mucus production, which is not influenced by BD use, this result seems to be reasonable. Important structural changes in CB includes airway wall thickening due to remodeling. This phenomena make airways difficult to “open”, reducing response to bronchodilator agents.43 Baldi et al suggested that airway distensibility is reduced in COPD and that airway smooth muscle contributes to the increased airway stiffness in COPD subjects with prevailing bronchitis,44 but not in those with more emphysema.
Bronchodilator use decreased the value of R4-R20 in patients with emphysema and ACOS (Figure 1C), revealing an improvement in the ventilation homogeneity.45 Although R4-R20 was reduced with the BD use, this parameter still presented increased values compared to healthy individuals. This indicates that not all imbalances in the time constants were eliminated with the BD use.46 In a similar way to what was observed in R4 (Figure 1A) and R20 (Figure 1B), the R4-R20 values did not change with the use of the bronchodilator. We can speculate that the same reasons described earlier for the lack of response in terms of R4 and R20 may also be involved in these results.43,44
A comparative analysis of the reactive parameters before BD use with the control group showed significant changes in all studied parameters and phenotypes (Figure 2). This can be explained by the increased ventilation non-homogeneity in the respiratory system of these patients, which occurs due to the increase in imbalances in the time constants of the different regions of the lungs of patients with COPD.47 These changes may also be associated with abnormalities in lung tissue, chest wall, airway distensibility and increased resistance,47 as well as with the reduction in the apparent compliance.48
The use of bronchodilator medication significantly improved almost all reactive parameters in the emphysema and ACOS groups (Figure 2). The bronchodilator use relax the smooth muscles of the bronchi, improving the compliance of the airway wall.49 A previous study showed an increase in Cdyn after the use of salbutamol in patients with obstruction due to asthma and COPD.50 The increase in compliance reflects the improvement in lung expansion, associated with a reduction in the peripheral airways resistance resulting in an improvement in lung homogeneity and a decrease in hyperinflation after drug inhalation.50 The cited factors explain the improvements observed in Cdyn, AX, Z4 and Xm (Figure 2).
In contrast, the use of BD did not result in discernible changes in patients with CB (Figure 2). Although persistent airflow limitation occurs in patients with COPD, ACOS and asthma, the flow limitation phenomena may have distinct characteristics, resulting in different responsiveness to bronchodilators.51 Chronic bronchitis is caused by the hypersecretion of mucus by the goblet cells, which leads to the worsening of resistance to airflow by obstructing the lumen of the small airways. The presence of inflammation in the epithelium of the central airways is another important characteristic, which may introduce epithelial remodelling.52,53 On the other hand, the main characteristic of emphysema refers to the destruction of the lung parenchyma, leading to loss of elastic recoil.54 Previous works hypothesize that an inflamed and thick airway may appear more rigid than an airway subjected to the devastation of proteases.55,56 This factor could explain, at least in part, observed differences between CB and emphysema, since relaxation in the airways smooth muscles could have lesser effects in more rigid airways, such as those present in CB than in the airways of patients with emphysema.
Asthma is characterized by bronchial muscle contraction and airway narrowing that is highly reversible using BD medication.35 The greater response to BD observed in patients with ACOS compared to those with CB (Figure 2) is probably related to this characteristic.
Respiratory abnormalities represent only one aspect of the multifaceted complications associated with this COPD. In this context, muscle dysfunction emerges as a primary anomaly linked to diminished functionality.57 The evaluation of peripheral muscle strength in COPD patients carries paramount importance due to its correlation with various factors, including exercise intolerance, challenges in performing daily activities, and a diminished quality of life.57 This study observed substantial reductions in manual grip strength for both the dominant (Figure 3A) and non-dominant hands (Figure 3B) within the emphysema, chronic bronchitis, and ACOS groups when contrasted with predicted values for each respective group. The etiology of muscle dysfunction and exercise impediments observed in COPD patients is explicable through systemic inflammation originating from the pulmonary system and a reduction in the bulk of musculature within the lower extremities.10 Musculoskeletal dysfunction, characterized by the loss of muscle strength and endurance, is principally attributed to diminished muscle area, reduced lean body mass, compromised muscle stamina, and an augmented susceptibility to fatigue.58,59 Holden et al described a relation between reduced handgrip strength and diminished quality of life, heightened vulnerability to exacerbated COPD morbidity and increased risk of mortality.58
Significant reductions in manometry were observed for PiMax (Figure 4A) and PeMax (Figure 4B) in the emphysema, chronic bronchitis, and ACOS groups. Muscle fatigue resulting from the detrimental effects of COPD not only affects peripheral muscles but can also compromise respiratory muscle function.60 Another explanation is associated with airway obstruction and pulmonary hyperinflation, which position the diaphragm at a mechanical disadvantage, resulting in a chronic decrease in contact area, leading to reduced respiratory muscle efficiency in these individuals.61
Significant increases in the time taken to complete the Glittre-ADL test were observed in the emphysema, chronic bronchitis, and ACOS groups compared to predicted values (Figure 5). These limitations are associated with several factors, including gas exchange inefficiency, ventilatory limitation, peripheral muscle weakness, alterations in metabolism, and peripheral muscle composition.62 Dynamic hyperinflation may contribute to these findings, potentially worsening when respiratory demand increases during exercise and creating a sensation of dyspnea as respiratory work intensifies.63
Table 2 shows that functional capacity was more sensitive to changes in airway obstruction (R4 and R12) and elastic properties (fr, Cdyn, Ax and Z4) than changes in ventilation heterogeneity (R4-R20). The moderate to good correlations observed among oscillometry with ADL-Glittre test and Handgrip analysis reinforce the notion that oscillatory indices are associated with physical performance and are valuable for predicting reduced exercise tolerance in individuals with COPD.64 The results are also consistent with that obtained recently using the 6-minute walking distance65,66 and during cycle ergometer tests.67 These associations agree with the involved physiology, describing an increase in ADL-Glittre test time with airway obstruction and Cdyn reduction. This reflects the systemic effects due to lung abnormalities, including the presence of airflow limitation and dynamic hyperinflation.
Considering the analysis performed specifically in the studied COPD phenotypes, it was interesting to note significant relationships in the emphysema group (Table 3) describing a decrease in ADL-Glittre test performance with reductions in Cdyn and increases in Z4. These associations agree with the typical changes observed in COPD, describing abnormalities in elastic properties (Cdyn), and respiratory work (Z4). The inverse associations observed among resistive properties (R4) and ventilation heterogeneity (fr) with Handgrip analysis are also consistent with the cited principles.
A recent review points out that oscillometry adds insight into the pathophysiology of COPD, and that we still need more data to assess how this method relates to clinical phenotypes of COPD.37 COPD phenotypes should be able to classify patients into distinct subgroups that provide prognostic information and allow us to better determine the appropriate therapy that alters clinically significant outcomes.3,37 In this sense, the current study provides evidence that oscillometry may help to accurately discriminate ACOS from emphysema and chronic bronchitis (AUC>0.80). This high performance probably reflects the higher impact of bronchodilator use in the ventilation heterogeneity of the ACOS group due to the asthmatic component of this phenotype.
The differential diagnosis between groups of emphysema predominance and chronic bronchitis predominance are likely to be complex, and their clarification needs further investigation. It is important to emphasize that promising values of AUC were observed in these analyses, with values around 0.65. Previous studies from our research group have demonstrated that the application of artificial intelligence methods can enhance the accuracy of oscillometry parameters in the early diagnosis of smoking-induced respiratory changes68 and in the diagnosis69 and classification70 of COPD. In this manner, the application of these methods for the proper discrimination between emphysema and chronic bronchitis is one of the upcoming steps planned by our research group in this line of investigation.
A thorough examination of potential limitations of the current study is necessary. Firstly, the study concentrated on whole-breath impedance measurements and did not evaluate within-breath analysis.71 In future research, it is advisable to explore similar analyses focusing on within-breath impedance parameters. This avenue represents a promising research direction. Another noteworthy point; plethismographic exams were not feasible in all patients due to their clinical condition or inability to cooperate during the test. This resulted in the loss of important information that could have helped to clarify the differences between the phenotypes. Lastly, this is a single-center study, thus the outcomes may lack broad applicability to the entire patient demographic. This emphasizes the necessity for future investigations with a higher number of volunteers. Despite these limitations, this preliminary analysis significantly contributes to a critical discussion concerning the use of oscillometric parameters to evaluate COPD phenotypes.
Conclusion
In conclusion, this study set out to examine COPD phenotypes in-depth via respiratory oscillometry. It has been shown initially that oscillometry provided a description of the COPD phenotypes consistent with the involved physiopathology. The use of BD medication introduced clear changes in ACOS and Emphysema, but not in patients with predominance of CB. The correlation analysis unveiled a clear relationship between oscillometry and functional capacity, notably within the emphysema phenotype. ROC analysis further indicated that oscillometric parameters displayed sufficient accuracy in discriminating ACOS from Emphysema and CB. These findings offer evidence that oscillatory indices have the potential to enhance our understanding and identification of COPD phenotypes.
Acknowledgments
The research presented in this study received support from the Brazilian Council for Scientific and Technological Development (CNPq), the Rio de Janeiro State Research Supporting Foundation (FAPERJ), and was partially funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES) under Finance Code 001.
Disclosure
The authors report no conflicts of interest in this work.
References
1. The global strategy for the diagnosis, management and prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2023. Available from: http://wwwgoldcopdorg.
2. Vestbo J. COPD: definition and Phenotypes. Clinics Chest Med. 2014;35(1):1–6. doi:10.1016/j.ccm.2013.10.010
3. Han M, Agusti A, Calverley P, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi:10.1164/rccm.200912-1843CC
4. Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish COPD Guidelines (GesEPOC) 2017. Pharmacological treatment of stable chronic obstructive pulmonary disease. Archivos de Bronconeumología. 2017;53(6):324–335. doi:10.1016/j.arbres.2017.03.018
5. King G, Bates J, Berger K, et al. Technical standards for respiratory oscillometry. Europ resp J. 2020;55(2):1900753. doi:10.1183/13993003.00753-2019
6. Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99(5):696–702. doi:10.1164/arrd.1969.99.5.696
7. Marcos L, Bichinho GL, Panizzi EA, Storino KKG, Pinto DV. Analysis of chest radiography of individuals with COPD and its correlation with functional testing. Fisioterapia e Movimento. 2012;25:629–637. doi:10.1590/S0103-51502012000300018
8. Cheung CL, Nguyen US, Au E, Tan KC, Kung AW. Association of handgrip strength with chronic diseases and multimorbidity: a cross-sectional study. Age. 2013;35(3):929–941. doi:10.1007/s11357-012-9385-y
9. Strandkvist V, Backman H, Röding J, Stridsman C, Lindberg A. Hand grip strength is associated with forced expiratory volume in 1 second among subjects with COPD: report from a population-based cohort study. Int J Chron Obstruct Pulmon Dis. 2016;11:2527–2534. doi:10.2147/COPD.S114154
10. Jeong M, Kang H, Song P, et al. Hand grip strength in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:2385–2390. doi:10.2147/COPD.S140915
11. Albarrati A, Gale N, Enright S, Munnery M, Cockcroft J, Shale D. A simple and rapid test of physical performance in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:1785–1791. doi:10.2147/COPD.S106151
12. Karloh M, Karsten M, Pissaia F, Araujo C, Mayer A. Physiological responses to the Glittre-ADL test in patients with chronic obstructive pulmonary disease. J Rehabil Med. 2014;46(1):88–94. doi:10.2340/16501977-1217
13. Gulart A, Munari A, Klein S, Santos da Silveira L, Mayer A. The glittre-ADL Test cut-off point to discriminate abnormal functional capacity in patients with COPD. COPD. 2018;15(1):73–78. doi:10.1080/15412555.2017.1369505
14. Teixeira EM, Lopes AJ, Melo PL Differences in respiratory mechanics in emphysema and chronic bronchitis evaluated by forced oscillations. In:
15. Izquierdo-Alonso JL, Rodriguez-Gonzalezmoro JM, De lucas-ramos P, et al. Prevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD). Respir Med. 2013;107(5):724–731. doi:10.1016/j.rmed.2013.01.001
16. Golpe R, Suárez-Valor M, Martín-Robles I, et al. Mortality in COPD patients according to clinical phenotypes. Int J Chron Obstruct Pulmon Dis. 2018;13:1433–1439. doi:10.2147/COPD.S159834
17. Cheng Y, Tu X, Pan L, et al. Clinical characteristics of chronic bronchitic, emphysematous and ACOS phenotypes in COPD patients with frequent exacerbations. Int J Chron Obstruct Pulmon Dis. 2017;12:2069–2074. doi:10.2147/COPD.S140231
18. Koblizek V, Chlumsky J, Zindr V, et al. Chronic Obstructive Pulmonary Disease: official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented care. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157(2):189–201. doi:10.5507/bp.2013.039
19. Soler-Cataluña JJ, Cosío B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012;48(9):331–337. doi:10.1016/j.arbres.2011.12.009
20. Miller M, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
21. Graham B, Steenbruggen I, Miller M, et al. Standardization of spirometry 2019 update. an official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi:10.1164/rccm.201908-1590ST
22. Pereira C, Sato T, Rodrigues S. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol. 2007;33(4):397–406. doi:10.1590/S1806-37132007000400008
23. de Melo PL, Werneck MM, Giannella-Neto A. New impedance spectrometer for scientific and clinical studies of the respiratory system. Rev Sci Instrum. 2000;71(7):2867–2872. doi:10.1063/1.1150705
24. Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg. 1984;9(2):222–226. doi:10.1016/S0363-5023(84)80146-X
25. Novaes RD, Miranda AS, Silva JO, Tavares BVF, Dourado VZ. Reference equations for predicting of handgrip strength in Brazilian middle-aged and elderly subjects. Fisioter Pesq. 2009;16(3):217–222. doi:10.1590/S1809-29502009000300005
26. Skumlien S, Hagelund T, Bjortuft O, Ryg M. A field test of functional status as performance of activities of daily living in COPD patients. Respir Med. 2006;100(2):316–323. doi:10.1016/j.rmed.2005.04.022
27. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exercise. 1982;14(5):377???381. doi:10.1249/00005768-198205000-00012
28. Reis C, Karloh M, Fonseca F, Biscaro R, Mazo G, Mayer A. Functional capacity measurement: reference equations for the Glittre Activities of Daily Living test. J Bras Pneumol. 2018;44(5):370–377. doi:10.1590/s1806-37562017000000118
29. Dawson B, Trapp RG. Basic & Clinical Biostatistics.
30. Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285–1293. doi:10.1126/science.3287615
31. Ribeiro F, Lopes A, Melo P. Reference values for respiratory impedance measured by the forced oscillation technique in adult men and women. Clin Respir J. 2018;12(6):2126–2135. doi:10.1111/crj.12783
32. Bohadana A, Teculescu D, Martinet Y. Mechanisms of chronic airway obstruction in smokers. Respir Med. 2004;98(2):139–151. doi:10.1016/j.rmed.2003.09.005
33. Loring SH, Garcia-Jacques M, Malhotra A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J Appl Physiol. 2009;107(1):309–314. doi:10.1152/japplphysiol.00008.2009
34. Widysanto A, Mathew G. Chronic Bronchitis. In: StatPearls. StatPearls Publishing; 2021.
35. Van Noord J, Clement J, Van de Woestijne K, Demedts M, Taussig LM. Total respiratory resistance and reactance in patients with asthma, chronic bronchitis, and emphysema. J Am Rev Respir Dis. 1991;143(5 Pt 1):922–927. doi:10.1164/ajrccm/143.2.312
36. Karayama M, Inui N, Yasui H, et al. Physiological and morphological differences of airways between COPD and asthma–COPD overlap. J Sci Rep. 2019;9(1):7818. doi:10.1038/s41598-019-44345-6
37. Kaminsky D, Simpson S, Berger K, et al. Clinical significance and applications of oscillometry. Eur Respir Rev. 2022;31(163):210208. doi:10.1183/16000617.0208-2021
38. Su Z-Q, Guan W-J, S-Y L, et al. Significances of spirometry and impulse oscillometry for detecting small airway disorders assessed with endobronchial optical coherence tomography in COPD. J Int J Chron Obstruct Pulm Dis. 2018;Volume 13:3031–3044. doi:10.2147/COPD.S172639
39. Spiro SG, Silvestri GA, Agustí A. Clinical respiratory medicine e-book: expert consult-online and print. Elsevier Health Sci. 2012;2:1.
40. Borrill ZL, Houghton C, Woodcock A, Vestbo J, Singh D. Measuring bronchodilation in COPD clinical trials. J Br Clin Pharmacol. 2005;59(4):379–384. doi:10.1111/j.1365-2125.2004.02261.x
41. da Costa GM, Faria AC, Di mango AM, Lopes AJ, Lopes de Melo P. Respiratory impedance and response to salbutamol in healthy individuals and patients with COPD. Respiration. 2014;88(2):101–111. doi:10.1159/000362691
42. Costa G, Faria A, Mango A, Lopes A, Jansen J, Melo P. Bronchodilation in COPD: beyond FEV1-The effect of albuterol on resistive and reactive properties of the respiratory system. J Bras Pneumol. 2009;35(1):1. doi:10.1590/s1806-37132009000100001
43. Jones RL, Noble PB, Elliot JG, James AL. Airway remodelling in COPD: it’s not asthma! Respirology. 2016;21(8):1347–1356. doi:10.1111/resp.12841
44. Baldi S, Dellacà R, Govoni L, et al. Airway distensibility and volume recruitment with lung inflation in COPD. J Appl Physiol. 2010;109(4):1019–1026. doi:10.1152/japplphysiol.00147.2010
45. Zerah F, Lorino A-M, Lorino H, Harf A, Macquin-Mavier I. Forced oscillation technique vs spirometry to assess bronchodilatation in patients with asthma and COPD. J Chest. 1995;108(1):41–47. doi:10.1378/chest.108.1.41
46. Costa GM, Faria ACD, Mango D, et al. Broncodilatação na DPOC: muito além do VEF1-efeito do salbutamol nas propriedades resistivas e reativas do sistema respiratório. J Brasil de Pneumol. 2009;35(4):325–333. doi:10.1590/S1806-37132009000400006
47. Di Mango A, Lopes A, Jansen J, Melo P. Changes in respiratory mechanics with increasing degrees of airway obstruction in COPD: detection by forced oscillation technique. Respir Med. 2006;100(3):399–410. doi:10.1016/j.rmed.2005.07.005
48. Faria ACD, da Costa AA, Lopes AJ, Jansen JM, de Melo PL. Forced oscillation technique in the detection of smoking‐induced respiratory alterations: diagnostic accuracy and comparison with spirometry. Clinics. 2010;65(12):1295–1304. doi:10.1590/S1807-59322010001200012
49. Delacourt C, Lorino H, Herve-Guillot M, Reinert P, Harf A, Housset B. Use of the Forced Oscillation Technique to Assess AirwayObstruction and Reversibility in Children. American Journal of Respiratory and Critical Care Medicine. 2000;161(3):730–736. doi:10.1164/ajrccm.161.3.9904081
50. Lorino A, Zerah F, Mariette C, Harf A, Lorino H. Respiratory resistive impedance in obstructive patients: linear regression analysis vs viscoelastic modelling. Europ resp J. 1997;10(1):150–155. doi:10.1183/09031936.97.10010150
51. Kitaguchi Y, Yasuo M, Hanaoka M. Comparison of pulmonary function in patients with COPD, asthma-COPD overlap syndrome, and asthma with airflow limitation. J Int J Chron Obstruct Pulm Dis. 2016;991–997. doi:10.2147/COPD.S105988
52. Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. J Am J Respir Crit Care Med. 2013;187(3):228–237. doi:10.1164/rccm.201210-1843CI
53. West J, Luks A. West’s Pulmonary Pathophysiology: The Essentials.
54. Márquez-Martín E, Ramos PC, López-Campos JL, et al. Components of physical capacity in patients with chronic obstructive pulmonary disease: relationship with phenotypic expression. J Int J Chron Obstruct Pulm Dis;2011. 105–112. doi:10.2147/COPD.S16646
55. Bates JH. Systems physiology of the airways in health and obstructive pulmonary disease. J Wiley Interdiscipl Rev. 2016;8(5):423–437.
56. Khan MA, Ellis R, Inman MD, Bates JH, Sanderson MJ, Janssen LJ. Influence of airway wall stiffness and parenchymal tethering on the dynamics of bronchoconstriction. Am J Physiol Lung Cell Mole Physiol. 2010;299(1):L98–L108. doi:10.1152/ajplung.00011.2010
57. Nunes MF, Hervé BB, Lukrafka JL, Monteiro MB. Handgrip strength and its relation to isokinetic dynamometry in COPD. J Fisioterapia Em Movime. 2020;3:33.
58. Holden M, Fyfe M, Poulin C, et al. Handgrip strength in people with chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Phys Ther. 2021;101:6.
59. Vieira RHG, Nogueira IDB, Queiroz NF, et al. Peripheral and respiratory muscle strength in chronic obstructive pulmonary disease. Rev bras cineantropom desempenho hum. 2018;20(2):125–133. doi:10.5007/1980-0037.2018v20n2p125
60. Souza RM, Cardim AB, Maia TO, Rocha LG, Bezerra SD, Marinho PM. Inspiratory muscle strength, diaphragmatic mobility, and body composition in chronic obstructive pulmonary disease. J Physiother Res Int. 2019;24:2.
61. Dellacà RL, Santus P, Aliverti A, et al. Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J. 2004;23(2):232–240. doi:10.1183/09031936.04.00046804
62. Corrêa KS, Karloh M, Martins LQ, Santos K, Mayer AF. Can the Glittre ADL test differentiate the functional capacity of COPD patients from that of healthy subjects? J Braz J Phy Ther. 2011;15(6):467–473. doi:10.1590/S1413-35552011005000034
63. Raskin J, Marks T, Miller A. Phenotypes and Characterization of COPD. J Cardiopul Rehabil Prevent. 2018;38(1):43–48. doi:10.1097/HCR.0000000000000271
64. Ribeiro CO, Lopes AJ, de Melo PL. Respiratory oscillometry in chronic obstructive pulmonary disease: association with functional capacity as evaluated by adl glittre test and hand grip strength test. Int J Chronic Obstr. 2022;Volume 17:1017–1030. doi:10.2147/COPD.S353912
65. Yamamoto A, Shirai T, Hirai K, et al. Oscillometry as a predictor of exercise tolerance in COPD. Copd. 2020;17(6):647–654. doi:10.1080/15412555.2020.1844176
66. Zeng G-S, Chen L-C, Fan H-Z, et al. The relationship between steps of 6MWT and COPD severity: a cross-sectional study. Int J Chronic Obstr. 2018;14:141–148.
67. Yamamoto Y, Miki K, Matsuki T, et al. Evaluation of exertional ventilatory parameters using oscillometry in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:1697–1711. doi:10.2147/COPD.S260735
68. Amaral JL, Lopes AJ, Jansen JM, Faria AC, Melo PL. An improved method of early diagnosis of smoking-induced respiratory changes using machine learning algorithms. Comput Methods Programs Biomed. 2013;112(3):441–454. doi:10.1016/j.cmpb.2013.08.004
69. Amaral JL, Lopes AJ, Jansen JM, Faria AC, Melo PL. Machine learning algorithms and forced oscillation measurements applied to the automatic identification of chronic obstructive pulmonary disease. Comput Methods Programs Biomed. 2012;105(3):183–193. doi:10.1016/j.cmpb.2011.09.009
70. Amaral JL, Lopes AJ, Faria AC, Melo PL. Machine learning algorithms and forced oscillation measurements to categorise the airway obstruction severity in chronic obstructive pulmonary disease. Comput Methods Programs Biomed. 2015;118(2):186–197. doi:10.1016/j.cmpb.2014.11.002
71. András L, Dorottya C, Zoltán G, et al. Airway dynamics in COPD patients by within-breath impedance tracking: effects of continuous positive airway pressure. Eur Respir J. 2017;49(2):1601270. doi:10.1183/13993003.01270-2016
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.