Back to Journals » Journal of Inflammation Research » Volume 8
Respiratory metapneumoviral infection without co-infection in association with acute and chronic lung allograft dysfunction
Authors Dosanjh A
Received 27 November 2014
Accepted for publication 5 February 2015
Published 19 March 2015 Volume 2015:8 Pages 79—82
DOI https://doi.org/10.2147/JIR.S78259
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Amrita Dosanjh
Department of Pediatrics, Rady Children’s Hospital, San Diego, CA, USA
Background: Metapneumoviral respiratory infection is a community-acquired respiratory viral (CARV) infection. Lung transplantation recipients exposed to CARV are at risk for development of allograft rejection. The cellular and molecular pathways initiated by viral infection leading to allograft dysfunction are not completely understood. The aim of this study was to identify human metapneumoviral (hMPV) cases in association with allograft rejection.
Methods: A literature search was conducted to identify cases of both hMPV and allograft rejection within 6 months of the initial infection. This resulted in 1,007 lung transplantation recipients, with a total of 2,883 samples identified. Of these, 57 demonstrated isolated hMPV without co-infection with other agents.
Results: The results of the study indicate that 35% of acute hMPV infections without co-infection, at the time of detection by molecular diagnostic platforms, were associated with acute cellular rejection within 3 months. There were 9.4% of the cases subsequently associated with chronic allograft dysfunction/bronchiolitis obliterans syndrome, which was collectively termed chronic rejection for purposes of analysis. In conclusion, the prompt identification of isolated hMPV from lung transplantation patients is an important treatable risk factor for subsequent allograft dysfunction. The cellular and molecular pathogenesis of viral-induced allograft rejection remains a topic of future study.
Keywords: viral infection, bronchiolitis obliterans, acute cellular rejection, allograft, lung, metapneumovirus
Introduction
Human metapneumovirus (hMPV) was discovered in 2001 as a frequent cause of upper and lower respiratory tract infections in the community. Among children, sero-positivity approaches 100% by school age.1 In one report, hMPV was detected among 6% of hospitalized children, 7% of those seen in outpatient clinics, and 7% of those seen in emergency departments. The overall incidence varied from one to two in 1,000 affected children. The risk of severe infection is higher among young infants who have developing immune systems.2 Among children with at least one underlying medical condition, the incidence of hospitalization is higher, and was reported by Hahn et al3 to be 68% of 238 (n=162) hospitalized children with hMPV. Children in the community may in turn expose immunocompromised patients to the virus. Other predisposing factors affecting severity include higher viral load and virulence factors associated with attachment to the airway epithelium.3 Most immunocompetent patients resolve their symptoms without any specific treatment. Among the lung transplant recipients though, the sequelae of this hMPV infection can be severe and contribute to organ failure and death. There are only limited studies describing hMPV specifically in the lung allograft rejection population. Since community-acquired viruses are known to be one possible risk factor for both acute cellular rejection and bronchiolitis obliterans syndrome (BOS)/chronic allograft dysfunction (CAD), the goal of this present study was to analyze acute rejection (AR) and chronic rejection (CR)/BOS related to metapneumoviral (MPV) infection in a lung transplantation population.
Methods
A literature search was conducted to identify studies describing hMPV infection in association with allograft rejection, among lung transplantation recipients of any age. The PubMed and MEDLINE databases, two different search engines, were used to search published citations from 2001 to 2014. The keywords used were “Metapneumoviral (both viral and virus)” and “Lung/Transplantation”.
Clinical case reports and any study with clinical patient data describing both lung allograft rejection and the identification of hMPV, without co-infection, were included for further analysis. Articles describing non-lung solid organ transplant patients only, or hematopoeitic stem cell transplantation patients only, and those without data identifying both allograft rejection and hMPV were excluded. The patients described in the articles that did not contain information on hMPV infection without co-infection and allograft rejection were not included in the analysis. There were 15 citations identified, and four were excluded for not fully meeting the inclusion criteria. There were eleven articles included therefore for further analysis.
Statistical analysis
This study identified the number (n) of total study samples and patients meeting the outlined criteria for analysis. The patients who experienced allograft rejection in association with isolated hMPV were described by number and percentage. The term meta-analysis refers to the analysis of a large dataset.
Results
All the studies included for analysis used molecular detection platforms to identify the messenger (m)RNA of hMPV. The mean age and post-transplantation day were not uniformly recorded in the studies and not included in the data summary. The data collection periods ranged from 6 months to 3 years. The follow-up period between hMPV detection and the diagnosis of rejection ranged from 0 to 3 months, with the exception of the Hopkins et al study,4 which identified BOS up to 6 months post-transplantation.
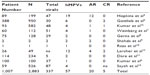
In one study of n=89 lung transplant patients with upper respiratory infection/lower respiratory tract infection, there were 19 polymerase chain reaction (PCR)-positive hMPV cases, of which 12/19 (63%) were associated with AR. There were no cases of CR described.4 In another larger study of 388 lung transplant patients screened for community-acquired respiratory virus (CARV), 30 patients were identified by PCR. Of these, 6/30 or 20% were proven positive for hMPV, and 2/6 or 33% had a history of BOS within 6 months of the identification of the hMPV.5
One study of 582 samples collected from 93 patients identified 81 samples by PCR, from 48 patients, with a positive PCR identification of viral mRNA. Of these, 4/48 patients (8.3%) were positive for hMPV. All four positive samples were from recipients with no symptoms. One of the four developed AR and then progressed to BOS/CR.6
Another study of 60 patients, with 112 respiratory infections, identified 51 samples with one or more CARV. Out of the 51, seven (13.7%) were hMPV positive, either alone or in combination with another virus, and four patients had only hMPV. Of the four patients, one had AR, and none had CR.7
Gerna et al collected 128 samples (56 nasopharyngeal and 72 bronchoalveolar lavage [BAL]) from 75 lung transplant recipients, over a 3-year period sampled either by nasal aspirate or BAL. hMPV was identified frequently in BAL (60%) either alone or in combination with other organisms.8 Four patients over the 3-year period had hMPV identified. Twenty-nine patients had a positive sample for hMPV. Two of the four patients with hMPV had an episode of acute cellular rejection, but there was co-infection in those patients with another virus (rhinovirus, cytomegalovirus). These two patients were not included due to hMPV-related rejection in this analysis.
Another study analyzed only positive cases of hMPV in an immunocompromised population. It identified two lung transplantation patients with hMPV infections, and two out of nine (22%) had hMPV.9 Both developed AR and subsequently died. The study did not describe the detection of other viruses. The summary of the results of all eleven studies is shown in Table 1.
In summary, there were 1,007 lung transplant recipients, with 2,883 samples analyzed for viruses. Out of 337 samples that identified viruses (any), 57 (17%) were positive for hMPV. Twenty out of the 57 (35%) cases of hMPV had AR within 3 months of detection of the virus. There were five cases of CR in association with hMPV.
Discussion and conclusion
This study is one of the largest meta-analyses to date analyzing hMPV respiratory infections and allograft rejection, among lung transplantation recipients. The results indicate that detection of hMPV from airway secretions may be a significant post-transplantation occurrence. There were 35% of cases of hMPV associated with AR and 9.4% associated with the development of CR.
Respiratory syncytial virus (RSV), adenovirus, and parainfluenza viruses are recognized as clinical risk factors for allograft rejection.10 There are fewer studies of hMPV infection without co-infection by bacteria, fungi, or other viruses as a risk factor for rejection and allograft dysfunction in the lung transplantation literature.11,12 The present study indicates that hMPV may be a significant risk factor for rejection episodes, since 35% of the patients with isolated hMPV respiratory infection developed AR and almost 10% developed CR within a short time frame of the initial infection. All studies, with the exception of one, included identified rejection within 3 months. The other study analyzed cases of CR within 6 months.4
Since respiratory infections can be prevalent in the community at any time during the year, the lung transplantation recipient is at risk during the initial and subsequent clinical course. Avoidance measures such as avoiding ill contacts, hand hygiene, and vaccinations are important in the clinical management.
hMPV was first identified in 2001 as a negative-stranded RNA virus, in the paramyxoviridae family. hMPV is closely related in structure, clinical presentation, and virulence to RSV. One contrasting feature though is that hMPV is much more difficult to detect by available rapid detection methods or standard viral culture. Molecular methods, such as PCR or luminex, remain the diagnostic test of choice. One study reported that 20% of false tests using a direct antigen platform were actually related to hMPV infections.4
The epidemiology in the community varies from 3% to 5%, as a cause of respiratory tract infection. Among children with severe respiratory tract infections, the rate is estimated to be 5%–7%.1,2
To date, there have been numerous studies of community-acquired viruses in lung transplantation patients, but very few focused on hMPV infection without co-infection with other organisms, as a risk factor for acute cellular rejection and CAD. The present study pooled data and analyzed the temporally related outcomes of AR and CR in this population.
Cellular and molecular pathways linking viral infection and CAD as well as acute cellular rejection suggest that the initial injury is to the epithelium, with subsequent release of inflammatory and immune factors which can lead to graft dysfunction. Viral attachment to the bronchial epithelium causes local damage at and around the point of viral attachment. The activation of cell surface receptors activates the innate immune system.13 The exact pathogenesis leading to AR and CAD is not fully identified. Many of the mediators of acute and chronic allograft rejection share common pathways with viral activation and epithelial cell injury. The antiviral cytokines involved in the immune response and CD8+ T lymphocytic activation are pro-inflammatory (Th1, tissue necrosis factor, and interferon families) and may induce fibrotic pathways leading to graft dysfunction.13 Other studies have reported that the functional and physiologic consequence of initial viral infection results in a decline in lung function.14,15
Our study showed that during or shortly after viral infection, the lung allograft response can lead to AR and CR in 35% and 9.4% of patients, respectively. One of the strengths of this study is that only cases of isolated hMPV identified by molecular diagnostics with short-term follow-up of events of rejection were analyzed further. The drawbacks of meta-analysis are common to most such studies, in that clinical information may not be uniformly presented between studies. By considering only isolated cases within a short period, this potential confounding factor was limited.
Respiratory viral infection affects the lung transplantation population more so than other solid organ transplantation recipients, since there is direct exposure of the lung graft to the contiguous upper airway, or portal of entry.
There were studies that were not identified by the search terms, since this study was focused on one virus, hMPV, specifically in the lung transplantation population. One such study of 343 bronchoscopic procedures among the lung transplantation population did not identify hMPV specifically. In this study, a temporal relationship was identified between acute respiratory symptoms and positive viral nucleic acid detection in the BAL fluid. It did not identify this temporal relationship between AR and acute phase of infection.16 Detection of severe hMPV infection among a variety of patients undergoing bronchoscopy identified two additional MPV-infected lung transplantation patients. One had co-infections with Streptococcus and Moraxella bacteria. This patient had no rejection but did demonstrate chronic interstitial inflammation. The other lung transplant patient had hMPV infection without co-infection. In this patient, there was no evidence of AR.17 While there may be additional literature regarding hMPV, this study focused on capturing those patients with isolated hMPV and temporally related allograft rejection.
In summary, this study of more than 1,007 patients and 2,883 viral samples identified that 17% of all respiratory viral infections are attributable to hMPV isolation. A significant portion of these patients developed AR or CR within less than 1 year of follow-up. Given the limited therapeutic options of ribavirin and pooled immunoglobulin to treat hMPV in lung transplantation patients, early diagnosis and recognition of the consequences remain important areas of future study. The present article highlights the importance of ordering hMPV molecular testing if allograft rejection is suspected.
Acknowledgment
The author thanks Lisa Nidu for her assistance.
Disclosure
The author reports no conflicts of interest in this work.
References
Principi N, Esposito S. Pediatric human metapneumovirus infection: epidemiology, prevention and therapy. J Clin Virol. 2014;59:141–147. | |
Ison M. Respiratory viral infections in transplant recipients. Antivir Ther. 2007;12:627–638. | |
Hahn A, Wang W, Jaggi P, et al. Human metapneumovirus infections area associated with severe morbidity in hospitalized children of all ages. Epidemiol Infect. 2013;141:2213–2223. | |
Hopkins P, McNeil K, Kermeen F, et al. Human metapneumovirus in lung transplant recipients and comparison to respiratory syncytial virus. Am J Respir Crit Care Med. 2008;178:876–881. | |
Gottlieb J, Schulz TF, Welte T, et al. Community acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation. 2009;87:1530–1537. | |
Kumar D, Erdman D, Keshavjee S, et al. Clinical impact of community acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5:2031–2036. | |
Weinberg A, Lyu DM, Li S, Marquesen J, Zamora MR. Incidence and morbidity of human matapneumovirus and other community acquired respiratory viruses in lung transplant recipients. Transpl Infect Dis. 2010;12:330–335. | |
Gerna G, Vitulo P, Rovida F, et al. Impact of human metapneumovirus and human cytomegalovirus versis other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol. 2006;78:408–416. | |
Shahda S, Carlos WG, Kiel PJ, Khan BA, Hage CA. The human meatpneumovirus: a case series and review of the literature. Transpl Infect Dis. 2011;13:324–328. | |
Raza K, Ismailjee SB, Crespo M, et al. Successful outcome of human metapneumovirus pneumonia in a lung transplant recipeint treated with intravenous ribavirin. J Heart Lung Transplant. 2007;26:862–864. | |
Dare R, Sanghavi S, Bullotta A, et al. Diagnosis of human metapneumovirus infection in immunosuppressed lung transplant recipients and children evaluated for pertussis. J Clin Microbiol. 2007;45:548–552. | |
Larcher C, Geltner C, Fischer H, et al. Human metapneumovrus infection in lung transplant recipients: clinical presentation and epidemiology. J Heart Lung Transplant. 2005;24:1891–1901. | |
Weight S, Gregson A, Deng J, Lynch JP 3rd, Belperio JA. Respiratory viral infections in hematopoietic stem cell and solid organ transplant recipients. Semin Respir Crit Care Med. 2011;32:471–492. | |
Sayah DM, Koff JL, Leard LE, Hays SR, Golden JA, Singer JP. Rhinovirus and other respiratory viruses exert different effects on lung allograft function that are not mediated through acute rejection. Clin Transplant. 2013;27:E64–E71. | |
Kumar D, Husain S, Chen MH, et al. A prospective molecular surveillance study evaluating the clinical impact of community acquired respiratory viruses in lung transplant recipients. Transplantation. 2010;89:1028–1033. | |
Soccal PM, Aubert JD, Bridevaux PO, et al. Upper and lower respiratory tract viral infections and acute graft rejection in lung transplant recipients. Clin Infect Dis. 2010;51:163–170. | |
Sumino KC, Agapov E, Pierce RA, et al. Detection of severe human metapneumoviru infection by real-time polymerase chain reaction and histopathological assessment. J Infect Dis. 2005;192:1052–1060. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.