Back to Journals » Journal of Inflammation Research » Volume 16
Resolvin D1 Attenuates Inflammation and Pelvic Pain Associated with EAP by Inhibiting Oxidative Stress and NLRP3 Inflammasome Activation via the Nrf2/HO-1 Pathway
Authors Zhang J, Chen J, Jiang Q, Feng R, Zhao X, Li H, Yang C, Hua X
Received 10 February 2023
Accepted for publication 17 July 2023
Published 8 August 2023 Volume 2023:16 Pages 3365—3379
DOI https://doi.org/10.2147/JIR.S408111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jiong Zhang,1,2,* Juan Chen,3,* Qing Jiang,1,* Rui Feng,2 Xiaohu Zhao,2 Haolin Li,2 Cheng Yang,2 Xiaoliang Hua1
1Department of Urology, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2Department of Urology, the First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 3The Ministry of Education Key Laboratory of Laboratory Medical Diagnostics, the College of Laboratory Medicine, Chongqing Medical University, Chongqing, 400016, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Cheng Yang, Department of Urology, the First Affiliated Hospital of Anhui Medical University, No. 218 Jixi Road, Shushan District, Hefei, 230022, People’s Republic of China, Tel +86 0551 6292 3440, Fax +86 551 6363 3742, Email [email protected] Xiaoliang Hua, Department of Urology, the Second Affiliated Hospital of Chongqing Medical University, No. 74 Linjiang Road, Yuzhong District, Chongqing, 400010, People’s Republic of China, Tel\Fax +86 023 6369 3222, Email [email protected]
Background: Resolvin D1 (RvD1), a member of the specialized pro-resolving lipid mediators family, has a potent anti-inflammatory effect and alleviates tissue damage. The purpose of the current research was to study the effect of RvD1 on CP/CPPS and the underlying mechanisms using a mouse model of experimental autoimmune prostatitis (EAP) mice.
Materials and Methods: The EAP mouse model was successfully established, and was used to test the therapeutic effect of RvD1. Hematoxylin-eosin staining and dihydroethidium staining were used to evaluate the histological changes and oxidative stress levels of prostate tissues. Chronic pelvic pain was assessed by applying von Frey filaments to the lower abdomen. The superoxide dismutase enzyme and malondialdehyde levels were detected using enzyme-linked immunosorbent assay (ELISA). The levels of inflammation-related cytokines, including IL-1β, IL-6, and TNF-α were detected by ELISA.
Results: RvD1 treatment ameliorated prostatic inflammation and the pelvic pain of EAP mice. RvD1 treatment could inhibit activation of the NLRP3 inflammasome and oxidative stress. RvD1 treatment could activate Nrf2/HO-1 signaling in mice with EAP. Blockade of Nrf2/HO-1 signaling abolished the RvD1-mediated inhibition of oxidative stress, NLRP3 inflammasome activation and the anti-inflammatory effect of RvD1 in EAP.
Conclusion: RvD1 treatment can reduce inflammatory cell infiltration in prostate tissue and attenuate pelvic pain associated with EAP by inhibiting oxidative stress and NLRP3 inflammasome activation via the Nrf2/HO-1 pathway. These results provide new insights that RvD1 has the potential as an effective agent in the treatment of EAP.
Keywords: chronic prostatitis and chronic pelvic pain syndrome, CP/CPPS, resolvin D1, inflammation, NLRP3 inflammasome, oxidative stress
Introduction
Chronic prostatitis, a very common disease of the genitourinary system, affected approximately 35–50% of men in their lifetime.1–3 Chronic prostatitis and chronic pelvic pain syndrome (CP/CPPS) is the most common type, accounting for more than 90% of all cases of chronic prostatitis among males under 50 years of age. CP/CPPS is a poorly understood syndrome characterized by perineal or pelvic pain, urinary irritation, psychological issues, and sexual dysfunction without infection, and it negatively affects sperm and quality of life.1–4 As the etiology of CP/CPPS is still not well understood, available therapeutic options including alpha blockers, anti-inflammatory drugs, physiotherapy, and neuroleptics are far from satisfactory to either patients or physicians.5,6 Therefore, a new treatment for CP/CPPS and a better understanding of its pathogenesis are urgently needed.
Recent studies have shown that the specialized pro-resolving lipid mediators deriving predominantly from ω-3 and ω-6 essential fatty acids, play a vital role in the chronic inflammatory diseases.7 Resolvin D1 (RvD1) is a specialized pro-resolving lipid mediator that is derived predominantly from docosahexaenoic acid. The potent effects of RvD1 on chronic inflammatory disease have attracted attention in recent years. The anti-inflammatory effects of RvD1 have been verified in multiple inflammatory diseases, including non-alcoholic steatohepatitis,8 cholestatic liver fibrosis,9 and rheumatoid arthritis.10 In addition, RvD1 has an inhibitory effect on oxidative stress and apoptosis, further reducing tissue damage.11,12 RvD1, as an endogenous metabolite, is expected to be safe and has great potential for clinical applications. However, the effects of RvD1 on the pathogenesis and development of CP/CPPS have not yet been elucidated.
NOD-like receptor protein 3 (NLRP3), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and caspase-1 comprise the NLRP3 inflammasome complex.13 The formation of the NLRP3 inflammasome complex triggers the cleavage of procaspase-1 into active caspase-1, which converts the cytokine precursors pro-IL-1β and pro-IL-18 into mature pro-inflammatory cytokines IL-1β and IL-18, causing inflammation and tissue damage.14 Recent studies have indicated that NLRP3 inflammasome activation exacerbates the inflammatory response of prostate tissue and pelvic pain in EAP.15,16 Therefore, strategies that inhibiting the activation of NLRP3 inflammasomes may represent a novel treatment option for patients suffering from CP/CPPS. Oxidative stress, namely imbalance in peroxide and antioxidant, is one of the pathogenesis of CP/CPPS.17 In CP/CPPS patients, high levels of oxidative stress can contribute to an inflammatory response, resulting in a variety of adverse effects, including infertility and erectile dysfunction.18,19 According to hypothesis, antioxidant therapy can be effective in treating patients with CP/CPPS.20
In present study, we explored the therapeutic effects of RvD1 on CP/CPPS on the basis of EAP mouse model. After RvD1 treatment, the histological appearance of prostate tissues was measured, the degree of pelvic pain was assessed, and the expression levels of inflammatory cytokines in plasma were detected. In addition, the potential mechanism of RvD1 inhibiting inflammatory response was systematically explored.
Materials and Methods
Animals
The NOD/LtJ nonobese diabetic (NOD) mice used in this study were 6 and 8 weeks and were purchased from the Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Adult male Sprague–Dawley rats (250–350 g body weight) were purchased from the Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). The NOD mice and Sprague–Dawley rats were housed in the Animal Center of Anhui Medical University under pathogen-free conditions, free access to water and food, and switched between light and dark cycles every 12 hours. All the animal protocols were approved by the Committee for Animal Care and Use of the Animal Center of Anhui Medical University, in accordance with the Chinese Guideline of Welfare and Ethics for Laboratory Animals, and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (LLSC20221130).
Reagents
According to the manufacturer, the reagents and antibodies used in this experiment are listed below: Complete Freund’s adjuvant (CFA), zinc protoporphyrin (ZnPP), and reactive oxygen species (ROS) staining solution were purchased from Sigma–Aldrich (St. Louis, MO, USA). RvD1 was purchased from Cayman Chemical (BIOMOL GmbH, Hamburg, Germany). ML385 was obtained from Selleck Chemicals (Houston, TX). The products of oxidative stress test kits, including the MDA and SOD test kits, were purchased from Solarbio (Beijing, China). The enzyme-linked immunosorbent assay (ELISA) kits to measure the levels of TNF-α (CSB-E04741m), IL-6 (CSB-E04639m) and IL-1β (CSB-E08054m) were purchased from CUSABIO (Wuhan, China). The primary antibodies used were anti-NLRP3 (DF7438; Affinity Biosciences), anti-Caspase-1 (22915-1-AP; ProteinTech), anti-IL-1β (AF5103; Affinity Biosciences), anti-Nrf2 (#12721; CST), anti-HO-1 (10701-1-AP; ProteinTech), and anti-GAPDH (#5174; CST). The secondary antibodies used was HRP-conjugated goat anti-rabbit (SA00001-2; ProteinTech).
Animal Treatment
The EAP mouse model was well established according to a previously modeling method.21 Briefly, adult male Sprague–Dawley rats were euthanized to obtain prostate tissue for tissue homogenization, and the supernatants were collected as prostate antigens (PAgs). We emulsified equal volumes of PAgs or phosphate buffer solution (PBS) thoroughly in CFA. The mice in EAP group and control group were subcutaneously injected with PAgs (300 μg/mouse) and PBS emulsified in CFA, respectively. We injected 150 μL total volume per mouse into different areas (bilateral hind footpads, lower back, and tail base). Twice antigen immunizations were used to establish the EAP mouse model; that is, the mice were immunized on Days 0 and 28 and then sacrificed on Day 42. RvD1 powder was dissolved in 0.9% saline. ML385 powder was dissolved in 100% DMSO to make a stock solution, diluted into a usable solution with a mixture (5% DMSO, 40% PEG300, 5% Tween 80, and 50% sterile water). In the dark, ZnPP powder was dissolved in 100% DMSO to make a stock solution, diluted into a usable solution with a mixture (5% DMSO, 40% PEG300, 5% Tween 80, and 50% sterile water). The experiment was carried out in two batches. In the first batch, the mice were randomly assigned to five groups: control, EAP, EAP treated with low-dose RvD1 (2.5 μg/kg), EAP treated with middle-dose RvD1 (5 μg/kg), and EAP treated with high-dose RvD1 (10 μg/kg). Each group included 6 mice. Treatment with RvD1 was initiated two days prior to the second immunization. In the second batch, the mice were randomly assigned to five groups: control, EAP, EAP treated with high-dose RvD1 (10 μg/kg), EAP treated with high-dose RvD1 (10 μg/kg) and ML385 (30 mg/kg), EAP treated with high-dose RvD1 (10 μg/kg) and ZnPP (5 mg/kg). Each group included 6 mice. Treatment with ML385 and ZnPP was initiated four days prior to the second immunization, and treatment with RvD1 was initiated two days prior to the second immunization. One hour before administration of RvD1, mice received intraperitoneal injections of ML385 or ZnPP, and mice in the control group were given vehicle instead.22
Behavioral Testing
Mice in different groups were evaluated for allodynia in the lower abdominal area near the prostate on Day 42 after immunization. As previously described,21 Von Frey force filaments were used for the tests, which were conducted in plastic chambers equipped with a wire grid floor in which the surface was isolated from the ambient environment. It took 30 minutes for the mice to acclimate to the new environment before the test began. Afterward, each mouse was tested for tactile allodynia and hyperalgesia using von Frey filaments of 0.04, 0.16, 0.4, 1.0, and 4.0 g. Each filament was applied for 1–2 s with an inter-stimulus interval of 5 s for a total of 20 times. In order to avoid “wind-up” effects, different areas within the region were stimulated. As a result, three types of responses were affirmed as positive responses to filament stimulation: (1) sharp retraction of the abdomen; (2) immediate licking or scratching of the filament stimulation area; and (3) jumping. The results are presented as the percentage of positive responses.
Histological Evaluation
During the experiment, prostate tissues were harvested from mice, fixed in paraformaldehyde for 24 hours, then dehydrated with alcohol and xylene before being embedded in paraffin wax and cut into 4 μm thick sections. The prostate tissues were stained with hematoxylin and eosin (H&E) to assess inflammation. Following the methods described in previous studies,23 the degree of inflammation was quantified on a four-point scale from 0 to 3. 0, no inflammation; 1, mild but definite perivascular cuffing with mononuclear cells; 2, moderate perivascular cuffing with mononuclear cells; 3, marked perivascular cuffing, hemorrhage, and numerous mononuclear cells in the parenchyma.
ROS Production Assay
The ROS levels in prostate tissues were evaluated using dihydroethidium (DHE) staining. Prostate tissue samples were stored at −80°C and frozen into sections with a thickness of 5 μm. Then, each frozen section was put into DHE (10 μM) at room temperature for one hour in the dark, and washed three times with PBS. In the dark, the nuclei were stained with DAPI solution for 10 minutes at room temperature. Images were captured using a confocal laser-scanning microscope (Olympus Fluoview FV1000, Tokyo, Japan), and the mean fluorescence intensity was quantitative analysed by Image J software (National Institutes of Health, Bethesda, MD).
Estimation of Oxidative Stress
The superoxide dismutase enzyme (SOD) is a marker of scavenging capacity for free radicals. The malondialdehyde (MDA) is an indicator of severe membrane damage. MDA can be used as an indicator of oxidative stress and free radical levels as well.24 The blood of NOD mice was obtained by eyeball blood collection and serum was obtained by centrifugation at 2000 rpm/min at 4°C for 15 minutes after blood coagulation. According to the manufacturer’s instructions, we measured the levels of SOD and MDA in mouse serum using commercial kits. The concentrations were calculated according to the manufacturer’s instructions and normalized to the total protein concentration.
Western Blotting Assay
After the mice were sacrificed, the prostate tissues of mice were harvested, flash frozen in liquid nitrogen, and stored at −80°C. To extract total protein from prostate tissues, prostate tissues were homogenized and dissolved in radio-immunoprecipitation assay (RIPA) protein lysis buffer (Beyotime Biotech, Jiangsu, China) containing protease and phosphatase inhibitors and PMSF for 20 minutes, and centrifuged at 12,000 rpm/min at 4°C for 15 minutes to collect the supernatant. Total protein samples from prostate tissues were denatured in boiling water with SDS–PAGE sample loading buffer. The denatured samples were electrophoresed in sodium dodecyl sulfate-polyacrylamide gels, followed by transfer to nitrocellulose membranes using semi dry transfer methods. Defatted milk (5% (w/v)) was used to block nonspecific binding sites. After that, the membranes were incubated with primary antibodies overnight at 4 °C. After three washes with TBST solution, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 2 hours. The membranes were visualized using an EZ‐ECL Kit (Biological Industries, Israel) on a ChemiScope 5600 (Clinx Science Instruments, China). For proteins of similar molecular weight, stripping buffer (Beyotime Biotech, Jiangsu, China) was used according to the manufacturer’s instructions. Three prostate tissues in each group were randomly selected for Western Blotting assay. The density of the bands was quantified using Image J software (National Institutes of Health, Bethesda, MD), and the data was normalized by GAPDH expression.
ELISA
Cytokine levels in mouse serum were measured using ELISA kits for IL-1β (CSB-E08054m, Cusabio, Wuhan, China), IL-6 (CSB-E04639m, Cusabio, Wuhan, China) and TNF-α (CSB-E04741m, Cusabio, Wuhan, China). All the procedures were performed according to the manufacturer’s instructions.
Data and Statistical Analysis
The statistical analysis was done by a one-way ANOVA analysis as a comparison among multiple groups, a Kruskal–Wallis non-parametric test for a comparison of ranked data among multiple groups, or a two-way ANOVA analysis for a comparison of two-factor experimental data using GraphPad Prism version 6.0 software. The results are expressed as the mean ± standard deviation (SD). P < 0.05 was considered statistically significant. In the figures, “ns” indicates P > 0.05; * indicates P < 0.05; ** indicates P < 0.01; *** indicates P < 0.001; **** indicates P < 0.0001.
Results
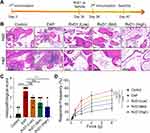
RvD1 Treatment Reduces Prostatic Inflammation and Pelvic Pain in Mice with EAP
The EAP mouse model was successfully induced, and the therapeutic effects of RvD1 on EAP mice were evaluated. The detailed RvD1 treatment procedure is shown in Figure 1A. H&E staining indicated that RvD1 treatment reduced the extent and number of intraprostatic inflammatory cell infiltration in EAP mice (Figure 1B). The histopathological inflammation scores were calculated. Results showed that high-dose RvD1 treatment significantly decreased inflammation score in EAP mice (Figure 1C). In CP/CPPS patients, pain in the perineum, rectum, or prostate is one of the major clinical symptoms, which has a serious negative impact on the quality of life.25 Therefore, the impact of RvD1 on pain symptoms was evaluated. Compared with the EAP group, the response frequencies to tactile allodynia in the RvD1 treatment group were significantly decreased (Figure 1D). These results suggested that RvD1 treatment reduced the pelvic pain associated with the inflammatory response of mice with EAP.
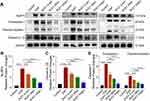
RvD1 Treatment Inhibits Activation of the NLRP3 Inflammasome
Emerging evidences suggest that NLRP3 inflammasome activation plays an important role in the pathogenesis and development of CP/CPPS, and drugs inhibiting NLRP3 inflammasome activation may be novel therapeutic options.15–17 Hence, we investigated whether RvD1 treatment could inhibit NLRP3 inflammasome activation. After treating EAP mice with different doses of RvD1, the expression levels of NLRP3, pro-caspase-1, cleaved-caspase-1, and cleaved-IL-1β were measured using Western blotting assay (Figure 2A). Results showed that NLRP3 inflammasome‐associated proteins, including NLRP3 (Figure 2B), cleaved-IL-1β (Figure 2C), pro-caspase-1, and cleaved-caspase-1 (Figure 2D) increased in the EAP group, and RvD1 treatment reduced the expression levels of NLRP3 (Figure 2B), cleaved-IL-1β (Figure 2C), pro-caspase-1, and cleaved-caspase-1 (Figure 2D). These results suggested that RvD1 treatment inhibits the activation of the NLRP3 inflammasome.
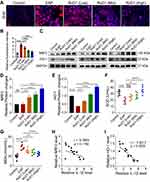
RvD1 Inhibits Oxidative Stress and Activates Nrf2/HO-1 Signaling in Mice with EAP
Previous studies have shown CP/CPPS patients are characterized by oxidative stress at the local and systemic levels, which plays an important role in the pathogenesis of CP/CPPS.26 Therefore, we evaluated the production of ROS using DHE staining. DHE staining indicated that the ROS production increased in EAP group, and RvD1 treatment effectively reduced ROS production (Figure 3A and B). Nrf2, a key factor regulating oxidative stress, regulates the expression of multiple antioxidants, including HO-1, to reduce ROS production.27 Hence, we measured the expression of Nrf2 and HO-1 using Western blotting assays (Figure 3C). We found that the expression levels of Nrf2 and HO-1 were increased in the RvD1 treatment group (Figure 3D and E). SOD, a marker of antioxidant capacity, converts superoxide radicals into harmless hydrogen peroxide and oxygen molecules. The SOD level in serum was dramatically reduced in mice with EAP, while RvD1 markedly increased the SOD level (Figure 3F). MDA measurement provides an assessment of the severity and extent of membrane damage. The MDA level in serum was increased in mice with EAP, while RvD1 markedly reduced the MDA level (Figure 3G). These results suggested that RvD1 inhibits oxidative stress and activates Nrf2/HO-1 signaling in mice with EAP. The correlation between Nrf2/HO-1 signaling and cleaved-IL-1β was evaluated based on the results of Western blotting assay. A negative correlation between the Nrf2 and IL-1β levels was observed in the prostate tissues (Figure 3H). A significantly negative correlation between the HO-1 and IL-1β levels was observed in the prostate tissues (Figure 3I). Therefore, we hypothesized that RvD1 treatment might inhibit NLRP3 inflammasome activation through activating Nrf2/HO-1 signaling pathway, and then alleviate inflammation in mice with EAP.
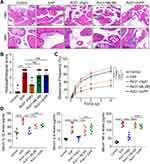
Blockade of Nrf2/HO-1 Signaling Abolished the RvD1-Mediated Inhibition of Oxidative Stress
To further determine whether the Nrf2/HO-1 signaling contributed to the inhibitory effect of RvD1 on oxidative stress, we intraperitoneally administered an inhibitor of Nrf2 ML385 (30 mg/kg) and an inhibitor of HO-1 ZnPP (5 mg/kg) to mice one hour before RvD1 treatment, inhibiting the activity of Nrf2 and HO-1, respectively.22 The mice in the control group were treated with vehicle. The detailed procedure for RvD1, ML385 or ZnPP treatment is shown in Figure 4A. DHE staining showed that the inhibition of ROS production by RvD1 was effectively antagonized by pretreatment with ML385 and ZnPP (Figure 4B and C). Western blotting assays showed that RvD1-mediated Nrf2 transcriptional activity was successfully blocked by ML385 (Figure 4D and E). ZnPP can effectively inhibit HO-1 expression, and the effect of RvD1 in activating HO-1 was offset by pretreatment with ZnPP (Figure 4D and F). We found that the serum SOD levels in EAP mice were reduced compared with control mice. RvD1 treatment increased the serum SOD levels, and pretreated with ML385 or ZnPP effectively antagonized the RvD1-mediated activation on SOD levels (Figure 4G). The serum MDA levels in EAP mice were increased compared with control mice; RvD1 treatment reduced the serum MDA levels, and pretreated with ML385 or ZnPP effectively antagonized the RvD1-mediated inhibition on MDA levels (Figure 4H). These results suggested that RvD1 inhibits oxidative stress via the Nrf2/HO-1 pathway, and that inhibiting the Nrf2/HO-1 pathway blocked the inhibitory effect of RvD1 on oxidative stress.
Blockade of Nrf2/HO-1 Signaling Abolished the RvD1-Mediated Inhibition of Inflammasome Activation
After pretreatment with ML385 or ZnPP, we measured the protein levels of NLRP3, and caspase-1 in the prostate tissues of mice with EAP to determine whether the Nrf2/HO-1 pathway is involved in the RvD1-mediated inhibitory effect on inflammasome activation. The results of Western blotting assays were showed in Figure 5A. We found that RvD1 treatment could effectively inhibit the expressions of NLRP3 inflammasome‐associated proteins, and the inhibitory effect of RvD1 was offset by pretreatment with ML385 or ZnPP. The expression levels of NLRP3 (Figure 5B), cleaved-caspase-1 (Figure 5C), pro-caspase-1, and cleaved-caspase-1 (Figure 5D) increased in the mice pretreated with ML385 or ZnPP compared with those treated with RvD1. The results suggest that RvD1 inhibits inflammasome activation by activating the Nrf2/HO-1 pathway and that inhibiting the Nrf2/HO-1 pathway reverses this inhibitory effect of RvD1 on inflammasome activation.
Blockade of Nrf2/HO-1 Signaling Abolished the Anti-Inflammatory Effect of RvD1 in EAP
We also determined whether ML385 and ZnPP could block RvD1’s anti-inflammatory effect on mice with EAP. H&E staining indicated that RvD1 treatment reduced the numbers of infiltrating inflammatory cells in the prostates of mice with EAP, and pretreatment with ML385 or ZnPP in EAP mice prior to RvD1 treatment increased the numbers of infiltrating inflammatory cells in the prostate (Figure 6A). The histopathological scores showed no significantly difference in mice pretreated with ML385 or ZnPP compared with EAP mice (Figure 6B). The response frequencies to tactile allodynia were also increased in mice pretreated with ML385 or ZnPP compared with EAP mice treated with RvD1 (Figure 6C). ELISA results showed that the serum levels of the inflammatory cytokines IL-1β, IL-6, and TNF-α were significantly increased in the EAP group compared with the control group. Treatment with RvD1 decreased the serum levels of the inflammatory cytokines IL-1β, IL-6, and TNF-α. Pretreating EAP mice with ML385 or ZnPP prior to RvD1 treatment increased the serum levels of IL-1β, IL-6, and TNF-α (Figure 6D). According to these findings, RvD1 inhibited the prostatic inflammatory response by activating the Nrf2/HO-1 pathway, and that inhibiting the Nrf2/HO-1 pathway blocked the anti-inflammatory effect of RvD1 on EAP mice.
Discussion
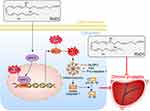
This is the first study to investigate the therapeutic effect of RvD1 on CP/CPPS in an EAP mouse model. RvD1 treatment could attenuate inflammation and pelvic pain associated with EAP. RvD1 treatment can inhibit oxidative stress and the NLRP3 inflammasome activation to reduce the production of ROS as well as pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. Mechanistic studies have shown that Nrf2/HO-1 signaling mediates the inhibitory effect of RvD1 on oxidative stress and the NLRP3 inflammasome activation (Figure 7). Blockade of Nrf2/HO-1 signaling offset the corresponding anti-inflammatory effects of RvD1 on EAP mice.
CP/CPPS, a common condition of urinary system, can lead to recurrent pain in the perineum, decrease in sperm quality, and has a considerable negative impact on quality of life.28 The pathological feature of CP/CPPS is characterized by the infiltration of inflammatory cells.29,30 Previous studies found that the levels of inflammatory cytokines IL-1β, IL-6, and TNF-α are increased in CP/CPPS patients and EAP model.31,32 In addition, there is a correlation between histological inflammation of prostate and symptomatic progression of CP/CPPS.33 Therefore, neutralizing or reducing the levels of inflammatory cytokines, including IL-1β, IL-6, and TNF-α, may be an effective therapeutic strategy for CP/CPPS. In this study, an EAP mouse model that mimics the inflammatory changes and pelvic pain of CP/ CPPS patients was successfully established by subcutaneous injection of a mixture of PAgs and CFA, and was used to test the therapeutic effect of RvD1. RvD1, a lipid mediator derived from docosahexaenoic acid, possesses anti-inflammatory and antioxidant properties. However, the effects of RvD1 on EAP remain uncertain. In this study, we found that RvD1 treatment alleviated the degree of inflammation in prostate tissues, ameliorated pelvic pain, and decreased pro-inflammatory cytokines expression, showing good therapeutic potential in EAP.
The activation of NLRP3 inflammasome is the key to the initiation and progression of inflammatory response in a variety of inflammatory diseases. Once NLRP3 inflammasome activated, it can recruit the adapter ASC, leading to caspase-1 activation; activated caspase-1 converts the precursor forms of IL-1β and IL-18 into mature pro-inflammatory cytokines.13 Current therapies that inhibit NLRP3 inflammasome activation have been developed to prevent or treat diseases.34,35 The activation of NLRP3 inflammasome has been implicated in the development of EAP, and blocking NLRP3 inflammasome activation with MCC950 ameliorates inflammation and pelvic pain associated with EAP.15 Therefore, other compounds targeting NLRP3 inflammasome activation may be a novel strategy for the treatment of CP/CPPS. Recent studies showed that RvD1 can inhibit NLRP3 inflammasome activation, and shows potent anti-inflammatory effects in diseases, including glomerular injury,36 blood-brain barrier disruption after subarachnoid hemorrhage37 and atrial fibrillation.38 In present study, we found that the expression of NLRP3 inflammasome‐associated proteins increased, suggesting that the NLRP3 inflammasome was activated in EAP mice. RvD1 treatment effectively inhibited the activation of the NLRP3 inflammasome.
Studies have shown a direct correlation between CP/CPPS progression and oxidative stress.26 Cells are protected against potential cytotoxicity due to the critical balance between production and degradation of ROS. When ROS is imbalanced, it interacts with lipids, proteins, carbohydrates, and nucleic acids interfering with cell signaling and homeostasis.39,40 As ROS concentration increases, pathological changes occur in proteins, lipids, and DNA within the cells.41 Excessive oxidative stress, under certain conditions, such as prostatitis, can cause cellular death and damage to the extracellular matrix, which can lead to tissue damage and fibrosis.42 There is evidence that free radicals/oxidative species such as inducible nitric oxide, reactive nitric species, and ROS are released when prostate tissue is exposed continuously to inflammation.43 These active substances can lead to significant changes in protein structure and function, as well as DNA changes.44 In addition, antioxidant compounds may activate nuclear factor Nrf2 to increase endogenous cellular antioxidant defenses, as well as directly act against free radicals directly.45,46 Therefore, treatment strategies to increase antioxidant capacity and alleviate oxidative stress might be promising options for CP/CPPS patients. In this study, we found that RvD1 treatment could inhibit oxidative stress, increase antioxidant capacity, and reduce oxidative damage in prostate tissues in mice with EAP.
ROS is a small highly reactive molecule, and an excessive production of ROS can cause the balance between the anti-oxidative and pro-oxidative systems to be upset.47 Nrf2 is a key transcription factor that regulates the expression of a series of antioxidant enzymes to scavenge endogenous ROS.48 Specifically, the elevated production of ROS promotes the nuclear translocation of Nrf2, binding to antioxidant response element (ARE) fragments in DNA to enhance the transcription of antioxidant enzymes, thus mitigating oxidative stress-related damage. Therapeutic strategies to activate Nrf2 may be an important way to alleviate inflammation and reduce oxidative stress-related damage. Zhao et al showed that lycopene attenuates oxidative stress and inflammation associated with CP/CPPS by activating Nrf2 signaling pathways.49 Lin et al showed that strategies targeting ferroptosis attenuates prostatitis by activating Nrf2/HO-1 signaling.50 HO-1, an important antioxidant controlled by Nrf2, can catalyze the heme reaction to produce metabolite carbon monoxide, having anti-inflammatory, anti-apoptotic, and diastolic blood vessels and other tissue protection effects. In this study, RvD1 treatment increased the expression of the antioxidant enzymes HO-1 and SOD, inhibited the activation of NLRP3 inflammasome, attenuated inflammation and pelvic pain associated with EAP. We also determine that the inhibition of NLRP3 inflammasome activation by RvD1 was due to Nrf2-mediated ROS clearance. Blockade of Nrf2/HO-1 signaling abolished oxidative stress, NLRP3 inflammasome activation, and anti-inflammatory effects in RvD1-treated mice. These results suggested that RvD1 may exert its protective effects via the Nrf2/HO-1 signaling in mice with EAP.
Conclusion
In summary, our study first demonstrated a therapeutic effect of RvD1 in EAP mice. RvD1 treatment could attenuate prostatic inflammation and pelvic pain by inhibiting oxidative stress and NLRP3 inflammasome activation via activating Nrf2/HO-1 signaling in the prostate tissue of EAP. As an endogenous anti-inflammatory substance, RvD1 may serve as a promising pharmaceutical preparation for treating CP/CPPS.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Approval and Informed Consent
All animal procedures were approved by the Committee for Animal Care and Use of the Animal Center of Anhui Medical University, in accordance with the Chinese Guideline of Welfare and Ethics for Laboratory Animals, and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (LLSC20221130).
Consent to Publication
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. All authors agree to the publication of this work.
Funding
This study was funded by the Research Fund of Anhui Institute of Translational Medicine (No. ZHYX2020A003).
Disclosure
The authors declare no competing interests.
References
1. Collins MM, Stafford RS, O’Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol. 1998;159(4):1224–1228. doi:10.1097/00005392-199804000-00037
2. Schwartz ES, Xie A, La JH, Gebhart GF. Nociceptive and inflammatory mediator upregulation in a mouse model of chronic prostatitis. Pain. 2015;156(8):1537–1544. doi:10.1097/j.pain.0000000000000201
3. Rees J, Abrahams M, Doble A, Cooper A; Prostatitis Expert Reference Group (PERG). Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: a consensus guideline. BJU Int. 2015;116(4):509–525. doi:10.1111/bju.13101
4. Schaeffer AJ. Clinical practice. Chronic prostatitis and the chronic pelvic pain syndrome. N Engl J Med. 2006;355(16):1690–1698. doi:10.1056/NEJMcp060423
5. Magistro G, Wagenlehner FM, Grabe M, Weidner W, Stief CG, Nickel JC. Contemporary Management of Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Eur Urol. 2016;69(2):286–297. doi:10.1016/j.eururo.2015.08.061
6. Polackwich AS, Shoskes DA. Chronic prostatitis/chronic pelvic pain syndrome: a review of evaluation and therapy. Prostate Cancer Prostatic Dis. 2016;19(2):132–138. doi:10.1038/pcan.2016.8
7. Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi:10.1038/nature13479
8. Li J, Deng X, Bai T, Wang S, Jiang Q, Xu K. Resolvin D1 mitigates non-alcoholic steatohepatitis by suppressing the TLR4-MyD88-mediated NF-κB and MAPK pathways and activating the Nrf2 pathway in mice. Int Immunopharmacol. 2020;88:106961. doi:10.1016/j.intimp.2020.106961
9. Abshagen K, Hartmann A, Grüner L, Liebig M, Vollmar B. Limited potential of resolvin D1 in treatment of cholestatic liver fibrosis. Hepatobiliary Surg Nutr. 2020;9(5):587–596. doi:10.21037/hbsn.2019.08.07
10. Sun W, Ma J, Zhao H, et al. Resolvin D1 suppresses pannus formation via decreasing connective tissue growth factor caused by upregulation of miRNA-146a-5p in rheumatoid arthritis. Arthritis Res Ther. 2020;22(1):61. doi:10.1186/s13075-020-2133-2
11. Lee HN, Surh YJ. Resolvin D1-mediated NOX2 inactivation rescues macrophages undertaking efferocytosis from oxidative stress-induced apoptosis. Biochem Pharmacol. 2013;86(6):759–769. doi:10.1016/j.bcp.2013.07.002
12. Saito P, Melo CPB, Martinez RM, et al. The Lipid Mediator Resolvin D1 Reduces the Skin Inflammation and Oxidative Stress Induced by UV Irradiation in Hairless Mice. Front Pharmacol. 2018;9:1242. doi:10.3389/fphar.2018.01242
13. o EK J, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13(2):148–159. doi:10.1038/cmi.2015.95
14. Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. doi:10.1038/nature10759
15. Zhang LG, Chen J, Meng JL, et al. Effect of alcohol on chronic pelvic pain and prostatic inflammation in a mouse model of experimental autoimmune prostatitis. Prostate. 2019;79(12):1439–1449. doi:10.1002/pros.23866
16. Zang L, Tian F, Yao Y, et al. Qianliexin capsule exerts anti-inflammatory activity in chronic non-bacterial prostatitis and benign prostatic hyperplasia via NF-κB and inflammasome. J Cell Mol Med. 2021;25(12):5753–5768. doi:10.1111/jcmm.16599
17. Chen H, Xie Y, Deng C, et al. The Anti-Inflammatory and Antioxidative Effects of Ningmitai Capsule in the Experimental Autoimmune Prostatitis Rat Model. Evid Based Complement Alternat Med. 2020;2020:5847806. doi:10.1155/2020/5847806
18. Hu Y, Niu X, Wang G, Huang J, Liu M, Peng B. Chronic prostatitis/chronic pelvic pain syndrome impairs erectile function through increased endothelial dysfunction, oxidative stress, apoptosis, and corporal fibrosis in a rat model. Andrology. 2016;4(6):1209–1216. doi:10.1111/andr.12273
19. Pasqualotto FF, Sharma RK, Potts JM, Nelson DR, Thomas AJ, Agarwal A. Seminal oxidative stress in patients with chronic prostatitis. Urology. 2000;55(6):881–885. doi:10.1016/s0090-4295(99)00613-5
20. Shahed AR, Shoskes DA. Oxidative stress in prostatic fluid of patients with chronic pelvic pain syndrome: correlation with gram positive bacterial growth and treatment response. J Androl. 2000;21(5):669–675. doi:10.1002/j.1939-4620.2000.tb02135
21. Rudick CN, Schaeffer AJ, Thumbikat P. Experimental autoimmune prostatitis induces chronic pelvic pain. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1268–1275. doi:10.1152/ajpregu.00836.2007
22. Liu X, Zhu Q, Zhang M, et al. Isoliquiritigenin Ameliorates Acute Pancreatitis in Mice via Inhibition of Oxidative Stress and Modulation of the Nrf2/HO-1 Pathway. Oxid Med Cell Longev. 2018;2018:7161592. doi:10.1155/2018/7161592
23. Breser ML, Motrich RD, Sanchez LR, Rivero VE. Chronic Pelvic Pain Development and Prostate Inflammation in Strains of Mice With Different Susceptibility to Experimental Autoimmune Prostatitis. Prostate. 2017;77(1):94–104. doi:10.1002/pros.23252
24. Wei LF, Zhang HM, Wang SS, et al. Changes of MDA and SOD in Brain Tissue after Secondary Brain Injury with Seawater Immersion in Rats. Turk Neurosurg. 2016;26(3):384. doi:10.5137/1019-5149.JTN.8265-13.1
25. Vral A, Magri V, Montanari E, et al. Topographic and quantitative relationship between prostate inflammation, proliferative inflammatory atrophy and low-grade prostate intraepithelial neoplasia: a biopsy study in chronic prostatitis patients. Int J Oncol. 2012;41(6):1950–1958. doi:10.3892/ijo.2012.1646
26. Kullisaar T, Türk S, Punab M, Mändar R. Oxidative stress--cause or consequence of male genital tract disorders? Prostate. 2012;72(9):977–983. doi:10.1002/pros.21502
27. Hyeon S, Lee H, Yang Y, Jeong W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic Biol Med. 2013;65:789–799. doi:10.1016/j.freeradbiomed.2013.08.005
28. Condorelli RA, Russo GI, Calogero AE, Morgia G, La Vignera S. Chronic prostatitis and its detrimental impact on sperm parameters: a systematic review and meta-analysis. J Endocrinol Invest. 2017;40(11):1209–1218. doi:10.1007/s40618-017-0684-0
29. Theyer G, Kramer G, Assmann I, et al. Phenotypic characterization of infiltrating leukocytes in benign prostatic hyperplasia. Lab Invest. 1992;66(1):96–107.
30. Penna G, Amuchastegui S, Cossetti C, et al. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006;177(12):8504–8511. doi:10.4049/jimmunol.177.12.8504
31. John H, Barghorn A, Funke G, et al. Noninflammatory chronic pelvic pain syndrome: immunological study in blood, ejaculate and prostate tissue. Eur Urol. 2001;39(1):72–78. doi:10.1159/000052415
32. Motrich RD, Breser ML, Molina RI, Tissera A, Olmedo JJ, Rivero VE. Patients with chronic prostatitis/chronic pelvic pain syndrome show T helper type 1 (Th1) and Th17 self-reactive immune responses specific to prostate and seminal antigens and diminished semen quality. BJU Int. 2020;126(3):379–387. doi:10.1111/bju.15117
33. Nickel JC, Freedland SJ, Castro-Santamaria R, Moreira DM. Chronic Prostate Inflammation Predicts Symptom Progression in Patients with Chronic Prostatitis/Chronic Pelvic Pain. J Urol. 2017;198(1):122–128. doi:10.1016/j.juro.2017.01.035
34. Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res. 2015;8:15–27. doi:10.2147/JIR.S51250
35. Jiang H, He H, Chen Y, et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med. 2017;214(11):3219–3238. doi:10.1084/jem.20171419
36. Li G, Chen Z, Bhat OM, et al. NLRP3 inflammasome as a novel target for docosahexaenoic acid metabolites to abrogate glomerular injury. J Lipid Res. 2017;58(6):1080–1090. doi:10.1194/jlr.M072587
37. Wei C, Guo S, Liu W, et al. Resolvin D1 ameliorates Inflammation-Mediated Blood-Brain Barrier Disruption After Subarachnoid Hemorrhage in rats by Modulating A20 and NLRP3 Inflammasome. Front Pharmacol. 2021;11:610734. doi:10.3389/fphar.2020.610734
38. Yarmohammadi F, Hayes AW, Karimi G. Possible protective effect of resolvin D1 on inflammation in atrial fibrillation: involvement of ER stress mediated the NLRP3 inflammasome pathway. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(8):1613–1619. doi:10.1007/s00210-021-02115-0
39. Brieger K, Schiavone S, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142:w13659. doi:10.4414/smw.2012.13659
40. Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12(4):274–277. doi:10.1016/s0899-9007(96)00000-8
41. Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem. 2014;395(2):203–230. doi:10.1515/hsz-2013-0241
42. Kennett EC, Chuang CY, Degendorfer G, Whitelock JM, Davies MJ. Mechanisms and consequences of oxidative damage to extracellular matrix. Biochem Soc Trans. 2011;39(5):1279–1287. doi:10.1042/BST0391279
43. Hamid AR, Umbas R, Mochtar CA. Recent role of inflammation in prostate diseases: chemoprevention development opportunity. Acta Med Indones. 2011;43(1):59–65.
44. Baltaci S, Orhan D, Gögüs C, Türkölmez K, Tulunay O, Gögüs O. Inducible nitric oxide synthase expression in benign prostatic hyperplasia, low- and high-grade prostatic intraepithelial neoplasia and prostatic carcinoma. BJU Int. 2001;88(1):100–103. doi:10.1046/j.1464-410x.2001.02231.x
45. Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;15(11):7792–7814. doi:10.3390/molecules15117792
46. Kang HJ, Hong YB, Kim HJ, Wang A, Bae I. Bioactive food components prevent carcinogenic stress via Nrf2 activation in BRCA1 deficient breast epithelial cells. Toxicol Lett. 2012;209(2):154–160. doi:10.1016/j.toxlet.2011.12.002
47. Bao L, Li J, Zha D, et al. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int Immunopharmacol. 2018;54:245–253. doi:10.1016/j.intimp.2017.11.021
48. Chen H, Xie K, Han H, et al. Molecular hydrogen protects mice against polymicrobial sepsis by ameliorating endothelial dysfunction via an Nrf2/HO-1 signaling pathway. Int Immunopharmacol. 2015;28(1):643–654. doi:10.1016/j.intimp.2015.07.034
49. Zhao Q, Yang F, Meng L, et al. Lycopene attenuates chronic prostatitis/chronic pelvic pain syndrome by inhibiting oxidative stress and inflammation via the interaction of NF-κB, MAPKs, and Nrf2 signaling pathways in rats. Andrology. 2020;8(3):747–755. doi:10.1111/andr.12747
50. Lin D, Zhang M, Luo C, Wei P, Cui K, Chen Z. Targeting Ferroptosis Attenuates Inflammation, Fibrosis, and Mast Cell Activation in Chronic Prostatitis. J Immunol Res. 2022;2022:6833867. doi:10.1155/2022/6833867
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.