Back to Journals » Infection and Drug Resistance » Volume 14
Resistance Patterns from Urine Cultures in Children Aged 0 to 6 Years: Implications for Empirical Antibiotic Choice
Authors Montagnani C, Tersigni C, D'Arienzo S, Miftode A, Venturini E , Bortone B, Bianchi L, Chiappini E, Forni S, Gemmi F , Galli L
Received 30 November 2020
Accepted for publication 3 March 2021
Published 23 June 2021 Volume 2021:14 Pages 2341—2348
DOI https://doi.org/10.2147/IDR.S293279
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Carlotta Montagnani,1 Chiara Tersigni,2 Sara D’Arienzo,3 Andreea Miftode,4 Elisabetta Venturini,1 Barbara Bortone,1 Leila Bianchi,1 Elena Chiappini,1,5 Silvia Forni,3 Fabrizio Gemmi,3 Luisa Galli1,5
1Infectious Disease Unit, Meyer Children’s University Hospital, Florence, Italy; 2Postgraduate School of Paediatrics, University of Florence, Florence, Italy; 3Regional Health Agency of Tuscany, Florence, Italy; 4University of Florence, Florence, Italy; 5Department of Health Sciences, University of Florence, Florence, Italy
Correspondence: Luisa Galli
Infectious Disease Unit, Anna Meyer Children’s University Hospital, Viale Pieraccini 24, Florence, I-50139, Italy
Tel +39-055 566 2439
Email [email protected]
Purpose: Urinary tract infection (UTI) is a frequent disorder of childhood, caused mainly by Gram negative Enterobacterales. The aim of this study is to evaluate etiology and antimicrobial susceptibility patterns of bacterial isolates in urine cultures of children under the age of 6 and to analyze the relationship between previous hospitalization or antibiotic prescriptions and antimicrobial resistance rates.
Patients and Methods: A retrospective study on positive urine cultures from 13 public laboratories in Tuscany, Italy was conducted. Data were obtained by reviewing records of the “Microbiological and Antibiotic-Resistance Surveillance System” (SMART) in Tuscany, Italy. A total of 2944 positive urine cultures were collected from 2445 children.
Results: Escherichia coli represented the majority of isolates (54,2%), followed by Enterococcus faecalis (12,3%), Proteus mirabilis (10,3%) and Klebsiella pneumoniae (6,6%). Isolated uropathogens showed high resistance rates to amoxicillin-clavulanate (> 25%), particularly in children under one year of age or hospitalized within the 12 months before the sample collection. High susceptibility rates were reported of aminoglycosides, cephalosporins and quinolones (> 90%). Previous antibiotic prescriptions by general pediatricians did not increase resistance rates.
Conclusion: Our results show a rate of amoxicillin-clavulanate resistance of 25%. Higher resistance rates were reported in children under one year of age and with previous hospitalization. Hence, amoxicillin-clavulanate should be used carefully in young children and those with severe symptoms.
Keywords: urinary tract infections, infant, antimicrobial resistance, antibiotic therapy
Introduction
Urinary tract infections (UTIs) are one of the most common infections in children, caused in the majority of cases by Gram negative bacteria with Escherichia coli as the most common isolated pathogen.1,2 The gold standard for diagnosis is represented by urinary culture, which, however, presents several difficulties especially in young children and takes time to obtain results.3 In case of clinical suspicion and with the help of rapid urinary tests (urine dipstick) and urine microscopy, it is recommended to start, as quickly as possible, an empirical antibiotic therapy based on the age of the child, the clinical presentation and local epidemiology.3–5 It is therefore pivotal to establish local patterns of antibiotic resistance to guide the choice of the empirical antibiotic therapy.3,6,7 In fact, a strong correlation between the use of antibiotics and the development of antibiotic resistance has been clearly demonstrated.8–10 In addition, the need to modify empirical treatment as quickly as possible has been recognized as one of the key elements of antimicrobial stewardship programs.11
The main aim of the present study was to evaluate the epidemiology of isolated pathogens from urine culture in children aged 0 to 6 years both in hospital and in community settings in Tuscany, Italy. The role of risk factors, such as previous hospitalization or previous antibiotic prescriptions (six months beforehand) on antibiotic-sensitivity patterns was also evaluated.
Patients and Methods
Urine cultures from outpatients and hospitalized children aged 0 to 6 years collected in the years 2017 and 2018 in Tuscany were evaluated retrospectively.
Data were obtained by reviewing records of the “Microbiological and Antibiotic-Resistance Surveillance System” (SMART) in Tuscany, Italy. The system contains information regarding isolated pathogens and sensitivity tests from blood, liquor and urine culture from 13 public laboratories in Tuscany.
Urine cultures replicated within 28 days were excluded.
The following data were collected according to the European Antimicrobial Resistance Surveillance Network (EARS-net) criteria: demographic characteristics (age, sex), isolated pathogens, antimicrobial susceptibility tests, data regarding hospital admissions (at the time of sample collection, four days before or after sample collection, one year before sample collection), antimicrobial prescriptions by the general pediatrician six months before the sample collection.12
Data on hospitalization and antibiotic use were obtained from Hospital Discharge Abstract.
To define children treated with antibiotics, the Anatomical Therapeutic Chemical classification system was used (J01, antibacterials for systemic use). Data regarding antibiotic consumption in Tuscany included: drugs supplied by both private and public pharmacies, molecules dispensed under medical prescription and pharmaceutical services provided directly by public structures. At least one prescription of these drugs in the period between 0 and 180 days prior to the urine culture collection was included in the analysis.
This study was conducted in accordance with the Helsinki Declaration.
According to Italian legislation (legislative decree 211/2003) and regional procedures, the study does not need ethics approval as it is a purely observational study on routine collected anonymous data. Moreover, informed consent to participate in the study is not required since data were obtained by an anonymous regional surveillance system. Furthermore, because this was an observational retrospective study, patients had already been treated when the study protocol was written; therefore, it could not have modified their life-trajectories or care pathways in any way.
Antimicrobial prescriptions by the general pediatrician in the last six months (in non-hospitalized children) before the sample collection and hospital admissions in the year before were considered as risk factors for the development of UTIs caused by a resistant pathogen.
Children were classified in three categories:
- Those hospitalized during the last year before urine culture collection (difference between data of collection of the urine sample and data of hospital discharge between 0 and 365 days)
- Those with antibiotic prescriptions in the six months before urine culture collection
- Those without hospitalization history or antibiotic prescriptions
Statistical analysis was performed using STATA (version 14.0). The χ-square test and Fisher test were performed when appropriate.
Results
Overall, 2944 positive urine cultures were evaluated in 2445 children. One thousand three hundred and sixteen (54%) were female. The median age of enrolled children was 13 months (Interquartile range [IQR]: 4–34 months). Children under one year of age accounted for the highest proportion of positive urine cultures (1184 patients, 48%).
A statistically significant difference in sex distribution regarding positive urine cultures was observed (p <0.001). In particular, regarding children under one year of age, positive urine cultures were detected more frequently in males (658, 55%), whereas the highest proportion was observed in females (790/1261, 62.5%) in older children.
About 10% of children (258/2445) were hospitalized in the year before the urine sample collection, with 39% of patients under one year of age. In 34.3% of non-hospitalized children (751/2187) at least one course of antibiotic therapy in the 6 months before the collection of the urinary sample was prescribed.
Age and sex distribution in children with/without risk factors for UTIs is reported in Table 1.
 |
Table 1 Age and Sex Distribution of Enrolled Children According to Different Categories (Hospitalized Children and Outpatients) and Risk Factors |
Hospitalized children at the time of urine culture, four days before or four days after the sample collection, were most commonly under one year of age (340/1184 vs 121/1261, p<0.001).
Urine Culture results
During the study period, 3247 microorganisms were isolated in 2944 urine cultures. Of those, 498 (15.3%) were isolated in children with a history of hospitalization in the previous year. Regarding patients who had never been hospitalized, 1017 microorganisms (31.3%) were isolated in children who received antibiotic therapy in the previous 6 months and 1732 (53.3%) in patients with no history of previous antibiotic prescriptions.
Isolated Pathogens
The most frequently isolated microorganism was E. coli with 1759/3247 total cases (54.2%), followed by Enterococcus faecalis (400/3247,12.3%), Proteus mirabilis (336/3247, 10.3%) and Klebsiella pneumoniae (214/3247, 6.6%)
Pathogen distribution according to age groups was reported and risk factors were reported in Tables 2 and 3.
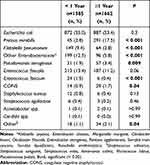 |
Table 2 Isolated Pathogens According to Age Groups |
 |
Table 3 Pathogen Distribution in Children with and without Risk Factors |
P. mirabilis and P. aeruginosa were isolated more frequently in children with previous hospitalization in the last year or an antibiotic prescription in the last 6 months (p <0.001 and p = 0, 006, respectively). On the contrary, isolation of K. pneumoniae was more frequent in children without risk factors (p<0.001).
Antibiotic Resistance Patterns and Antimicrobial Susceptibility Tests
Resistance patterns of isolated pathogens in urine cultures to the main classes of antibiotics are reported in Table 3. Isolated microorganisms in urine cultures of children under one year of age were more resistant (p <0.001 for aminoglycosides, cephalosporins and quinolones, p = 0.002 for amoxicillin-clavulanic acid) (Table 4).
 |
Table 4 Antibiotic Resistance Patterns Related to Age Groups, to Hospital Admissions in the Year Before the Sample Collection and to Children with and without Risk Factors |
Resistance patterns of isolated microorganisms in relation to previous hospitalization in the year preceding urine culture are reported in Table 4. In previously hospitalized children, a higher percentage of resistant strains to cephalosporins (p = 0.009), amoxicillin-clavulanic acid (p = 0.02) and quinolones (p = 0.005) was reported.
Higher resistance rates were detected in children with no history of hospitalization in the previous year or with an antibiotic prescription in the previous 6 months for aminoglycosides (p = 0.003), cephalosporins (p <0.001) and quinolones (p = 0.05). On the contrary, no statistically significant differences were reported for amoxicillin-clavulanic acid (Table 4).
E. coli showed a good susceptibility profile to cephalosporins and aminoglycosides (>90%). On the other hand, the percentages of resistance rates to ampicillin and amoxicillin-clavulanic acid were high (above 40 and 20% respectively) (Figure 1).
 |
Figure 1 Antimicrobial susceptibility patterns of the most common isolated pathogens. Abbreviations: S, susceptible; I, intermediate; R, resistant. |
E. faecalis, the second most frequently isolated microorganism, was largely sensitive to antibiotics for which its resistance profile was evaluated, with percentages >95% (Figure 1).
P. mirabilis showed sensitivity to amoxicillin-clavulanic acid, cephalosporins and to aminoglycosides in more than 90% of cases but higher resistance rates were reported for ampicillin and co-trimoxazole (>20%) (Figure 1).
K. pneumoniae exhibited resistance rates >20% against amoxicillin-clavulanic acid, piperacillin-tazobactam, gentamicin and levofloxacin. Sensitivity to cephalosporins was >90% (Figure 1).
Discussion
Although the diagnosis of UTI is based on the microbiological confirmation obtained through urine culture, the recommendation to start early empirical antibiotic treatment in suspected cases is widely accepted, in order to avoid potential complications, especially in younger children.3,6,7 Therefore, the knowledge of local epidemiology and susceptibility patterns of pathogens as a guide for empirical antibiotic choice is pivotal for correct management of UTIs in children.
Recently published Italian guidelines suggested as first-line oral treatment amoxicillin-clavulanic acid followed by III generation cephalosporins (cefixime and ceftibuten).7 The American Academy of Pediatrics (AAP) guidelines also reported the use of amoxicillin-clavulanic acid, cephalosporins of I (cefalexin), II (cefprozil and cefuroxime axetil) and III generation (cefixime and cefpodoxime) and of cotrimoxazole as first-line suggested treatments.3 In addition, the English National Institute for Health and Care Excellence guidelines suggested the use of oral cefalexin as first treatment option. The use of amoxicillin-clavulanic acid was reserved to situations in which sensitivity was demonstrated by urine culture.6
In case of intravenous treatment, both Italian and English guidelines, suggested the use of amoxicillin-clavulanic acid or ampicillin-sulbactam and, as alternative treatment, third-generation cephalosporins (cefotaxime or ceftriaxone) or aminoglycosides.6,7 The AAP recommended the use of piperacillin, third-generation cephalosporins (ceftriaxone, cefotaxime or ceftazidime) or aminoglycosides.3 The use of fluoroquinolones remains controversial and should be reserved to selected cases based on pathogen resistance patterns.7
Our retrospective study describes the susceptibility patterns of microorganisms isolated from urine cultures obtained from children in Tuscany in the years 2017–2018. Eighty-five percent of the enrolled children were not hospitalized at the time of sample collection. The most frequently isolated pathogens were E. coli (54.2%), followed by E. faecalis (12.3%) and P. mirabilis (10.3%). Resistance rates to amoxicillin-clavulanic acid in our setting was high (>25%), particularly in children under one year of age compared to the older ones (27,9% vs 23.4%, p=0.002) and with a history of hospitalization in the previous year (29,7% vs 25%, p=0.02).
These results were in line with a study published in 2016 by Calzi et al, in which the percentages of resistance rates for E. coli and other Enterobacterales to amoxicillin-clavulanic acid were over 30% (35.6 and 39.3% respectively).13 A greater susceptibility to amoxicillin-clavulanic acid however was found in children under one year (26.1% of resistance compared to 32.4% in patients aged ≥1 year).13
Based on antibiotic resistance patterns reported by Calzi et al and confirmed by our study, the choice of use of amoxicillin-clavulanic acid or ampicillin-sulbactam as empirical first-line treatment should be carefully evaluated, above all in children under one year of age, and limited to patients in good clinical conditions.13
Cephalosporins showed acceptable values of susceptibility rates (91%), representing a valid therapeutic option. A similar sensitivity pattern was reported in the study conducted by Calzi et al, with a resistance of E. coli to cefuroxime of about 11%. Aminoglycosides also showed a good susceptibility profile (93%).13 However, based on the nephrotoxicity of this category of drugs, they should be used with caution.14–17 A retrospective study conducted in 2011 showed that a percentage ranging from 20 to 30% of children receiving an aminoglycoside (amikacin, netilmicin and streptomycin) for more than 5 days developed acute kidney injury.15,16 Furthermore, in case of UTIs caused by P. aeruginosa, monotherapy with aminoglycosides was associated with an increased risk of resistance.17
In addition, in our study the highest percentages of resistance rates were reported in children under one year of age. This may be associated with peripartum exposure to maternal antibiotics, which increases the risk of resistant rods in the newborn.18,19 In fact, a significant increase in E. coli’s resistance rates to amoxicillin in newborns with a history of maternal antibiotic treatment was reported (81.8% compared to 35.5%, respectively).18,19 Furthermore, the increased resistance rates in children under one year of age could be related to the acquisition of nosocomial microorganisms at the time of birth.18,19
Moreover, high resistance rates to amoxicillin-clavulanic acid found in our study could also be related to the wide use of amoxicillin-clavulanic acid compared to other Italian regions (amoxicillin/amoxicillin clavulanic acid ratio of 0.2, compared to the national average of 0.3), particularly in central and northern Italy (ratio 0.5).20,21 A more rational use of amoxicillin-clavulanic acid for respiratory infections (pharyngotonsillitis, otitis and pneumonia) could lead to a decrease in resistance rates of Enterobacterales, as already reported for Streptococcus pyogenes to macrolides.22–24
Notably, isolated pathogens in children with a history of hospitalization in the previous year had a greater resistance to amoxicillin-clavulanic acid, cephalosporins and fluoroquinolones compared to non-hospitalized children (0.002, 0.009 and 0.005, respectively). Several studies showed a relationship between recent hospitalizations and the selection of multidrug resistant (MDR) microorganisms.25 Recent hospitalizations (1–3 months beforehand) were reported to be independent risk factors for the development of UTIs caused by strains producing extended-spectrum beta-lactamase.26–28 In a French study published in 2016, an association between hospitalization in the previous 6 months and selection of strains of E. coli ST131, a group of MDR clones resistant to cephalosporins and fluoroquinolones, was reported.29
An evaluation of antibiotic resistance profiles was also performed in relation to the presence of risk factors (hospitalization in the previous 12 months or previous antibiotic prescriptions by the general pediatrician in the 6 months beforehand). In children without risk factors, resistance rates were significantly higher for aminoglycosides, cephalosporins and quinolones. On the other hand, no statistically significant differences were reported for amoxicillin-clavulanic acid. A recent antibiotic prescription therefore was not associated with an increased risk of resistance rates.
This finding is in contrast to what emerged from previous published studies, in which a correlation between recent exposure to antibiotics and an increase in resistance rates of uropathogens was reported.9,26,30
Our study presents several limitations. Only antibiotics prescribed by general pediatricians were evaluated, whereas prescriptions in hospital settings and self-administered drugs by parents were not included in the analysis. Data regarding the real prescribed antibiotic consumption was unknown. In addition, only urine culture results were available without clinical correlation (ie urine analysis, symptoms), without information regarding sample collection (and possible contaminations) and regarding bacterial load. A crude analysis of positive urine cultures and antibiotic resistance patterns was carried out without clinical correlations.
Further studies on this topic are needed to evaluate the correlation between recent exposure to antibiotics and antibiotic resistance patterns in the pediatric population. However, in the light of rational use of antibiotics, the use of broad-spectrum antibiotics should be avoided if not indicated by clinical presentation and local epidemiology.
Conclusion
A high rate of resistance to amoxicillin-clavulanic acid in microorganisms isolated from urine cultures in children, especially in the first year of life and with a history of hospitalization in the previous year emerged from our study. On the contrary, cephalosporins showed an acceptable susceptibility profile. This data is fundamental for the choice of an empirical therapy of UTIs, especially in critically ill patients and infants.
An accurate knowledge of local epidemiology should be the basis for empirical therapy choices. A more judicious use of antibiotics, especially regarding the use of broad-spectrum molecules in infection typically caused by sensitive pathogens, is pivotal in order to reduce the spread of MDR microorganisms.
Abbreviations
AAP, American Academy of Pediatrics; IQR, interquartile range; MDR, multidrug resistant; UTIs, urinary tract infections.
Ethics Approval
According to the Italian legislation (legislative decree 211/2003) and the regional procedures, the study does not need ethics approval as it is a purely observational study on routine collected anonymous data. Furthermore, because this was an observational retrospective study, patients had already been treated when the study protocol was written; therefore, it could not have modified their life-trajectories or care pathways in any way.
Informed Consent
Informed consent to participate in the study is not required since data were obtained by anonymous regional surveillance system.
Author Contributions
CM, CT, LG and SF conceived the study. SF, SD’A and AM performed statistical analysis. CM and CT wrote the manuscript. EV, BB, EC and LB revised available literature on the topic. LG and FG revised the final draft of the manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
There is no funding source.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Millner R, Becknell B. Urinary tract infections. Pediatr Clin North Am. 2019;66:1–13. doi:10.1016/j.pcl.2018.08.002
2. Chang SL, Shortliffe LD. Pediatric urinary tract infections. Pediatr Clin North Am. 2006;53:379–400. doi:10.1016/j.pcl.2006.02.011
3. Roberts KB; Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595–610.
4. Okarska-Napierała M, Wasilewska A, Kuchar E. Urinary tract infection in children: diagnosis, treatment, imaging - comparison of current guidelines. J Pediatr Urol. 2017;13:567–573. doi:10.1016/j.jpurol.2017.07.018
5. Stein R, Dogan HS, Hoebeke P, et al. European society for pediatric urology. Urinary tract infections in children: EAU/ESPU guidelines. Eur Urol. 2015;67:546–558. doi:10.1016/j.eururo.2014.11.007
6. National Collaborating Centre for Women’s and Children’s Health. Urinary tract infection in children: diagnosis, treatment and long-term management; 2007. Available from: http://guidance.nice.org.uk/CG54.
7. Ammenti A, Alberici I, Brugnara M, et al. Italian society of pediatric nephrology. Updated Italian recommendations for the diagnosis, treatment and follow-up of the first febrile urinary tract infection in young children. Acta Paediatr. 2020;109:236–247. doi:10.1111/apa.14988
8. Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–433. doi:10.1128/MMBR.00016-10
9. Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. BMJ. 2016;352:i939. doi:10.1136/bmj.i939
10. Erol B, Culpan M, Caskurlu H, et al. Changes in antimicrobial resistance and demographics of UTIs in pediatric patients in a single institution over a 6-year period. J Pediatr Urol. 2018;14:
11. Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56–66. doi:10.1016/S1473-3099(18)30605-4
12. European Centre for Disease Prevention and Control. Surveillance atlas of infectious diseases. Available from: https://atlas.ecdc.europa.eu/public/index.aspx.
13. Calzi A, Grignolo S, Caviglia I, et al. Resistance to oral antibiotics in 4569 Gram-negative rods isolated from urinary tract infection in children. Eur J Pediatr. 2016;175:1219–1225. doi:10.1007/s00431-016-2763-1
14. McWilliam SJ, Antoine DJ, Smyth RL, Pirmohamed M. Aminoglycoside-induced nephrotoxicity in children. Pediatr Nephrol. 2017;32:2015–2025. doi:10.1007/s00467-016-3533-z
15. Zappitelli M, Moffett BS, Hyder A, Goldstein SL. Acute kidney injury in non-critically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transplant. 2011;26:144–150. doi:10.1093/ndt/gfq375
16. Begg EJ, Barclay ML. Aminoglycosides–50 years on. Br J Clin Pharmacol. 1995;39:597–603.
17. Daikos GL, Jackson GG, Lolans VT, Livermore DM. Adaptive resistance to aminoglycoside antibiotics from first-exposure down-regulation. J Infect Dis. 1990;162:414–420. doi:10.1093/infdis/162.2.414
18. Arshad M, Seed PC. Urinary tract infections in the infant. Clin Perinatol. 2015;42:17–28. doi:10.1016/j.clp.2014.10.003
19. Didier C, Streicher MP, Chognot D, et al. Late-onset neonatal infections: incidences and pathogens in the era of antenatal antibiotics. Eur J Pediatr. 2012;171:681–687. doi:10.1007/s00431-011-1639-7
20. Agenzia Regionale Sanità Toscana. I profili di antibiotico-resistenza: le urinocolture; 2018. Available from: https://www.ars.toscana.it/images/pubblicazioni/Collana_ARS/2019/set_diapositive_resistenze_2018_urinocolture.ppt.
21. Agenzia Italiana del Farmaco. L’uso degli antibiotici in Italia; 2018. Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2894_allegato.pdf.
22. De Rosa R, Avolio M, Stano P, Modolo ML, Camporese A. Disappearance of Streptococcus pyogenes macrolide resistance in an area of northeastern Italy: a possible link with rational long-acting macrolide consumption. Infez Med. 2009;17:82–87.
23. Montagnani F, Stolzuoli L, Croci L, et al. Erythromycin resistance in Streptococcus pyogenes and macrolide consumption in a central Italian region. Infection. 2009;37:353–357. doi:10.1007/s15010-008-8023-1
24. Gherardi G, Petrelli D, Di Luca MC, et al. Decline in macrolide resistance rates among Streptococcus pyogenes causing pharyngitis in children isolated in Italy. Eur J Clin Microbiol Infect Dis. 2015;34:1797–1802. doi:10.1007/s10096-015-2414-x
25. Mancini A, Pucciarelli S, Lombardi FE, Barocci S, Pauri P, Lodolini S. Differences between community - and hospital - acquired urinary tract infections in a tertiary care hospital. New Microbiol. 2020;43:17–21.
26. Fan NC, Chen HH, Chen CL, et al. Rise of community-onset urinary tract infection caused by extended-spectrum β-lactamase-producing Escherichia coli in children. J Microbiol Immunol Infect. 2014;47:399–405. doi:10.1016/j.jmii.2013.05.006
27. Albaramki JH, Abdelghani T, Dalaeen A, et al. Urinary tract infection caused by extended-spectrum β-lactamase-producing bacteria: risk factors and antibiotic resistance. Pediatr Int. 2019;61:1127–1132. doi:10.1111/ped.13911
28. Uyar Aksu N, Ekinci Z, Dündar D, Baydemir C. Childhood urinary tract infection caused by extended-spectrum β-lactamase-producing bacteria: risk factors and empiric therapy. Pediatr Int. 2016;59:176–180. doi:10.1111/ped.13112
29. Birgy A, Levy C, Bidet P, et al. ESBL-producing Escherichia coli ST131 versus non-ST131: evolution and risk factors of carriage among French children in the community between 2010 and 2015. J Antimicrob Chemother. 2016;71:2949–2956. doi:10.1093/jac/dkw219
30. Bryce A, Costelloe C, Wootton M, Butler CC, Hay AD. Comparison of risk factors for, and prevalence of, antibiotic resistance in contaminating and pathogenic urinary Escherichia coli in children in primary care: prospective cohort study. J Antimicrob Chemother. 2018;73:1359–1367. doi:10.1093/jac/dkx525
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
